Introduction
Ischemic stroke is one of the leading causes of
disability in adults worldwide, and the poor prognosis for stroke
is largely due to a lack of effective therapies (1). Ischemia and hypoxia lead to the
release of cytokines, glutamate excitotoxicity, calcium overload,
oxidative stress, disordered metabolism, inflammation and apoptosis
in nerve cells (2,3). It has been demonstrated that
oxidative stress, inflammation and apoptosis are significant in
ischemic injury (4). Reactive
oxygen species (ROS) induce lipid peroxidation, protein oxidation
and DNA damage (5). Nitric oxide
(NO) is one of the ROS and is generated from L-arginine by nitric
oxide synthase (NOS). Cerebral ischemia induces excessive NO
production, which may aggravate cell injury. Liu et al
investigated antioxidative or antiapoptotic strategies against
ischemic injury (6). Although
numerous agents have been demonstrated to exhibit neuroprotective
effects in animal experiments, the majority of these agents fail to
show efficacy in clinical trials. Therefore, the development of
novel agents remains an important issue. Natural products,
particularly medicinal plants, have been the subjects of
significant focus with regard to neuroprotection in ischemia
(7).
Pana quinquefolium, also known as American
ginseng, is a medicinal herb with a long history in China and other
countries. Pana quinquefolium has been described to possess
anti-stress, anti-diabetic and antitumor effects (8–11).
The major active components of Pana quinquefolium are
ginsenosides, which are divided into the protopanaxadriol,
protopanaxatriol and oleanolic acid ginsenosides, according to
their structure. Panax quinquefolium 20(S)-protopanaxadiol
saponins (PQDS) are extracts from the stems and leaf of Pana
quinquefolium L. and contain numerous ginsenosides, including
Rd, Rb2, Rb3, Rc, Rg3 and pF11 (12).
Recently, our laboratory demonstrated that PQDS were
able to reduce the infarct size in acute myocardial infarction in
rats (13). However, the effects
of PQDS on cerebral ischemia have not yet been elucidated. On the
basis of previous studies, we hypothesized that PQDS may have a
protective effect in cerebral ischemia. Therefore, in the present
study, a middle cerebral artery occlusion (MCAO) model of cerebral
ischemia was used to examine the protective effects of PQDS in
cerebral ischemia and to investigate the potential mechanism
underlying the effects of PQDS in rats.
Materials and methods
Chemicals and reagents
Malondialdehyde (MDA), superoxide dismutase (SOD),
NO, NOS, calcium (Ca2+) and
Na+-K+-ATP assay kits were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Antibodies against Bcl-2 were obtained from Cell Signaling
Technology, Inc. (Beverly, MA, USA), while antibodies against
β-actin were purchased from Tianjin Jinmai Gene Mapping Technology
Co., Ltd. (Tianjin, China). The PQDS were obtained from Dr Yanping
Chen and dissolved in physiological saline for use. All other
chemicals were analytical reagents.
Animals
Male Sprague-Dawley rats (weight, 230–280 g) were
purchased from the Experimental Animal Center of Jilin University
(Changchun, China). All rats were allowed free access to food and
water. The experiments were performed in accordance with the Guide
for the Care and Use of Laboratory Animals of Jilin University, and
approved by the Ethics Committee of Jilin University.
The rats were randomly divided into five groups (12
rats in each group): i) Sham surgery, where physiological saline
was administered to the rats by intraperitoneal (i.p.) injection at
a dose of 2 ml/kg; ii) model, where physiological saline was
administered to the rats by i.p. injection at a dose of 2 ml/kg;
iii) PQDS 12.5 mg/kg, where rats were treated with PQDS by i.p.
injection at a dose of 12.5 mg/kg; iv) PQDS 25.0 mg/kg, where rats
were treated with PQDS by i.p. injection at a dose of 25.0 mg/kg;
and v) PQDS 50.0 mg/kg, where rats were treated with PQDS by i.p.
injection at a dose of 50.0 mg/kg. The drug or saline treatment was
administered once a day for three consecutive days. Thirty minutes
following the final treatment, the rats were anesthetized for the
induction of cerebral ischemia.
Surgical procedures
The MCAO was performed with a modification, as
described previously (14).
Briefly, the rats in the model and PQDS treatment groups were
anesthetized with a 10% i.p. injection of 10% choral hydrate (350
mg/kg). The right common carotid artery, external carotid artery
and internal carotid were exposed through a neck incision. The
external carotid artery was cut and a monofilament nylon suture
with a heated-rounded tip was inserted into the external carotid
artery and gently advanced into the internal carotid artery, until
a slight resistance was felt. The sham-surgery rats underwent the
same surgical procedure with the exception of the MCAO ligation.
The suture was tightened around the intraluminal filament to
prevent bleeding and the suture was left in place until the rats
were sacrificed. The body temperature of the rats was monitored
with a rectal probe and was maintained at 37±0.5ºC by a heating pad
throughout the surgery.
