Introduction
Primary hepatocellular carcinoma (PHC), a prevalent
cancer, is the third leading cause of cancer related mortality
(1,2). Furthermore, the incidence of PHC is
increasing in many countries and regions, particularly in China
(2). Additionally, only ~20% of
patients are eligible for curative surgery, with limited
therapeutic options for those who are ineligible. Failure to
achieve a timely diagnosis, in addition to the limited efficacy of
palliative treatments, contributes to the poor prognosis for PHC
patients. Furthermore, PHC remains a highly lethal disease due to
the recurrence of metastasis, thereby leading to poor patient
prognosis (3).
Due to the scarcity of efficacious testing methods,
the identification of novel PHC biomarkers is necessary. To date,
several studies have focused on tests that are capable of detecting
and monitoring PHC, including tests for the ratio of glycosylated
α-fetoprotein (AFP; L3 fraction) to total AFP, and prothrombin
induced by vitamin K absence II (PIVKA II), α-fucosidase and HSP-70
levels (4). However, the
specificity and sensitivity of these serological markers are low
and have been demonstrated to be inadequate and impractical for the
purposes of PHC screening, even when they are combined (2).
Angiotensin II (AT-II) is a major peptide hormone of
the renin-angiotensin system (RAS), which is crucial for
maintaining cardiovascular homeostasis and mediating diverse
physiological functions such as cell growth, differentiation and
apoptosis (5). The majority of
AT-II actions are mediated by its two sub-receptors, which are the
AT-II type 1 receptor (AT-1R) and the AT-II type 2 receptor (AT-2R)
(6). These two subunits control
the effects of AT-II on various organs (5,7),
while AT-2R is less common than AT-1R and has been observed in
fetal cells (8).
Previous studies have revealed AT-II to have major
functions in several aspects of neoplastic diseases, which indicate
an anti-neoplastic action for AT-II by binding to activated AT-1R
(9). Activation of the AT-1R
associated with tumor development may be via various pathways.
AT-1R has the potential to stimulate tumor growth factors, which
results in the suppression of immune function (10). AT-1R assists vascular endothelial
growth factor (VEGF) to promote tumor vessel growth. Furthermore,
AT-1R is capable of mediating inflammation by stimulating various
inflammatory factors including interleukin 1β, tumor necrosis
factor-α, plasminogen activator inhibitor-1 and adrenomedullins
(11,12). These effects cause enduring tumor
vessel growth, tumor invasion and metastasis, and
immunosuppression, thereby leading to the development of tumors.
Kawamata et al(13)
transformed non-invasive esophageal cancer cells into AT-1R
overexpressed invasive esophageal cancer cells, and suggested that
nine inflammation-related genes in the cells were altered,
indicating that AT-1R promoted tumor growth via
inflammation-inducing factors.
Numerous studies have reported that AT-1R
overexpression is potentially associated with various malignancies
such as non-small cell lung cancer (14), gastric cancer (15,16),
breast cancer (17,18), ovarian cancer (19), bladder cancer (20,21),
pancreatic cancer (22,23) and prostate cancer (24–27).
However, currently there is limited literature regarding AT-1R
expression in patients with PHC and the results are frequently
contradictory. Di et al(28) demonstrated that AT-1R was
overexpressed in human hepatocellular carcinoma tissues by using
immunohistochemistry, and thus concluded it was a marker reflecting
the degree of malignancy of the hepatocellular carcinoma. However,
Wu et al(29) concluded
that the levels of AT-1Rs in normal tissues were markedly higher
compared with those in hepatocellular carcinoma tissues by
immunohistochemistry in a murine xenograft hepatocellular cancer
model. Nevertheless, the two studies used traditional
semi-quantitative methods, which leads to a certain degree of
subjectivity and possible inaccuracy. Additionally, subgroups of
PHC were not mentioned.
This study aimed to determine AT-1R mRNA and AT-1R
protein levels in PHC tissues, elucidate their association with the
clinicopathological characteristics of PHC and confirm the clinical
value of AT-1R as a biomarker for PHC in clinical diagnosis.
