Introduction
Chronic renal failure (CRF) is characterized by
progressive tubulointerstitial fibrosis and glomerulosclerosis
(1), which are associated with the
accumulation of extracellular matrix (ECM) proteins. The major
physiological regulators of ECM are matrix metalloproteinases
(MMPs). MMPs belong to a large family of proteolytic enzymes that
degrade various types of ECM components, such as collagens,
laminin, fibronectin, vitronectin, aggrecan, enactin, tenascin,
elastin and proteoglycans, and a number of non-ECM substrates,
including signaling molecules and cell adhesion molecules (2,3).
To date, six groups of MMPs have been identified
based on substrate and sequence homology. These are collagenases,
gelatinases, stromelysins, matrilysins, membrane-type MMPs and
other MMPs. MMP-9 is a gelatinase and cleaves denatured collagens
(gelatins) and laminin, as well as certain chemokines (2). MMP-9, like other MMPs, is expressed
in the kidney in a variety of vertebrates, including rabbits, and
appears to be mainly confined to the glomerulus (4). MMP-9 is also expressed in the
collecting duct of rabbits (5).
Due to their ability to degrade the structure of
most tissues, MMPs are responsible for the accumulation of ECM
proteins, which may lead to a fibrotic state in various organs,
including the kidney, when MMP activity and expression levels
decrease. The expression and activity of MMPs are tightly regulated
at multiple levels, including gene transcription,
post-transcriptional modification and, in particular, the
interaction with circulating inhibitors that serve to limit the
activity of the MMPs (6). Tissue
inhibitors of metalloproteinases (TIMPs) are endogenous, specific
inhibitors of MMPs. TIMPs non-covalently bind to MMPs to form
high-affinity complexes and block the binding of MMPs to their
substrates (7). Four TIMPs
(TIMP-1-4) have been identified in vertebrates. TIMP-1 is capable
of inhibiting the activity of most MMPs, with the exceptions of
MMP-14, 16 and 24 (8), and is
important in maintaining the balance between ECM deposition and
degradation. TIMP-1 expression has been observed in the glomeruli
of rats and humans (9,10).
Accumulating data have shown that MMP-9 and TIMP-1
function differentially in chronic kidney diseases (CKDs). The
downregulation of MMPs or upregulation of TIMPs leads to an
imbalance in the MMP/TIMP ratio, which results in ECM accumulation
(1). This may promote CKD
progression. Studies of various kidney disease models and patients
with CKD have demonstrated a reduction in the activity and
expression of MMP-9, in contrast with an increase in TIMP-1
expression (11,12). However, in diabetic nephropathy, a
marked increase in the MMP-9/TIMP-1 ratio has been identified
(13). The upregulation of MMP-9
has also been observed in diabetic CKD arteries, which was noted to
be associated with arterial stiffening, impaired angiogenesis and
endothelial dysfunction (14).
As a result of the contradictory observations
regarding MMP-9, in the present study the effects of MMP-9 on CRF
were examined through the injection of MMP-9 into rabbit renal
arteries in an adenine-induced model of CRF. Adenine-induced CRF
has been demonstrated to be accompanied by an increased expression
of inflammatory mediators, including cyclooxygenase-2 (COX-2)
(15); thus, the expression of
COX-2, as well as TIMP-1, was investigated in this study.
Materials and methods
Reagents
Adenine (A8626) was purchased from Sigma (St. Louis,
MO, USA). Anti-MMP-9 (sc-21733), anti-TIMP-1 (sc-21734), anti-COX-2
(sc-376861) and anti-β-actin (sc-47778) antibodies were obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
horseradish peroxidase-conjugated secondary antibody and
streptavidin-biotin complex (sABC) kits used in the study were
purchased from Wuhan Boster Biological Technology Co. Ltd., (Wuhan,
China), while the kits for serum creatinine (SCr) and blood urea
nitrogen (BUN) were purchased from Nanjing Jiancheng Technology
Company, Ltd. (Nanjing, China). MMP-9 protein (911-MP-010, purity
>90%) and enzyme-linked immunosorbent assay (ELISA) kits for
MMP-9 (DMP900) and TIMP-1 (DTM100) were purchased from R&D
Systems (Minneapolis, MN, USA). TRIzol® reagent
(15596-026) and SYBR®-GreenER™ qPCR SuperMix Universal
kit (11762-500) were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). Moloney murine leukemia virus (M-MLV) reverse
transcriptase was purchased from Promega Corp. (Madison, WI,
USA).