Score of neurological deficits
Twenty-four hours subsequent to the induction of the
ischemia, the neurological deficits in each rat were assigned a
score, using a scale as previously described (15): 0, no observable deficit; 1,
difficulty in fully extending the contralateral forelimb; 2, unable
to extend the contralateral forelimb; 3, mild circling to the
contralateral side; 4, severe circling; and 5, falling to the
contralateral side.
Measurement of infarct area
The infarct volume was assessed using the
2,3,5-triphenyltetrazolium chloride (TTC) method, as described
previously (16). The rats were
sacrificed and the brains were extracted following the measurement
of the neurological deficit score. Each brain was cut into five
coronal slices with a blade, and the brain slices were stained with
2% TTC solution at 37ºC for 30 min. Following staining, the areas
of cerebral infarction were identified using the different
color-tones (white for ischemic cerebral tissue and red for
non-ischemic cerebral tissue). The infarct size was calculated as a
percentage fraction of the ischemic cerebral tissue in the whole
brain.
Measurement of brain water content
The brain water content was measured using the
wet-dry method (17). Following
the measurement of the neurological deficit score, the rats were
anesthetized using chloral hydrate (350 mg/kg, i.p.) and then
sacrificed, prior to the rat brains being rapidly extracted. The
pons and olfactory bulb were removed and the wet weight of the
brains was measured using an electronic balance. Subsequently, the
brains were dried for 24 h at 100ºC in order to obtain the dry
weight. The brain water content (BW) was calculated as follows: BW
= [(wet weight - dry weight) / wet weight] × 100, and used as an
index for brain edema.
Analysis of MDA and Ca2+
levels and the activities of SOD and
Na+-K+-ATPase in the brain tissue
Following the collection of the blood samples, the
brains were removed, weighed and homogenized in ice-cold
phosphate-buffered saline (PBS). The homogenate was centrifuged
(2,500 × g, 15 min) and the supernatant was obtained to measure the
activities of SOD and Na+-K+-ATPase and the
levels of MDA and Ca2+ using assay kits and a
spectrophotometer (7202B; Unico (Shanghai) Instrument Co., Ltd.,
China), in accordance with the kit manufacturer's instructions.
Biochemical analysis
Having measured the neurological deficit score, the
rats were anesthetized with chloral hydrate (350 mg/kg, i.p.).
Blood samples were collected from the abdominal artery into
non-heparinized tubes, allowed to clot for 2 h at room temperature
and then centrifuged at 2,000 × g for 15 min. The NO level and the
activity of NOS were measured using diagnostic kits according to
the manufacturer's instructions.
Histopathological examination
Having measured the neurological deficit score, the
rats were sacrificed by decapitation. The brains were rapidly
removed and placed into 4% paraformaldehyde solution for one day
and then embedded in paraffin. Five-micrometer sections were cut
and the brain sections were stained with hematoxylin and eosin
(H&E). The brain sections were subsequently examined under a
microscope (Nikon ECLIPSE 80i; Nikon, Tokyo, Japan) and
photomicrographs were taken.
Western blotting
The expression of Bcl-2 protein in the rats was
analyzed using western blotting. Briefly, the brains were dissected
and homogenized with a lysis buffer [1× PBS, 1% NP-40, 0.5% sodium
deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and
phenylmethylsulfonyl fluoride (PMSF)] to extract the cellular
proteins, prior to being centrifuged at 1,200 × g for 15 min at
4ºC. The protein concentration was determined using the
bicinchoninic acid (BCA) method. Equal quantities of protein were
separated using 12% SDS-polyacrylamide gel electrophoresis (PAGE)
and transferred onto polyvinylidene difluoride (PVDF) membranes.