Patients and methods
Patient enrollment and tissue
samples
In total, 44 patients with PHC were enrolled between
January 2007 and June 2013 in the Department of Hepatobiliary
Surgery, The Third Affiliated Hospital of Soochow University
(Changzhou, China). All diagnoses were verified pathologically.
Clinical data were obtained by retrospective chart review. Survival
was determined from the date of the initial surgery. Follow-up was
available for all patients. The survival period ranged from 1–72
months (mean, 24.1±16.4 months). Of the 44 patients, 36 were male
and 8 were female. The ages ranged between 28–78 years with an
average age of 52 years. All enrolled patients were treated with
radical surgery for PHC and received no other treatments. A section
of tumor tissue 0.5×0.5×0.5 cm was obtained from each patient
immediately after the surgery. Additionally a section of normal
liver tissue, 0.5×0.5×0.5 cm and >5 cm away from the tumor
margin was obtained. All tissue samples were fixed in 10% formalin,
embedded in paraffin, and routinely stained with hematoxylin and
eosin. Specimens were assessed blindly and independently by two
pathologists. In case of interobserver disagreement, final
decisions were achieved by general consensus. The cancer grading
was determined by histology according to Edmondson’s criteria
(30). Edmondson’s grade I–II was
designated low-grade PHC and Edmondson grade III–IV was designated
high-grade PHC. All enrolled patients provided written consent. The
protocol was approved by the institutional ethics review board at
Soochow University. This study complies with the principles of the
Declaration of Helsinki and Good Clinical Practice Guidelines.
Quantitative polymerase chain reaction
(qPCR)
Unless indicated otherwise, all reagents for qPCR
were purchased from Fermentas-China Inc. (Shenzhen, China). Total
RNA was extracted from the tumor and normal tissues using an
extraction reagent (Shennengbocai Inc., Shanghai, China) according
to the manufacturer’s instructions. RNA samples were stored at
−70°C until required. PCR was performed using a RevertAid™ First
Strand cDNA Synthesis kit with PCR primers for synaptophysin
designed by TaqMan® Gene Expression Assays (Invitrogen,
Carlsbad, CA, USA). RNA (3 μg) was reverse transcribed using the
First Strand cDNA Synthesis kit with 0.5 μg oligo(dT)16
according to the manufacturer’s instructions. The reaction mixture
was incubated at 70°C for 5 min, and subsequently at 0°C for 30
sec. The cDNA concentration was determined by spectrophotometer.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
internal control. Specific primers for AT-1R were synthesized as
follows: forwards, 5′-AGACAGATGACGGCTGCTCG-3′; reverse,
5′-AACAATCTGGAACTCTCATCTCCTG-3′. Specific primers for GAPDH were
synthesized as follows: forwards, 5′-GGAAGGTGAAGGTCGGAGTC-3′;
reverse, 5′-CGTTCTCAGCCTTGACGGT-3′. The cycling conditions were as
follows: initial denaturation at 95°C for 3 min, followed by 40
cycles at 95°C for 15 sec, and a final extension for 45 sec at
60°C. The relative level of gene expression was evaluated using
2−ΔΔCt.
Immunohistochemistry
All reagents for immunohistochemistry were obtained
from R&D systems, Inc. (Minneapolis, MN, USA). Tissue sections
(5 μm) were deparaffinized in xylene, rehydrated in an ethanol
series and subsequently treated for 30 min with 0.3% hydrogen
peroxide, washed with phosphate-buffered saline (PBS) and unmasked
in a citrate antigen unmasking solution for 20 min at 120°C. The
sections were incubated with primary antibodies [rabbit anti-human
polyclonal antibody to AT-1R (1/50)] for 1 h at room temperature.