Animals
All animal use procedures were in strict accordance
with National Institutes of Health guidelines and were approved by
the Fourth Military Medical University (Xi’an, China). Male rabbits
(weight, 2.0–2.5 kg; age, 4–5 months) were provided by the Animal
Center of the Fourth Military Medical University. The rabbits were
maintained in separate cages at a constant humidity and
temperature, with food and water available ad libitum. The
animal room was on a 12/12-h light/dark cycle. The 30 male rabbits
were randomly divided into control, CRF and MMP-9 groups. The
rabbits in the CRF and MMP groups were treated with adenine (350
mg/kg body weight) once a day by oral gavage for a total of 10
weeks. The control rabbits were treated with an equal volume of
vehicle. At the 10th week following the adenine administration, the
rabbits in the MMP-9 group were anesthetized with a mixture of
diazepam, haloperidol and dihydroetorphine, and the bilateral renal
arteries were exposed. MMP-9 was subsequently injected into the
bilateral renal arteries (1 μg each artery). The rabbits in the
control and CRF groups underwent an identical surgical procedure
and were injected with an equal volume of vehicle. The surgery was
strictly conducted under sterile conditions. All rabbits were
treated with 4×105 units penicillin following the
surgery, and were kept in a room with specific pathogen free (SPF)
standards.
Measurements of MMP-9, TIMP-1, SCr, BUN
and proteinuria
The levels of SCr and BUN were measured with the
picric acid and Urease-Berthelot methods, respectively, in
accordance with the kit manufacturers’ instructions. The serum
levels of MMP-9 and TIMP-1 were measured using ELISA, according to
the recommended instructions. Proteinuria from sporadic urea was
measured with a regular medical examination method.
Kidney section and protein lysate
preparation
All rabbits were sacrificed at the 14th week (the
fourth week subsequent to MMP-9 injection) and the bilateral
kidneys were removed. A sample of renal tissue was fixed in
paraffin and cut into 5-μm-thick sections. The sections were used
for hematoxylin and eosin (H&E) staining to examine the renal
morphology. An additional sample of renal tissue was lysed in
radioimmunoprecipitation assay (RIPA) buffer and total protein was
extracted for immunoblotting, while a third sample was used for
mRNA extraction.
Quantitative reverse
transcription-polymerase chain reaction (qPCR)
qPCR was performed in order to evaluate the mRNA
expression of MMP-9, TIMP-1 and COX-2. Total RNA was extracted from
the renal tissues using TRIzol reagent, in accordance with the
manufacturer’s instructions, and the RNA was reverse-transcribed
into cDNA using M-MLV reverse transcriptase. qPCR was performed
using a SYBR-GreenER qPCR SuperMix Universal kit with an ABI
StepOnePlus™ real-time PCR system (Applied Biosystems; Life
Technologies, Carlsbad, CA, USA). The following cycling profile was
used subsequent to an initial denaturation at 95ºC for 5 min:
denaturation at 95ºC for 30 sec, annealing at 60ºC for 30 sec and
extension at 72ºC for 45 sec. Amplification was performed for 39
cycles. The primers specific for the examined genes are shown in
Table I. The results are presented
as the levels of expression relative to those of the controls
subsequent to normalizing to β-actin using the 2−ΔΔCt
method.
 | Table ISequences of primers. |
Table I
Sequences of primers.