Subsequent to blocking with 5% non-fat milk in PBS-Tween-20 (PBST)
for 1 h, the membranes were incubated overnight at 4ºC with
anti-Bcl-2, and anti-β-actin antibodies. The membranes were then
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibody (Tianjin Jinmai Gene Mapping Technology Co., Ltd.) against
rabbit for 1 h at room temperature. Immunoactive bands were
visualized with an enhanced chemiluminescence (ECL) detection
system using X-ray film. β-actin was used as an internal
control.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical significance was determined using one-way
analysis of variance (ANOVA) followed by Dunnett's test. In all
cases, P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of PQDS on neurological
deficits
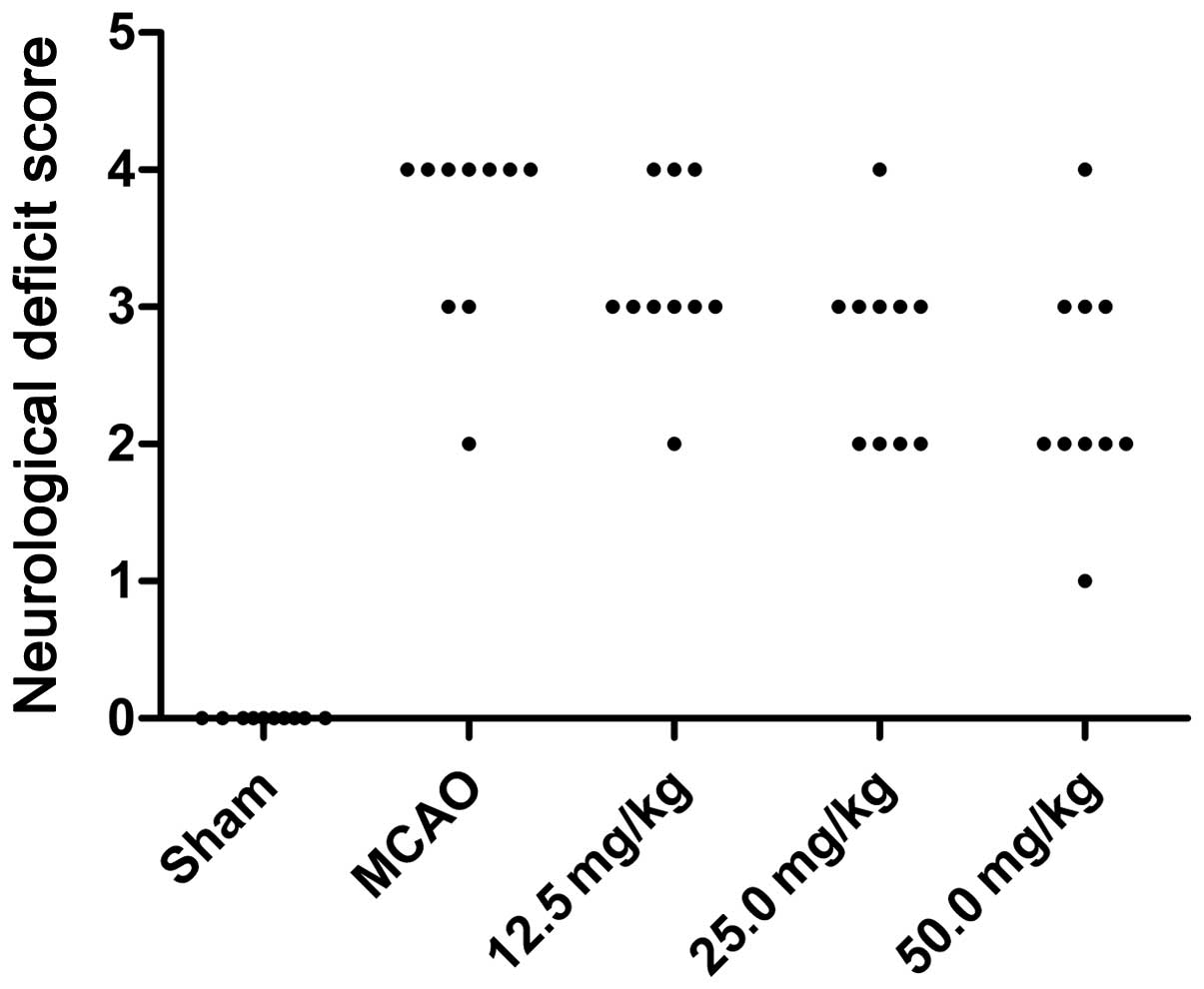
Twenty-four hours subsequent to ischemia, the
neurological deficits of the rats were assessed. The neurological
deficit scores for the sham, MCAO and PQDS 12.5, 25.0 and 50.0
mg/kg groups were 0, 3.6±0.70, 3.2±0.63, 2.7±0.67 and 2.4±0.84,
respectively. The behavioral abnormalities were particularly
apparent in the MCAO group, while PQDS treatment (25.0 and 50.0
mg/kg) significantly suppressed the development of the behavioral
abnormalities (P<0.05; Table I
and Fig. 1).
 | Table IEffect of PQDS on neurological deficit
scores in rats. |
Table I
Effect of PQDS on neurological deficit
scores in rats.