The bound primary antibodies were detected by adding secondary
antibodies (peroxidase labeled goat anti-human IgG) and
avidin/biotin/horseradish peroxidase complex (Dako, Carpinteria,
CA, USA) for 30 min at room temperature. The sections were
visualized using solid diaminobenzidine diluted with PBS,
counterstained with hematoxylin and mounted. Breast cancer tissue
was used as the positive control. Three independent investigators
assessed the positivity of AT-1R semiquantitatively without prior
knowledge of the clinical study. The intensity of cytoplasmic
staining was defined as negative (stained cells, <20%) or
positive (stained cells, ≥20%).
Statistical analysis
Data were analyzed using GraphPad Prism 5 (GraphPad
Software Inc., San Diego, CA, USA). The differences in AT-1R mRNA
levels between PHC tissues and normal tissues were compared using
the Wilcoxon test. The differences in AT-1R mRNA among various
subgroups of PHC were analyzed using the non-pairing t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline patient characteristics
Baseline patient characteristics, including gender,
age, pathological grade, HBS infection status, tumor size and
number as well as recurrence status are shown in Table I.
 | Table IBaseline patient characteristics
(n=44). |
Table I
Baseline patient characteristics
(n=44).
| Clinicopathologic
factors | No. patients | % |
|---|
| Gender |
| Female | 36 | 81.8 |
| Male | 8 | 18.2 |
| Age (years) |
| Average | 52 | |
| Range | 28–78 | |
| Edmondson’s
pathological grade |
| I–II | 24 | 54.5 |
| III–IV | 20 | 45.5 |
| HBsAg infection |
| Positive | 40 | 90.9 |
| Negative | 4 | 9.1 |
| AFP (ng/ml) |
| ≤20 | 6 | 13.6 |
| >20 | 38 | 86.4 |
| Hepatocirrhotic
nodule (cm) |
| ≤3 | 34 | 77.3 |
| >3 | 10 | 22.7 |
| Tumor size (cm) |
| ≤5 | 8 | 18.2 |
| >5 | 36 | 81.9 |
| Tumor
encapsulation |
| Yes | 26 | 59.1 |
| No | 18 | 40.9 |
| Tumor number |
| Single | 34 | 77.3 |
| Multiple | 10 | 22.7 |
| Cancerous
embolus |
| Yes | 14 | 31.8 |
| No | 30 | 68.2 |
| Recurrence |
| Yes | 10 | 22.7 |
| No | 34 | 77.3 |
AT-1R mRNA expression
AT-1R mRNA and GAPDH mRNA were expressed in all
hepatocellular carcinoma tissues and normal liver tissues. In 40
cases (90.9%), the AT-1R mRNA expression level in normal tissues
was higher compared with that in the tumor tissues, and the
opposite was observed for the other four cases. The difference was
considered to be statistically significant (P=0.0033). The relative
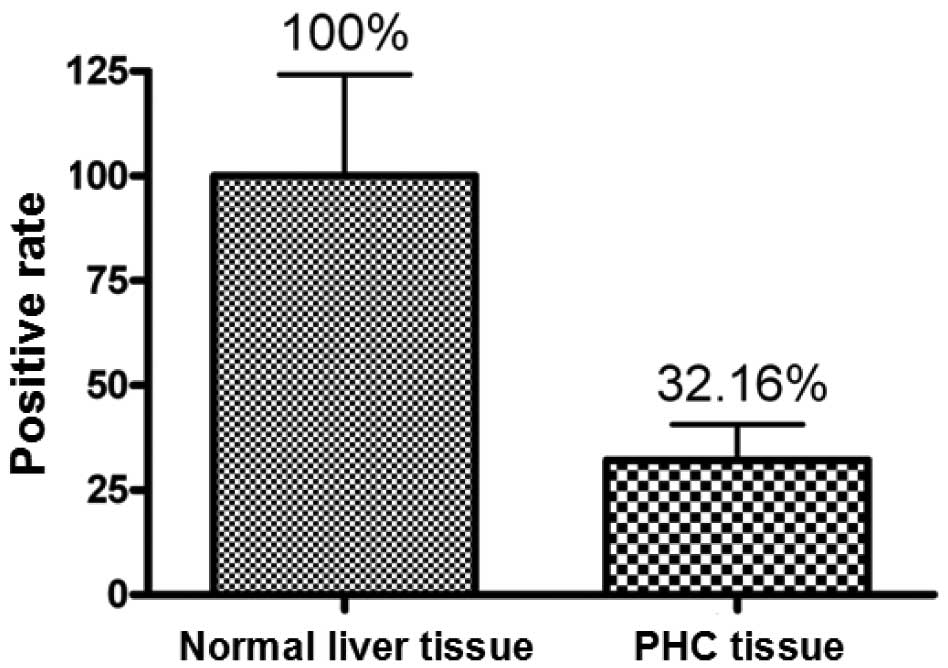
expression rates of AT-1R mRNA in normal tissues and in tumor
tissues were 100 and 32.16%, respectively (Fig. 1).