| Primer | Sense (5′-3′) | Antisense
(5′-3′) | Accession number |
|---|
| β-actin |
GTGAGATGCCATGTGACGGA |
TACACAAATGCGATGCTGCC | NM_001101683.1 |
| MMP-9 |
GGGCTACGTGAGCTTTGACA |
AAACTGGTCCCTTCCCCGTC | NM_001082203.1 |
| TIMP-1 |
CCGGACAGACGCTAGAGAATC |
AAGGTCGGAGTTGCAGAAGG | NM_001082232.2 |
| COX-2 |
TGAACTTCCAAGCTGGCCTC |
CCGATGCACAACTGAACTGG | NM_001082388.1 |
Western blot analysis
The protein levels of MMP-9, TIMP-1 and COX-2 in the
kidneys were assessed with western blotting, in accordance with a
previous study (16). Whole tissue
proteins were separated electrophoretically in 4–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, prior
to being transferred to nitrocellulose membranes. Following 30 min
of blocking with 2.5% non-fat milk, the membranes were incubated
with primary antibodies (1:2,000) at 4ºC overnight, prior to 1 h
incubation with horseradish peroxidase-conjugated secondary
antibody (1:2,000). The membranes were adequately washed with
phosphate-buffered saline (PBS) containing 0.5% Tween 20 subsequent
to each treatment with antibody. The membranes were developed with
Amersham ECL Western Blotting Analysis system (Cat. No.: RPN2109,
GE healthcare, Chalfont St Giles, UK) and then exposed to X-ray
film. The protein levels of MMP-9, TIMP-1 and COX-2 are expressed
as the ratio of the band optical intensity to that of β-actin.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. The statistical analysis was performed using SPSS 13
statistical software (SPSS, Inc., Chicago, IL, USA) and a Dunnett’s
test was utilized for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
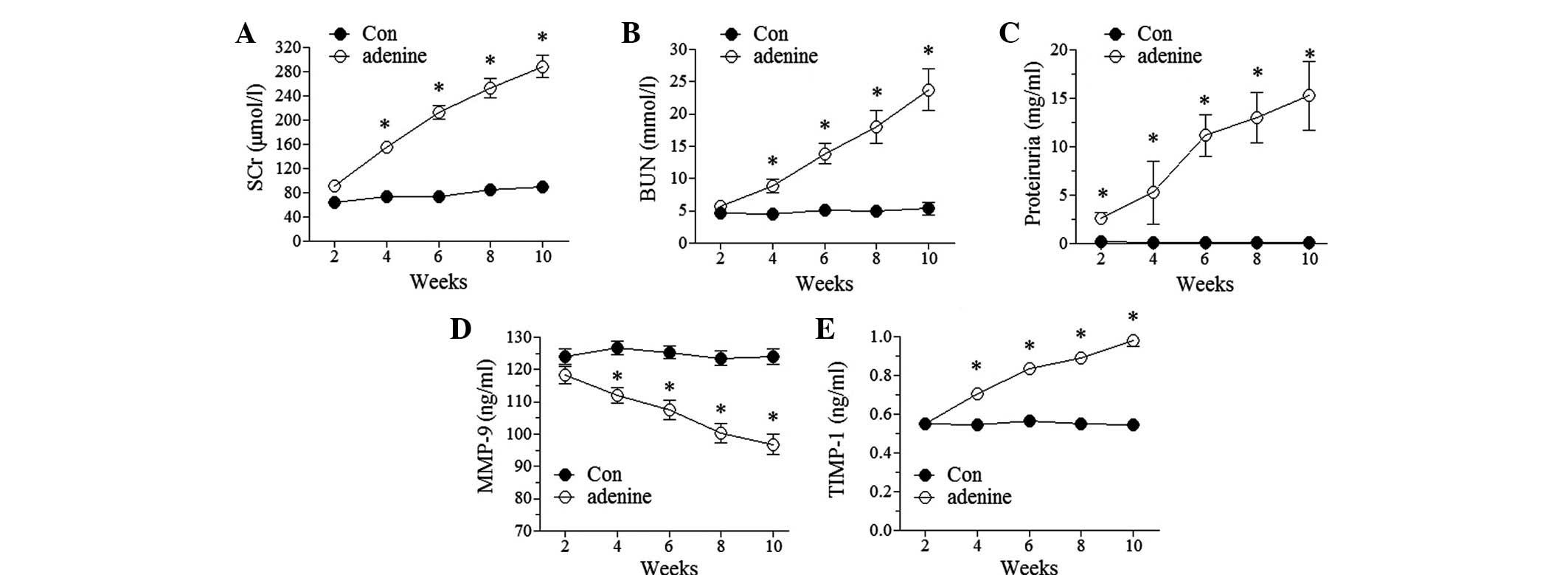
Induction of CRF by adenine
Adenine administration has been frequently used to
induce CRF models in various animals, including mice and rats
(15,17). In this study, the rabbits that were
treated with adenine exhibited a CRF-like change. The levels of SCr
and BUN were observed to significantly increase at the fourth week
following adenine administration (P<0.05 versus the control) and
the levels continually increased with time during the total period
of adenine administration (Fig. 1A and
B). Proteinuria was apparent early in the second week
subsequent to adenine administration and was also observed to
increase with time during the total period of adenine
administration (Fig. 1C). These
data indicated the presence of glomerular damage. By contrast,
there were no changes in the levels of SCr and BUN in the control
group and no proteinuria. These results were suggestive of the
successful induction of CRF. The increased levels of SCr, BUN and
proteinuria induced by adenine administration remained unchanged up
to four weeks subsequent to the cessation of adenine administration
in this study (data not shown).