| Score (n) | |
|---|
|
| |
|---|
| Group | 0 | 1 | 2 | 3 | 4 | 5 | Average score |
|---|
| Sham | 10 | - | - | - | - | - | - |
| MCAO | - | - | 1 | 2 | 7 | - | 3.6±0.70a |
| PQDS |
| 12.5 mg/kg | - | - | 1 | 6 | 3 | - | 3.2±0.63 |
| 25.0 mg/kg | - | - | 4 | 5 | 1 | - | 2.7±0.67b |
| 50.0 mg/kg | - | 1 | 5 | 3 | 1 | - | 2.4±0.84a |
Effect of PQDS on brain infarcted area
and brain water content
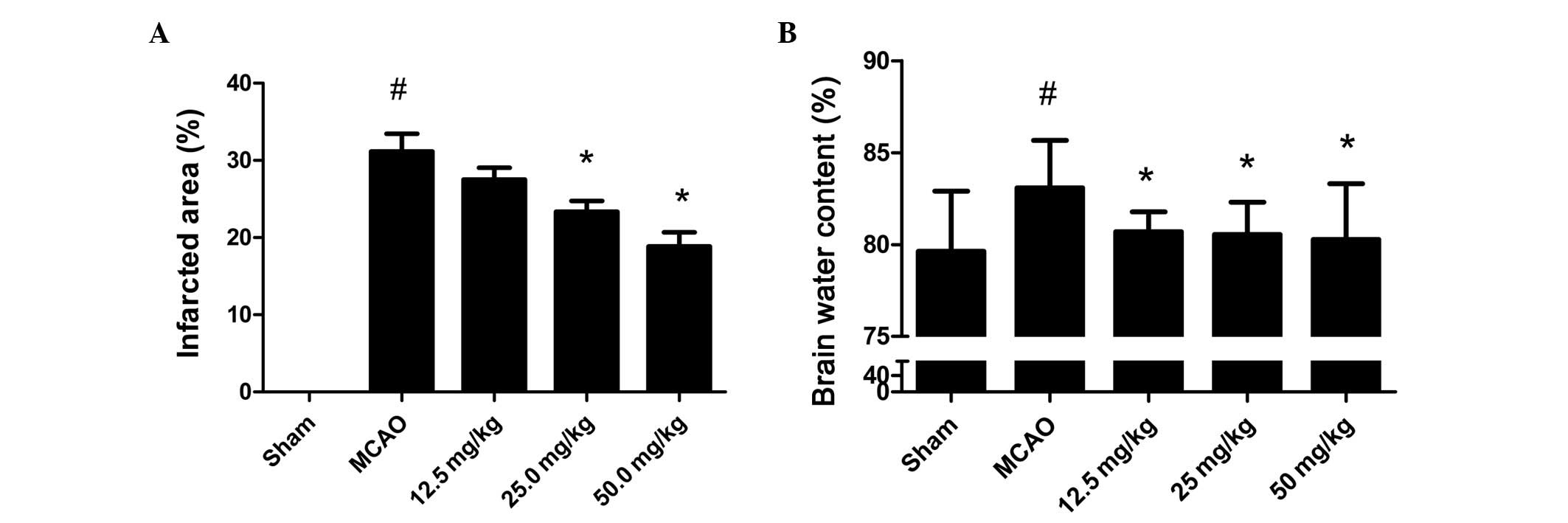
To investigate the effect of PQDS on cerebral
ischemia, the infarcted area and brain water content were measured.
As shown in Fig. 2A, no infarcted
area was observed in the sham group. The infarcted areas in the
PQDS 12.5, 25.0 and 50.0 mg/kg groups were 27.5±3.78, 23.33±3.50
and 18.83±4.49%, respectively, which were lower than that in the
MCAO group (31.17±5.56%). The infarcted area tended to be smaller
following treatment with 12.5 mg/kg PQDS, although the reductions
were not statistically significant.
The brain water content following 24 h ischemia is
shown in Fig. 2B. In the sham
group, the water content was 79.63±3.29%. Ischemia led to a
significant increase in water content in the MCAO group compared
with that in the sham group (83.09±2.57%, P<0.05). However, in
the PQDS treatment groups, the water content was significantly