AT-1R protein expression
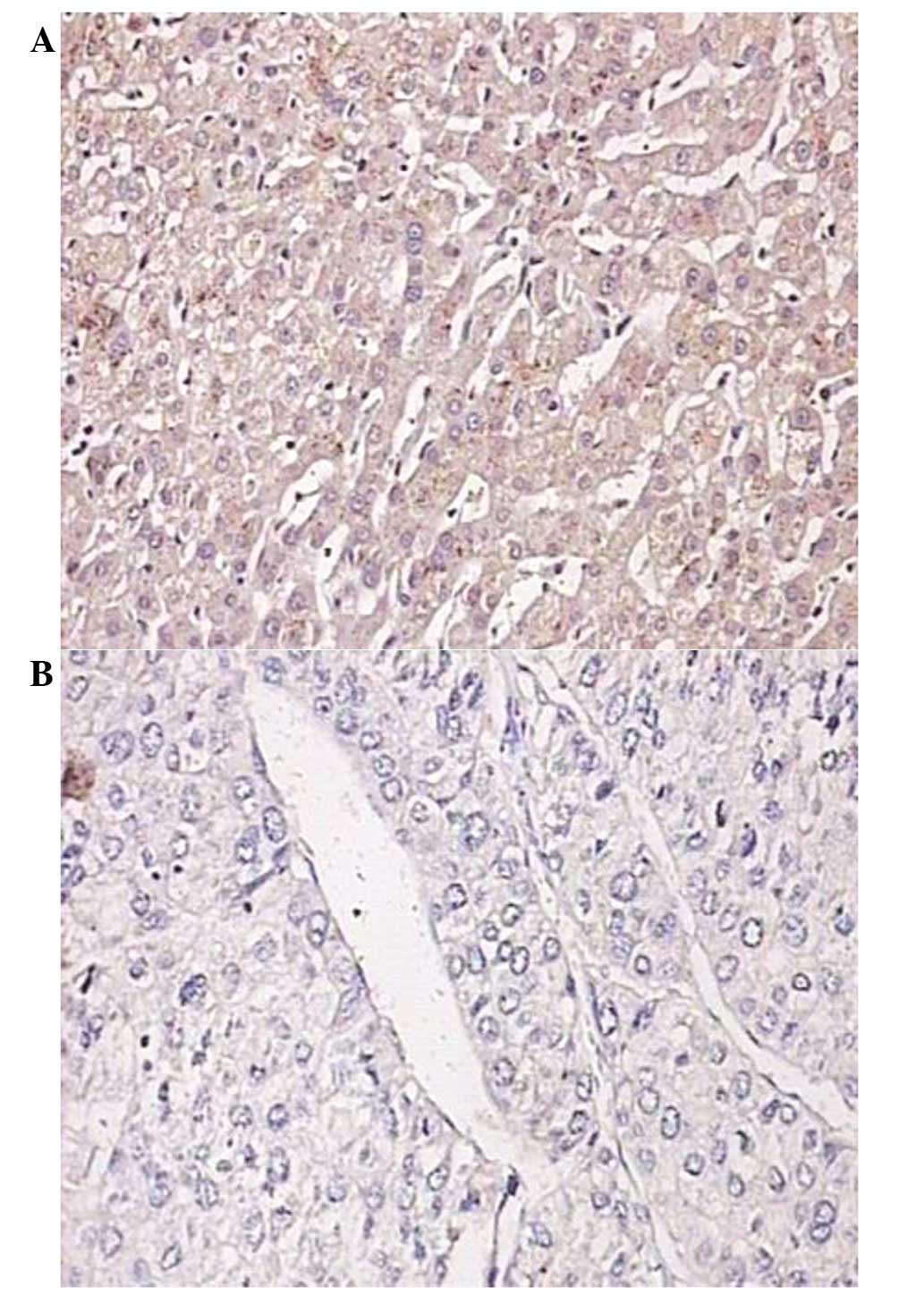
The majority of the AT-1R expression was detected in
the cytoplasm. The number of positively stained cells and the
staining density were markedly higher in the normal tissues
compared with those in the tumor tissues (Fig. 2).