Serum levels of MMP-9 and TIMP-1 in the
CRF model
Experiments were conducted to examine whether CRF
affected the serum levels of MMP-9 and TIMP-1. The results showed
that the levels of MMP-9 decreased and TIMP-1 increased in a
time-dependent manner in the adenine-treated rabbits, compared with
virtually no change in the control rabbits (Fig. 1D and E). The altered levels of
MMP-9 and TIMP-1 remained unchanged up to four weeks subsequent to
the cessation of adenine administration (data not shown).
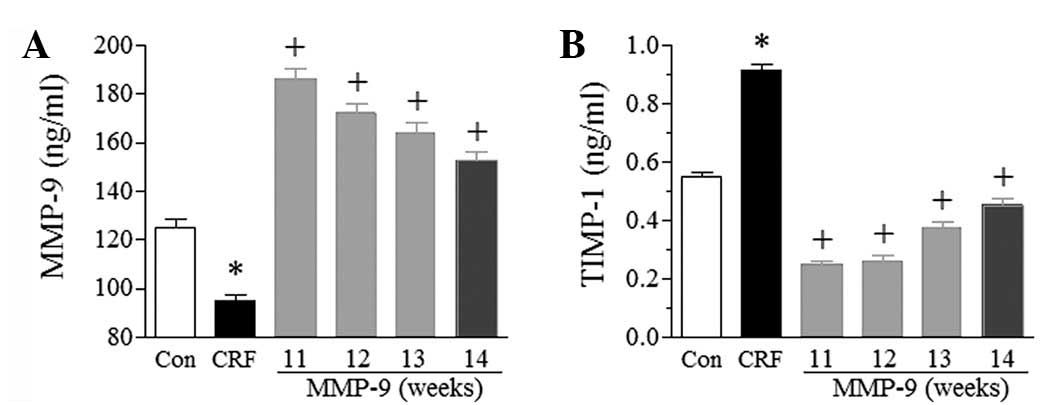
Exogenous MMP-9 improves renal function
and morphology
To confirm whether MMP-9 was involved in CRF, MMP-9
was injected into the bilateral renal arteries. The treatment was
demonstrated to significantly increase the serum level of MMP-9
(P<0.05). As shown in Fig. 2A,
at the 11th week (the first week following MMP-9 injection), the
serum level of MMP-9 was two-fold higher in the MMP-9 group than in
the CRF group, and more than two-fold higher than that in the
control group. The elevated serum level of MMP-9 in the MMP-9 group
gradually decreased; however, it remained higher than the levels in
the control and CRF groups throughout the total experimental period
of the study.
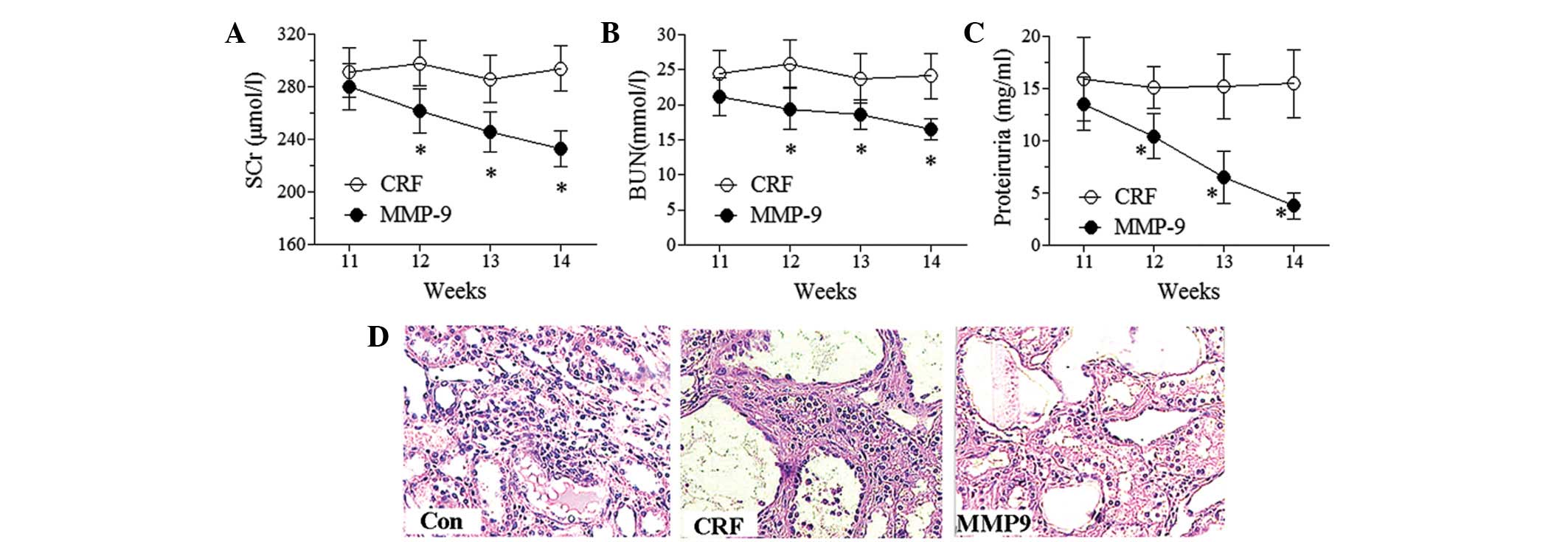
The levels of SCr, BUN and proteinuria were measured
to evaluate the effect of MMP-9 on renal function. MMP-9 treatment
time-dependently decreased the levels of SCr, BUN and proteinuria
(Fig. 3A–C). Significant effects
appeared early at the second week subsequent to MMP-9 treatment
(P<0.05). By contrast, no significant changes were observed in