decreased in a dose-dependent manner. The mean brain water contents
were 80.71±1.08, 80.55±1.75 and 80.27±3.03% in the 12.5, 25.0 and
50.0 mg/kg PQDS groups, respectively.
Effect of PQDS on the levels of MDA and
Ca2+ and the activities of SOD and
Na+-K+-ATPase in brain tissue
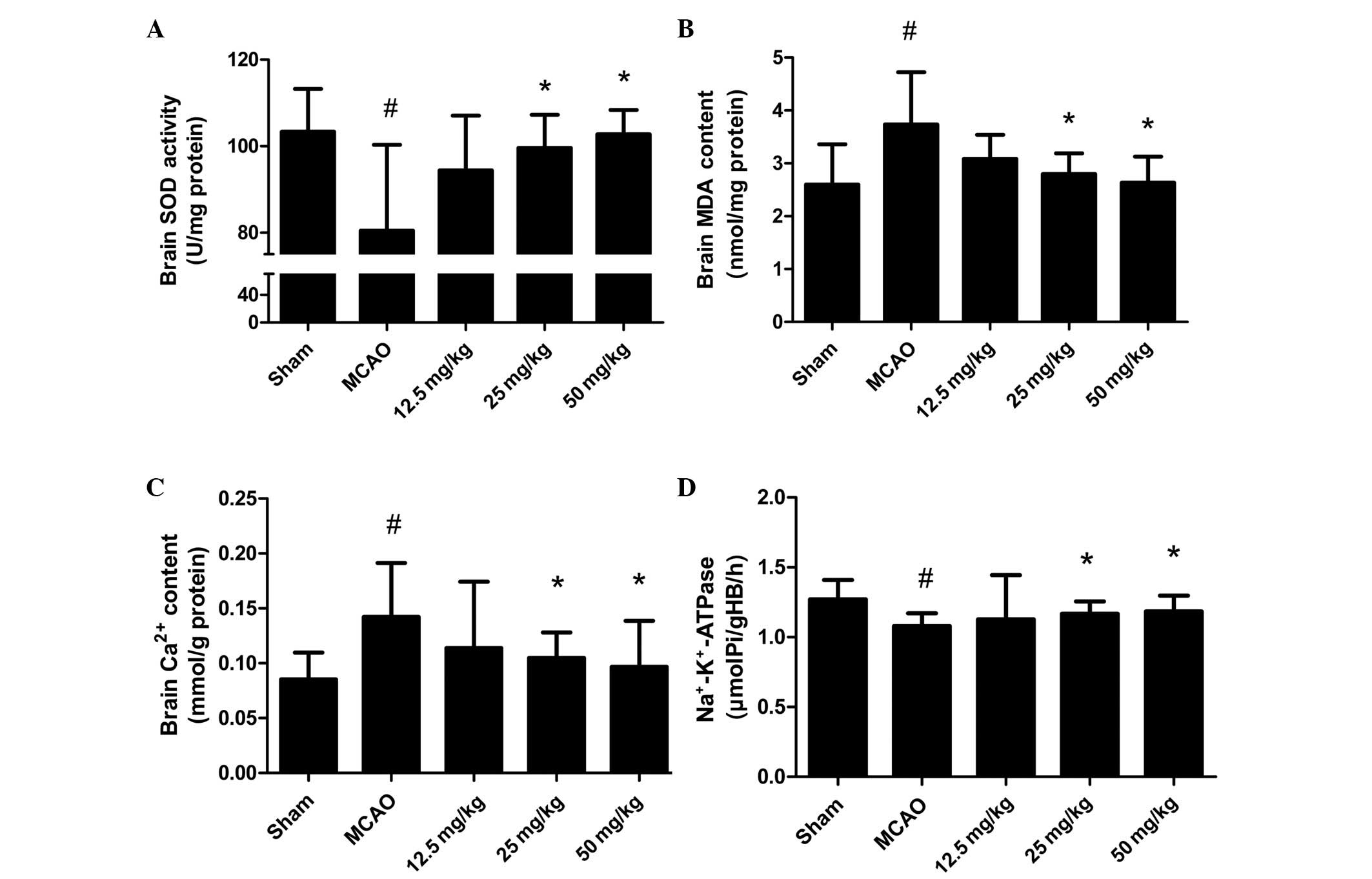
To illustrate the effect of PQDS on the oxidative
stress induced by MCAO, the levels of MDA and Ca2+ and
the activities of SOD and Na+-K+-ATPase were
measured. The levels of MDA and Ca2+ were increased in
the MCAO group compared with those in the sham group (Fig. 3). PQDS treatment (25.0 and 50.0
mg/kg) significantly reduced the levels of MDA and Ca2+
in a dose-dependent manner compared with those in the MCAO group.
Conversely, significant reductions in the activities of SOD and
Na+-K+-ATPase were observed in the MCAO
group, which were significantly attenuated by PQDS treatment (25.0
and 50.0 mg/kg).
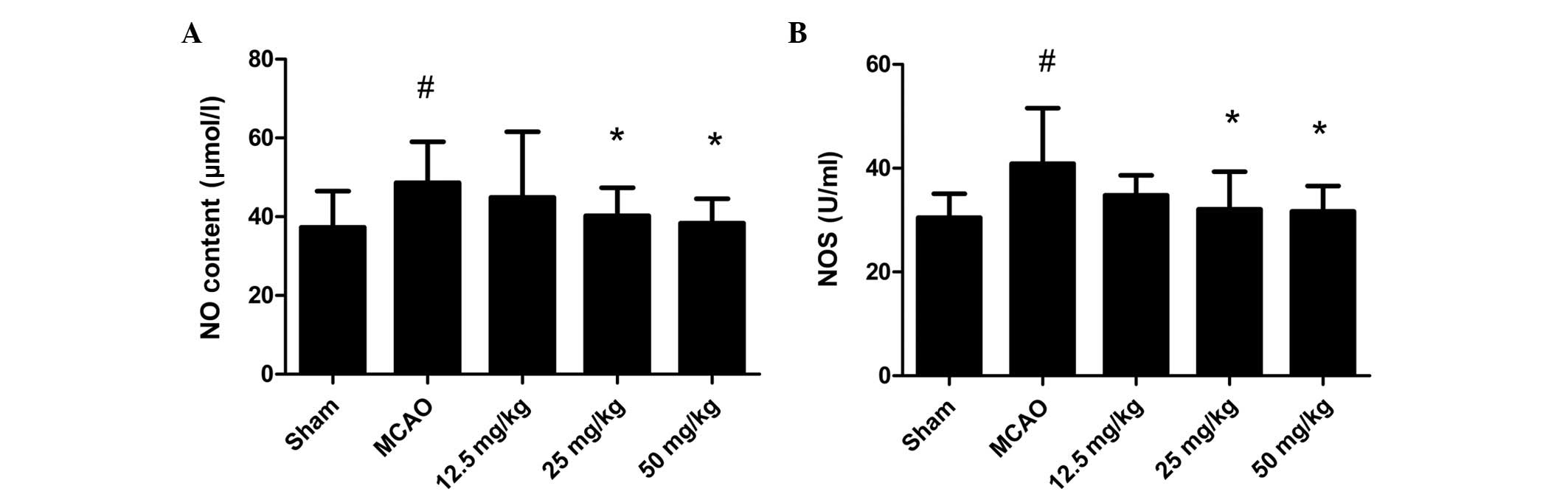
Effect of PQDS on the NO and NOS levels
in the serum
The changes in the NO and NOS levels are shown in
Fig. 4. The NO level and NOS
activity were increased in the MCAO group compared with those in
the sham group. PQDS treatment significantly reduced the NO level
and NOS activity in a dose-dependent manner compared with those in
the MCAO group.