Correlation between AT-1R mRNA expression
and clinicopathological factors
The AT-1R mRNA expression levels in cases which were
positive for HBsAg infection (40/44) were markedly higher compared
with those in cases which were negative for HBsAg infection (4/44)
(P=0.0005). The AT-1R mRNA expression levels in cases with normal
AFP levels (6/44) were markedly higher compared with those in cases
with aberrantly increased AFP levels (38/44) (P=0.0008). The AT-1R
mRNA expression levels in cases with Edmondson’s pathological grade
I–II (24/44) were markedly higher compared with that in cases with
Edmondson’s pathological grade III–IV (20/44) (P=0.0290; Table II).
 | Table IICorrelation between the
clinicopathological factors of PHC patients and AT-1R levels in
tumor tissues (n=44). |
Table II
Correlation between the
clinicopathological factors of PHC patients and AT-1R levels in
tumor tissues (n=44).
| Clinicopathological
factor | Cases | AT-1R/GAPDH (Mean ±
SD) | t-value | P-value |
|---|
| Gender |
| Male | 36 | 0.3199±0.4104 | 0.0407 | 0.9680 |
| Female | 8 | 0.3292±0.4140 | | |
| Age (years) |
| ≤50 | 18 | 0.3341±0.2943 | 0.1183 | 0.9070 |
| >50 | 26 | 0.3130±0.4728 | | |
| HBsAg |
| Negative | 4 | 1.1660±0.8565 | 4.1670 | 0.0005a |
| Positive | 40 | 0.2371±0.2378 | | |