the levels of SCr, BUN and proteinuria in the CRF group during the
experimental period. These results demonstrated the protective
effect of MMP-9 on renal function. MMP-9 is an ECM proteolytic
enzyme. The accumulation of ECM is an important pathological
feature in the development of glomerulosclerosis and
tubulointerstitial fibrosis. Thus, renal morphology was examined to
further investigate the effect of MMP-9 (Fig. 3D). H&E staining showed no
abnormal morphological changes in the control group. However, in
the CRF group, a number of glomeruli were observed to have
developed focal segmental glomerulosclerosis, basement membrane
thickening, mesangial cell proliferation with ECM expansion, fibrin
accumulation in the renal capsule, tubulointerstitial fibrosis and
tubular basement membrane thickening. MMP-9 treatment decreased the
glomerular lesions and, more prominently, largely prevented fibrin
accumulation in the renal capsule and reduced the development of
tubulointerstitial thickness.
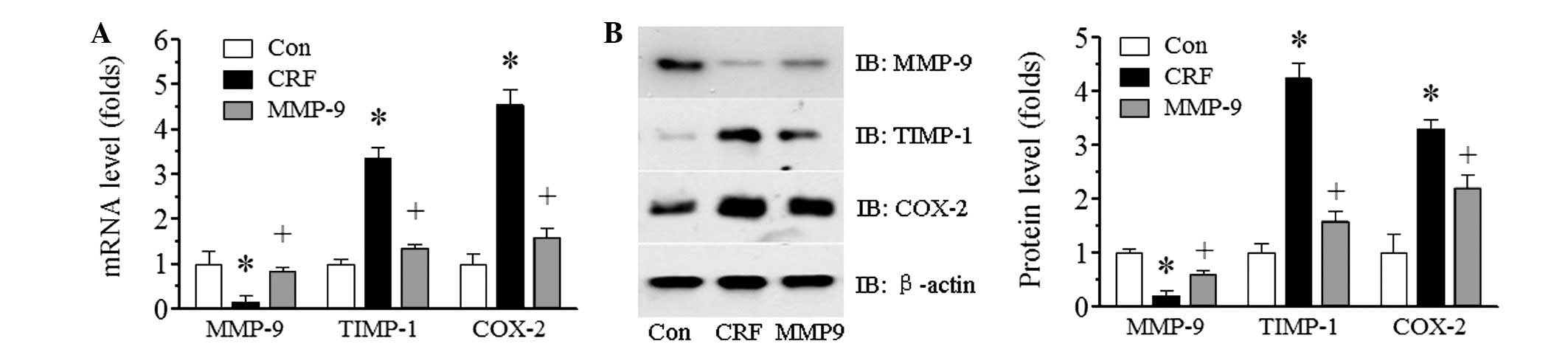
Exogenous MMP-9 increases the renal
expression of MMP-9
In order to examine whether exogenous MMP-9
treatment affected the endogenous expression of MMP-9, the renal
mRNA and protein expression of MMP-9 following a four-week
treatment period of MMP-9 in adenine-induced CRF rabbits was
examined using qPCR (Fig. 4A) and
immunoblotting (Fig. 4B),
respectively. There was abundant MMP-9 expression of mRNA and
protein in the control group; however, in the adenine-induced CRF
group, a marked reduction in MMP-9 expression was observed
(P<0.05). The low level of MMP-9 was significantly improved by
exogenous MMP-9 treatment (P<0.05).