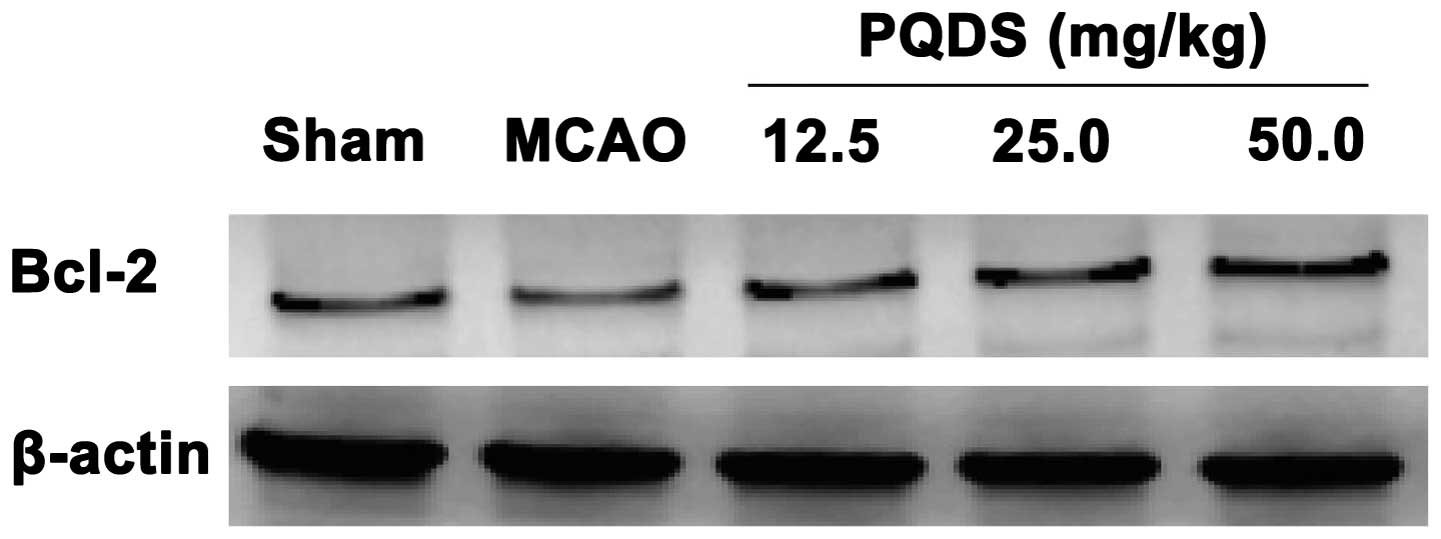
Effect of PQDS on Bcl-2 expression
To gain an insight into the apoptotic signaling, the
expression of the antiapoptotic protein Bcl-2 was analyzed. The
level of Bcl-2 protein expressed in the MCAO group was markedly
decreased compared with that in the sham group, which was
consistent with previous studies (18). Pretreatment with PQDS significantly
increased the expression of Bcl-2 compared with that in the MCAO
group, suggesting that the protective effects of PQDS may be
mediated by the inhibition of apoptosis (Fig. 5).
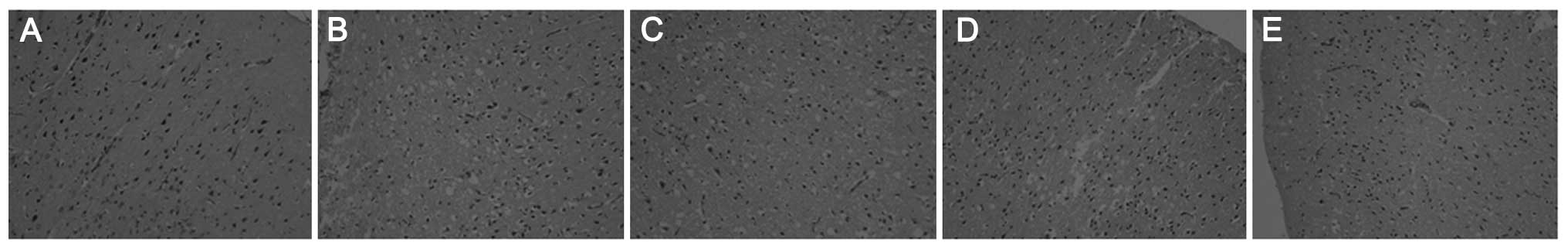
Histopathological examination of the
brain tissues
As shown in Fig. 6,
numerous pyramidal neurons were observed in the sham-surgery group,
while marked morphological changes were observed in the model
group, such as neuronal cell loss, nuclear shrinkage, neuronal
vacuolization and dark staining of the neurons. Pretreatment with
PQDS (25.0 and 50.0 mg/kg) markedly attenuated these pathological
changes; however, 12.5 mg/kg PQDS exhibited no effect.