| AFP (ng/ml) |
| ≤20 | 6 | 0.9730±0.7784 | 3.9340 | 0.0008a |
| >20 | 38 | 0.2188±0.1961 | | |
| Hepatocirrhotic
nodule (cm) |
| ≤3 | 34 | 0.3426±0.4142 | 0.4431 | 0.6624 |
| >3 | 10 | 0.2504±0.3873 | | |
| Tumor size
(cm) |
| ≤5 | 8 | 0.1225±0.0714 | 1.1030 | 0.2829 |
| >5 | 36 | 0.3659±0.4317 | | |
| Tumor
encapsulation |
| No | 18 | 0.1999±0.1491 | 1.1960 | 0.2456 |
| Yes | 26 | 0.4059±0.4979 | | |
| Edmondson’s
pathological grade |
| I–II | 24 | 0.4881±0.4841 | 2.3520 | 0.0290a |
| III–IV | 20 | 0.1218±0.0867 | | |
| Tumor number |
| Single | 34 | 0.3323±0.4257 | 0.2250 | 0.8243 |
| Multiple | 10 | 0.2853±0.3429 | | |
| Cancerous
embolus |
| No | 30 | 0.3840±0.4636 | 1.0730 | 0.2962 |
| Yes | 14 | 0.1878±0.1759 | | |
| Recurrence |
| No | 34 | 0.3413±0.4370 | 0.4163 | 0.6816 |
| Yes | 10 | 0.2546±0.2710 | | |
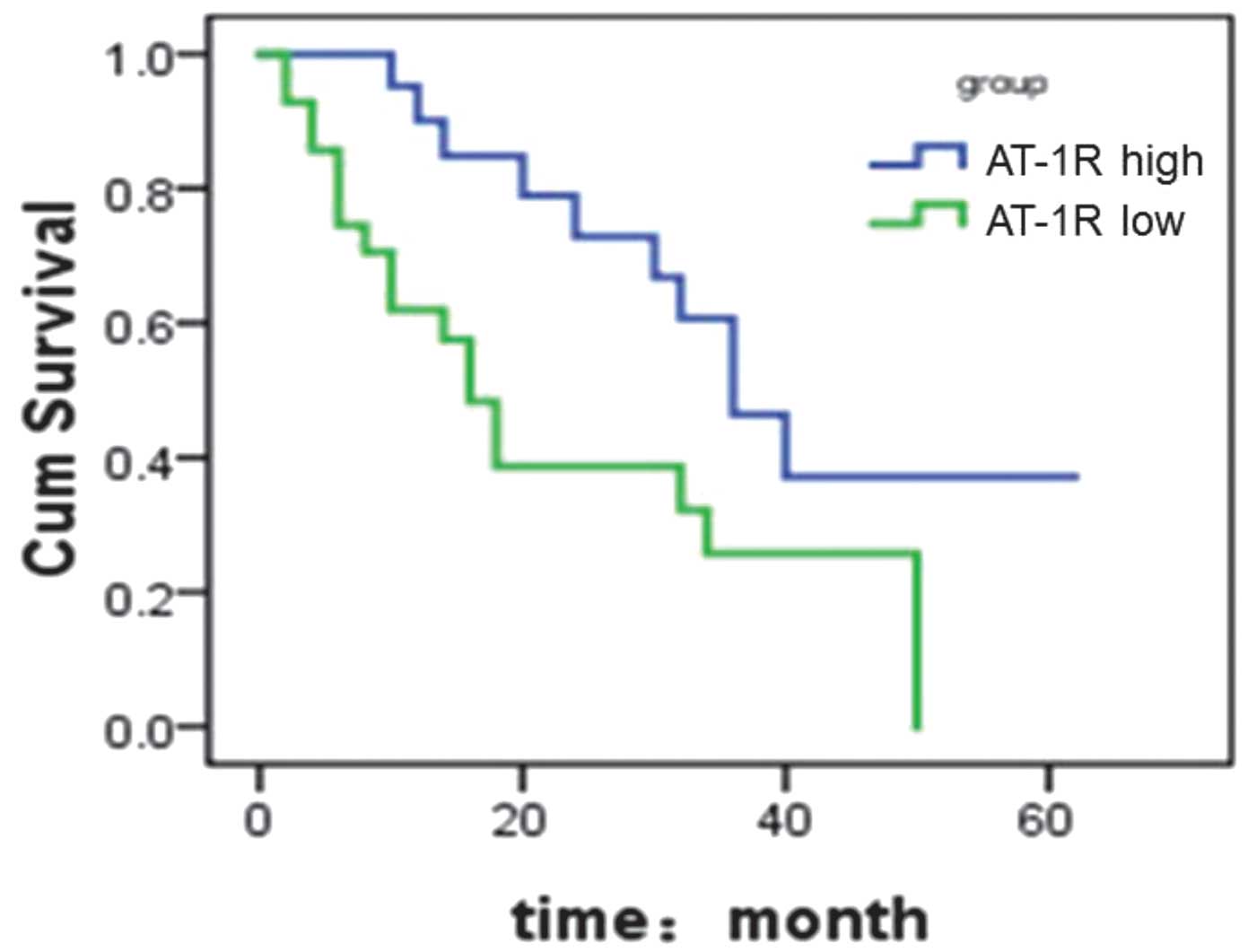
Notably, the data from the 5-year follow-up
demonstrated a correlation between AT-1R mRNA expression level and
patient survival rate. In cases with high levels of AT-1R mRNA
expression, the 3-year survival rate was 46%, with a median
survival time of 35.5 months. However, in cases with low levels of
AT-1R mRNA expression, the 3-year survival rate was 26%, with a
median survival time of 15.6 months (Fig. 3).