MMP-9 decreases the expression of TIMP-1
and COX-2
It has been indicated that TIMP-1 may be positively
correlated with renal function damage (11). In the current study, the serum
level of TIMP-1 was significantly increased in adenine-induced CRF
(P<0.05 versus the control, Fig.
1E). Thus, serum levels of TIMP-1 were examined to investigate
whether the improvement in renal function due to MMP-9 was
accompanied by decreased serum levels of TIMP-1. The results showed
that MMP-9 treatment significantly decreased the serum level of
TIMP-1 at the 11th week (the first week subsequent to MMP-9
treatment) compared with that in the CRF group (P<0.05, Fig. 2B). The serum level of TIMP-1 in the
MMP-9-treated group increased gradually with time; however, it
remained lower than that in the CRF group. By contrast, the serum
level of TIMP-1 in the control and CRF groups remained virtually
unchanged (data not shown). Following this, whether MMP-9 treatment
affected the renal expression of TIMP-1 was examined using qPCR and
immunoblotting. Consistent with the results from the serum, the
renal mRNA and protein expression levels of TIMP-1 were increased
in the CRF group compared with those in the control group. MMP-9
treatment significantly decreased TIMP-1 expression (P<0.05,
Fig. 4B).
The expression of the proinflammatory factor COX-2
in the kidney was also examined. The COX-2 mRNA and protein
expression levels were markedly increased in the CRF group compared
with those in the control group (P<0.05); however, MMP-9
treatment significantly reduced the expression (P<0.05, Fig. 4).
Discussion
As a key enzyme involved in ECM degradation, MMP-9
has been observed to be differentially expressed in acute and
chronic renal disease models. In acute glomerulonephritis, MMP-9
expression increases, in parallel with the development of abnormal
glomerular histology (18).
However, in numerous CKD models, the expression and activity of
MMPs have been shown to decrease (19,20).
The decreased expression and activity of MMPs have been suggested
to be associated with the development of tubulointerstitial
fibrosis and glomerulosclerosis (19,20),
in addition to the exacerbation of renal function (21). Consistent with these observations,
our results in an adenine-induced model of CRF revealed decreased
MMP-9 expression in the kidney, impaired renal function,
proteinuria, tubulointerstitial fibrosis and
glomerulosclerosis.
To date, there has been no direct and conclusive
experimental evidence to support the presumed protective role of
MMPs in CKD. The data in this study revealed that exogenous MMP-9
decreased the levels of SCr and BUN, reduced proteinuria and
improved kidney morphology. Our data also revealed that exogenous
MMP-9 treatment induced MMP-9 mRNA and protein expression in the
kidney, which demonstrated that MMP-9 stimulated endogenous MMP-9
expression. The endogenous MMP-9 levels in the present study,
including the serum level during adenine administration and the
tissue level prior to or following MMP-9 treatment, were negatively
correlated with the impairment of renal function and morphology.
With regard to the involvement of exogenous MMP-9, the results of
the present study have, to the best of our knowledge, for the first
time, directly demonstrated the protective role of MMP-9 in CRF.
Endogenous MMP-9 levels may be a useful marker for the evaluation
of CRF. Moreover, MMP-7, another member of the MMP family, has been
considered as a noninvasive biomarker of profibrotic signaling in
obstructive nephropathy and focal segmental glomerulosclerosis
(22).
In general, TIMP-1 shows contrasting changes to
MMP-9 in CKD. The abundance of TIMP-1 in the kidneys has been shown
to significantly increase in the majority of experimental models
and several human renal diseases, showing positive correlation with
the extent of fibrosis (23,24).
The overexpression of TIMP-1 in a transgenic mice model promoted
renal interstitial fibrosis through the inflammatory pathway, which
may have been partly induced by the upregulation of intercellular
adhesion molecule-1 (ICAM-1), a non-ECM substrate of MMPs (3). In the present study, the TIMP-1
levels in the serum and kidney increased during adenine-induction.
While renal function and morphology were improved by MMP-9
treatment, the TIMP-1 level was significantly decreased. These
results further demonstrated the positive correlation between
TIMP-1 expression and renal damage. The decreased serum level and
renal expression of TIMP-1 may mediate the protective effects of
MMP-9 in CRF.
Following the initial reduction at the first week
subsequent to MMP-9 treatment, the serum level of TIMP-1 gradually
increased, although the renal function gradually improved. The
inverse correlation between the serum level of TIMP-1 and renal
function impairment was not consistent with the positive
correlation mentioned previously. In this study, the injection of
MMP-9 into the renal arteries notably increased the serum level of
MMP-9, which was accompanied by rapid and marked decreases in the
serum level of TIMP-1. Furthermore, the contrasting changes in
TIMP-1 and MMP-9 levels were apparent during adenine
administration. As the tissue inhibitors of MMPs, TIMPs bind to
MMPs to form high-affinity complexes (7). It has been indicated that the
high-affinity complexes of MMP-9 and TIMP-1 may contribute to
TIMP-1 clearance. However, further investigation is required.