Discussion
In our previous study, we demonstrated that PQDS
reduced the infarct size in acute myocardial infarction in rats and
dogs (13). In the present study,
the effects of PQDS on brain damage following MCAO were
investigated. The results showed that the MCAO group had
significantly decreased neurological function and increased infarct
size and brain water content compared with the sham-surgery group.
The administration of PQDS effectively reduced the infarct size and
brain water content and improved the neurological function and
morphological changes in a dose-dependent manner, when
administration commenced three days prior to MCAO. These results
suggested that PQDS may attenuate cerebral injury in rats. This
effect was associated with decreased MDA, NO and Ca2+
levels and with increased SOD and
Na+-K+-ATPase activity. It also appeared that
PQDS increased the expression of Bcl-2 in cerebral ischemia.
Oxidative stress is significant in ischemic injury.
It has been indicated that ROS, such as the superoxide anion and
the hydroxyl radical, are produced during ischemia, and that these
attack lipids, proteins and DNA in ischemic brain tissue (19). ROS are scavenged by endogenous
antioxidant enzymes, such as SOD, catalase (CAT) and
glutathione-S-transferase (GST), as well as
Na+-K+-ATPase (20). Therefore, measuring the levels of
such enzymes enables the amount of oxidative stress to be
estimated. The results of the present study showed that cerebral
ischemia increased the lipid peroxidation and decreased the
activity of endogenous antioxidant enzymes, which was consistent
with the results of a previous study (21). Pretreatment with PQDS significantly
reduced the level of MDA and increased the activities of SOD and
Na+-K+-ATPase in the brain tissue. These
results indicated that the antioxidant properties of PQDS may act
as a protective mechanism, by increasing the levels of antioxidant
enzymes, such as SOD, to combat the oxidative stress induced by
MCAO.
In addition to disordered free radical production
and lipid metabolism, Ca2+ overload has also been
demonstrated to be a risk factor for cerebral ischemic injury
(22). During cerebral ischemia,
increases in cytoplasmic Ca2+ levels are capable of
activating phospholipases, endonuclease and proteases, as well as
activating enzymes that generate ROS and NO, which participate in
cell death (23). Pretreatment
with PQDS significantly reduced the level of Ca2+ in the
brain tissue. This result indicated that the
Ca2+-lowering property of PQDS might act as a protective
mechanism
In addition, the present study demonstrated that the
administration of PQDS increased the level of Bcl-2 expression
following cerebral ischemia. Oxidative stress, ionic imbalance and
excitotoxicity result in nerve cell apoptosis. The Bcl-2 family has
been considered to be the most important regulator of apoptosis.
The antiapoptotic protein Bcl-2 is capable of preventing cytochrome
c release into the cytoplasm (24), while the pro-apoptotic protein,
Bcl-2-associated X protein (Bax), promotes cell death, unless it is
bound by Bcl-2 or Bcl-xL (25).
The balance between Bcl-2 and Bax maintains mitochondrial
stabilization. The Bcl-2 expression level in the MCAO group was
markedly decreased compared with that in the sham group, which is
consistent with the results of a previous study (18). Pretreatment with PQDS significantly
increased the expression of Bcl-2 compared with that in the MCAO
group, suggesting that PQDS may mediate the protective effect
against cerebral ischemia by inhibiting apoptosis.
In conclusion, the present study demonstrated the
protective effect of PQDS in a rat model of ischemia. The
mechanisms were associated with reductions in free radical
formation, lipid peroxidation and calcium overload, and an increase
in antiapoptotic protein expression. These results suggest that
PQDS may have therapeutic potential in the treatment of cerebral
ischemic injury. However, further study is required before this is
able to be transferred to clinical practice.
Acknowledgements
This study was sponsored by The National 863 High
-Tech Research and Development Program (grant no.
2004AAZ23861).
References
|
1
|
Zhang HL, Xu M, Wei C, Qin AP, Liu CF,
Hong LZ, Zhao XY, Liu J and Qin ZH: Neuroprotective effects of
pioglitazone in a rat model of permanent focal cerebral ischemia
are associated with peroxisome proliferator-activated receptor
gamma-mediated suppression of nuclear factor-κB signaling pathway.