However, no correlation was observed between AT-1R
mRNA expression and other clinical features, such as age, gender,
tumor number, tumor size, cirrhosis status, tumor encapsulation,
cancerous embolus and recurrence (Table II).
Discussion
PHC represents a paradigm of the correlation between
the tumor microenvironment and tumor development (31). It has been demonstrated that
controlling the growth of tumor vessels is an important modality
for the treatment of PHC.
To date, the function of the VEGF family for
generating tumor vessel growth has been relatively well clarified
(32). VEGF is crucial in the
development of PHC by inducing tumor vessel growth in the early
stages, and VEGF levels have been observed to correlate positively
with microvessel density (33).
However, the mechanism by which VEGF regulates PHC cells growth has
not been fully elucidated. Fujiyama et al(34) demonstrated that AT-II promoted the
expression of VEGF by endothelial cells.
The results of the present study indicated that the
level of AT-1R expression in normal liver tissues was higher than
that in tumor tissues, potentially due to that fact that the
majority of PHC cases had HBV-related hepatocirrhosis (36/44). This
indicates that the upregulation of AT-1R expression is correlated
with hepatocyte proliferation. Furthermore, it was observed that
the AT-1R mRNA expression level correlated negatively with
hepatocyte differentiation. Once PHC formed an invasive cancer,
which broke through the basement membrane, AT-1R expression was
downregulated. Takeda et al(35) demonstrated that positive rates of
AT-1R expression in well-differentiated, moderately differentiated
and poorly differentiated squamous cell carcinomas were 81, 72 and
0%, respectively, which were consistent with the results of the
present study.
De Paepe et al(36) applied immunohistochemistry and
in situ hybridization to investigate the expression of AT-1R
in various stages of breast cancer, and the results revealed that
AT-1R was overexpressed in neoplasms with a relatively low level of
malignancy. These results are consistent with those of the present
study. De Paepe et al(36)
hypothesized that AT-1R was an important mediator for the
precursors of breast cancer but not a necessary protein for
invasive breast cancer. Similarly, we considered that AT-1R is
unnecessary for PHC. Lower expression levels of AT-1R in PHC
tissues leave the blood supply for PHC cells unaffected by AT-II,
leading to the sustained growth of PHC; this is a difference
between PHC and normal vessels.
Notably, the data from the present study
demonstrated that patients with higher levels of AT-1R mRNA
expression have an improved survival rate, indicating that AT-1R is
a novel prognostic factor in hepatic carcinoma.
In conclusion, AT-1R mRNA is expressed in normal
liver tissue and PHC tissue. AT-1R mRNA levels correlate negatively
with the degree of malignancy of PHC, which is a potential cause of
the increased blood supply in PHC tissues. AT-1R mRNA expression
correlates with PHC cell differentiation, but does not correlate
with gender, age, hepatocirrhotic nodules, tumor size, tumor
number, cancerous embolus, tumor encapsulation or tumor recurrence.
These results suggest that AT-1R expression correlates with PHC
development, and inhibits AT-1R expression prior to invasive tumor
formation, which may prevent PHC from growing progressively. Future
studies concerning the correlation between AT-1R and other ligands
are warranted.
Acknowledgements
The authors would like to thank Dr Chun Yang for
technical support. This work was funded by grants from the Jiangsu
Health International Exchange Supporting Program provided by
Xiao-Dong Li.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases. Management of
hepatocellular carcinoma: an update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Boix L, Sala M and Llovet JM:
Focus on hepatocellular carcinoma. Cancer Cell. 5:215–219. 2004.