Inflammation is another important factor involved in
CRF. An increased expression of COX-2 was observed in an
adenine-induced model of CRF (15). Furthermore, celecoxib, a selective
COX-2 inhibitor, was demonstrated to attenuate cisplatin-induced
nephrotoxicity (25). In the
present study, MMP-9 treatment significantly decreased
adenine-stimulated COX-2 expression in the kidney, suggesting that
an anti-inflammatory effect may be another action mechanism
underlying the protective role of MMP-9 in CRF.
With the advance in biomedicine, the role of
MMPs/TIMPs in various organs is becoming clear. The imbalance in
the MMPs/TIMPs ratio is important in the early stage of
osteoarthritis (26),
bleomycin-induced pulmonary fibrosis (27) and liver fibrosis (28). A variety of treatments are being
developed to improve fibrosis by interfering with the imbalance in
MMPs/TIMPs, such as hepatocyte growth factor in liver fibrosis
(12) and all-trans retinoic acid
in glomerulosclerosis (29). With
regard to the protective role of MMP-9 in CRF explored in the
present study, investigating methods to increase MMP-9 levels is
likely to be beneficial in the search for efficient therapies for
tubulointerstitial fibrosis and glomerulosclerosis in CRF.
In conclusion, the results presented in this study
demonstrate the protective role of MMP-9 in CRF. MMP-9 may exert
the protective effect directly via its ability to degrade the ECM
or through the suppression of its endogenous inhibitor, TIMP-1, and
the proinflammatory response.
Acknowledgements
This study was supported by the Science and
Technology Innovation and Development Foundation, Fourth Military
Medical University (grant no. TDCX2011001).
References
|
1
|
Lenz O, Elliot SJ and Stetler-Stevenson
WG: Matrix metalloproteinases in renal development and disease. J
Am Soc Nephrol. 11:574–581. 2000.PubMed/NCBI
|
|
2
|
Catania JM, Chen G and Parrish AR: Role of
matrix metalloproteinases in renal pathophysiologies. Am J Physiol
Renal Physiol. 292:F905–F911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cai G, Zhang X, Hong Q, et al: Tissue
inhibitor of metalloproteinase-1 exacerbated renal interstitial
fibrosis through enhancing inflammation. Nephrol Dial Transplant.
23:1861–1875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartleroad JY, Beharry KD, Hausman N,
Stavitsky Y, Asrat T and Modanlou HD: Effect of maternal
administration of selective cyclooxygenase (COX)-2 inhibitors on
renal size, growth factors, proteinases, and COX-2 secretion in the
fetal rabbit. Biol Neonate. 87:246–253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piedagnel R, Murphy G, Ronco PM and
Lelongt B: Matrix metalloproteinase 2 (MMP2) and MMP-9 are produced
by kidney collecting duct principal cells but are differentially
regulated by SV40 large-T, arginine vasopressin, and epidermal
growth factor. J Biol Chem. 274:1614–1620. 1999. View Article : Google Scholar
|
|
6
|
Siefert SA and Sarkar R: Matrix
metalloproteinases in vascular physiology and disease. Vascular.
20:210–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rémy L: Current data on
metalloproteinases, obligatory partners of tumor progression.
Pathol Biol (Paris). 45:759–765. 1997.(In French).
|
|
8
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tomita M, Koike H, Han GD, Shimizu F and
Kawachi H: Decreased collagen-degrading activity could be a marker
of prolonged mesangial matrix expansion. Clin Exp Nephrol. 8:17–26.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carome MA, Striker LJ, Peten EP, et al:
Human glomeruli express TIMP-1 mRNA and TIMP-2 protein and mRNA. Am
J Physiol. 264:F923–F929. 1993.PubMed/NCBI
|
|
11
|
Musiał K and Zwolińska D: Matrix
metalloproteinases (MMP-2,9) and their tissue inhibitors (TIMP-1,2)
as novel markers of stress response and atherogenesis in children
with chronic kidney disease (CKD) on conservative treatment. Cell
Stress Chaperones. 16:97–103. 2011.PubMed/NCBI
|
|
12
|
Liu Y, Rajur K, Tolbert E and Dworkin LD:
Endogenous hepatocyte growth factor ameliorates chronic renal
injury by activating matrix degradation pathways. Kidney Int.