Neuroscience. 176:381–395. 2011.PubMed/NCBI
|
|
2
|
Barone FC: Ischemic stroke intervention
requires mixed cellular protection of the penumbra. Curr Opin
Investig Drugs. 10:220–223. 2009.PubMed/NCBI
|
|
3
|
Mehta SL, Manhas N and Raghubir R:
Molecular targets in cerebral ischemia for developing novel
therapeutics. Brain Res Rev. 54:34–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui L, Zhang X, Yang R, Wang L, Liu L, Li
M and Du W: Neuroprotection of early and short-time applying
atorvastatin in the acute phase of cerebral ischemia:
down-regulated 12/15-LOX, p38MAPK and cPLA2 expression, ameliorated
BBB permeability. Brain Res. 1325:164–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Warner DS, Sheng H and Batinić-Haberle I:
Oxidants, antioxidants and the ischemic brain. J Exp Biol.
207:3221–3231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Zhang XJ, Yang CH and Fan HG:
Oxymatrine protects rat brains against permanent focal ischemia and
downregulates NF-kappaB expression. Brain Res. 1268:174–180. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim H: Neuroprotective herbs for stroke
therapy in traditional eastern medicine. Neurol Res. 27:287–301.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li G and Wang Z, Sun Y, Liu K and Wang Z:
Ginsenoside 20(S)-protopanaxadiol inhibits the proliferation and
invasion of human fibrosarcoma HT1080 cells. Basic Clin Pharmacol
Toxicol. 98:588–592. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishijo H, Uwano T, Zhong YM and Ono T:
Proof of the mysterious efficacy of ginseng: basic and clinical
trials: effects of red ginseng on learning and memory deficits in
an animal model of amnesia. J Pharmacol Sci. 95:145–152. 2004.
View Article : Google Scholar
|
|
10
|
Shin JY, Song JY, Yun YS, Yang HO, Rhee DK
and Pyo S: Immunostimulating effects of acidic polysaccharides
extract of Panax ginseng on macrophage function. Immunopharmacol
Immunotoxicol. 24:469–482. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang LC and Lee TF: Effect of ginseng
saponins on cold tolerance in young and elderly rats. Planta Med.
66:144–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beveridge TH, Li TS and Drover JC:
Phytosterol content in American ginseng seed oil. J Agric Food
Chem. 50:744–750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu H, Yu X, Qu S, Chen Y, Wang Z and Sui
D: In vive and in vitro cardioprotective effects of panax
quinquefolium 20(S)-protopanaxadiol saponins (PQDS), isolated from
panax quinquefolium. Pharmazie. 68:287–292. 2013.PubMed/NCBI
|
|
14
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee EJ, Chen HY, Wu TS, Chen TY, Ayoub IA
and Maynard KI: Acute administration of Ginkgo biloba
extract (EGb 761) affords neuroprotection against permanent and
transient focal cerebral ischemia in Sprague-Dawley rats. J
Neurosci Res. 68:636–645. 2002.
|
|
16
|
Li Y, He D, Zhang X, Liu Z, Zhang X, Dong
L, Xing Y, Wang C, Qiao H, Zhu C and Chen Y: Protective effect of
celastrol in rat cerebral ischemia model: down-regulating p-JNK,
p-c-Jun and NF-κB. Brain Res. 1464:8–13. 2012.PubMed/NCBI
|
|
17
|
Vakili A, Kataoka H and Plesnila N: Role
of arginine vasopressin V1 and V2 receptors for brain damage after
transient focal cerebral ischemia. J Cereb Blood Flow Metab.
25:1012–1019. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheyuo C, Jacob A, Wu R, et al:
Recombinant human MFG-E8 attenuates cerebral ischemic injury: its
role in anti-inflammation and anti-apoptosis. Neuropharmacology.
62:890–900. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noh SJ, Lee SH, Shin KY, Lee CK, Cho IH,
Kim HS and Suh YH: SP-8203 reduces oxidative stress via SOD
activity and behavioral deficit in cerebral ischemia. Pharmacol
Biochem Behav. 98:150–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dinkova-Kostova AT and Talalay P: Direct
and indirect antioxidant properties of inducers of cytoprotective
proteins. Mol Nutr Food Res. 52(Suppl 1): S128–S138.
2008.PubMed/NCBI
|
|
21
|
Yin J, Tu C, Zhao J, Ou D, Chen G, Liu Y
and Xiao X: Exogenous hydrogen sulfide protects against global
cerebral ischemia/reperfusion injury via its anti-oxidative,
anti-inflammatory and anti-apoptotic effects in rats. Brain Res.
1491:188–196. 2013. View Article : Google Scholar
|
|
22
|
Ban JY, Cho SO, Choi SH, Ju HS, Kim JY,
Bae K, Song KS and Seong YH: Neuroprotective effect of Smilacis
chinae rhizome on NMDA-induced neurotoxicity in vitro and focal
cerebral ischemia in vivo. J Pharmacol Sci. 106:68–77. 2008.
|
|
23
|
Kristian T and Siesjo BK: Calcium in
ischemic cell death. Stroke. 29:705–718. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakka VP, Gusain A, Mehta SL and Raghubir
R: Molecular mechanisms of apoptosis in cerebral ischemia: multiple
neuroprotective opportunities. Mol Neurobiol. 37:7–38. 2008.
View Article : Google Scholar : PubMed/NCBI
|