58:2028–2024. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rysz J, Banach M and Stolarek RA: Serum
matrix metalloproteinases MMP-2 and MMP-9 and metalloproteinase
tissue inhibitors TIMP-1 and TIMP-2 in diabetic nephropathy. J
Nephrol. 20:444–452. 2007.PubMed/NCBI
|
|
14
|
Chung AW, Yang HH, Sigrist MK, et al:
Matrix metalloproteinase-2 and -9 exacerbate arterial stiffening
and angiogenesis in diabetes and chronic kidney disease. Cardiovasc
Res. 84:494–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nicholas SB, Yuan J, Aminzadeh A, Norris
KC, Crum A and Vaziri ND: Salutary effects of a novel oxidative
stress modulator on adenine-induced chronic progressive
tubulointerstitial nephropathy. Am J Transl Res. 4:257–268.
2012.
|
|
16
|
Dai YQ, Jin DZ, Zhu XZ and Lei DL:
Triptolide inhibits COX-2 expression via NF-kappa B pathway in
astrocytes. Neurosci Res. 55:154–160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morishita Y, Ohnishi A and Watanabe M:
Establishment of acute kidney injury mouse model by 0.75% adenine
ingestion. Ren Fail. 33:1013–1018. 2011.PubMed/NCBI
|
|
18
|
Kuroda T, Yoshida Y, Kamiie J, et al:
Expression of MMP-9 in mesangial cells and its changes in anti-GBM
glomerulonephritis in WKY rats. Clin Exp Nephrol. 8:206–215. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
González-Avila G, Iturria C,
Vadillo-Ortega F, Ovalle C and Montaño M: Changes in matrix
metalloproteinases during the evolution of interstitial renal
fibrosis in a rat experimental model. Pathobiology. 66:196–204.
1998.PubMed/NCBI
|
|
20
|
Uchio-Yamada K, Manabe N, Goto Y, et al:
Decreased expression of matrix metalloproteinases and tissue
inhibitors of metalloproteinase in the kidneys of hereditary
nephrotic (ICGN) mice. J Vet Med Sci. 67:35–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang HR, Yang SF, Li ML, Lin CC, Hsieh YS
and Lian JD: Relationships between circulating matrix
metalloproteinase-2 and -9 and renal function in patients with
chronic kidney disease. Clin Chim Acta. 366:243–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He W, Tan RJ, Li Y, et al: Matrix
metalloproteinase-7 as a surrogate marker predicts renal
Wnt/β-catenin activity in CKD. J Am Soc Nephrol. 23:294–304.
2012.PubMed/NCBI
|
|
23
|
Duymelinck C, Dauwe SE, De Greef KE, et
al: TIMP-1 gene expression and PAI-1 antigen after unilateral
ureteral obstruction in the adult male rat. Kidney Int.
58:1186–1201. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hörstrup JH, Gehrmann M, Schneider B, et
al: Elevation of serum and urine levels of TIMP-1 and tenascin in
patients with renal disease. Nephrol Dial Transplant. 17:1005–1013.
2002.PubMed/NCBI
|
|
25
|
Suddek GM, El-Kenawi AE, Abdel-Aziz A and
El-Kashef HA: Celecoxib, a selective cyclooxygenase-2 inhibitor,
attenuates renal injury in a rat model of Cisplatin-induced
nephrotoxicity. Chemotherapy. 57:321–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shibakawa A, Yudoh K, Masuko-Hongo K, Kato
T, Nishioka K and Nakamura H: The role of subchondral bone
resorption pits in osteoarthritis: MMP production by cells derived
from bone marrow. Osteoarthritis Cartilage. 13:679–687. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JY, Choeng HC, Ahn C and Cho SH: Early
and late changes of MMP-2 and MMP-9 in bleomycin-induced pulmonary
fibrosis. Yonsei Med J. 50:68–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng WH, Tien YC, Huang CY, et al:
Fraxinus rhynchophylla ethanol extract attenuates carbon
tetrachloride-induced liver fibrosis in rats via down-regulating
the expressions of uPA, MMP-2, MMP-9 and TIMP-1. J Ethnopharmacol.
127:606–613. 2010. View Article : Google Scholar
|
|
29
|
Qin YH, Lei FY, Hu P, et al: Effect of
all-trans retinoic acid on renal expressions of matrix
metalloproteinase-2, matrix metalloproteinase-9 and tissue
inhibitor of metalloproteinase-1 in rats with glomerulosclerosis.
Pediatr Nephrol. 24:1477–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|