View Article : Google Scholar
|
|
4
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases.
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
5
|
Paul M, Poyan Mehr A and Kreutz R:
Physiology of local renin-angiotensin systems. Physiol Rev.
86:747–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang CH, Li F and Takahashi N: The renin
angiotensin system and the metabolic syndrome. Open Hypertens J.
3:1–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tahmasebi M, Barker S, Puddefoot JR and
Vinson GP: Localisation of renin-angiotensin system (RAS)
components in breast. Br J Cancer. 95:67–74. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jethon A, Pula B, Piotrowska A, et al:
Angiotensin II type 1 receptor (AT-1R) expression correlates with
VEGF-A and VEGF-D expression in invasive ductal breast cancer.
Pathol Oncol Res. 18:867–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deshayes F and Nahmias C: Angiotensin
receptors: a new role in cancer? Trends Endocrinol Metab.
16:293–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kobie JJ, Wu RS, Kurt RA, et al:
Transforming growth factor beta inhibits the antigen-presenting
functions and antitumor activity of dendritic cell vaccines. Cancer
Res. 63:1860–1864. 2003.PubMed/NCBI
|
|
11
|
Suzuki Y, Ruiz-Ortega M, Lorenzo O, et al:
Inflammation and angiotensin II. Int J Biochem Cell Biol.
35:881–900. 2003. View Article : Google Scholar
|
|
12
|
Tsutamoto T, Wada A, Maeda K, et al:
Angiotensin II type 1 receptor antagonist decreases plasma levels
of tumor necrosis factor alpha, interleukin-6 and soluble adhesion
molecules in patients with chronic heart failure. J Am Coll
Cardiol. 35:714–721. 2000. View Article : Google Scholar
|
|
13
|
Kawamata H, Furihata T, Omotehara F, et
al: Identification of genes differentially expressed in a newly
isolated human metastasizing esophageal cancer cell line, T.Tn-AT1,
by cDNA microarray. Cancer Sci. 94:699–706. 2003. View Article : Google Scholar
|
|
14
|
Wilop S, von Hobe S, Crysandt M, et al:
Impact of angiotensin I converting enzyme inhibitors and
angiotensin II type 1 receptor blockers on survival in patients
with advanced non-small-cell lung cancer undergoing first-line
platinum-based chemotherapy. J Cancer Res Clin Oncol.
135:1429–1435. 2009. View Article : Google Scholar
|
|
15
|
Huang W, Wu YL, Zhong J, et al:
Angiotensin II type 1 receptor antagonist suppress angiogenesis and
growth of gastric cancer xenografts. Dig Dis Sci. 53:1206–1210.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang W, Yu LF, Zhong J, et al:
Angiotensin II type 1 receptor expression in human gastric cancer
and induces MMP2 and MMP9 expression in MKN-28 cells. Dig Dis Sci.
53:163–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inwang ER, Puddefoot JR, Brown CL, et al:
Angiotensin II type 1 receptor expression in human breast tissues.
Br J Cancer. 75:1279–1283. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Meng Q, Zhao Y, et al: Angiotensin
II type 1 receptor antagonists inhibit cell proliferation and
angiogenesis in breast cancer. Cancer Lett. 328:318–324. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ino K, Shibata K, Kajiyama H, et al:
Angiotensin II type 1 receptor expression in ovarian cancer and its
correlation with tumour angiogenesis and patient survival. Br J
Cancer. 94:552–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka N, Miyajima A, Kosaka T, et al:
Acquired platinum resistance enhances tumour angiogenesis through
angiotensin II type 1 receptor in bladder cancer. Br J Cancer.
105:1331–1337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shirotake S, Miyajima A, Kosaka T, et al:
Angiotensin II type 1 receptor expression and microvessel density
in human bladder cancer. Urology. 77:e19–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohta T, Amaya K, Yi S, et al: Angiotensin
converting enzyme-independent, local angiotensin II-generation in
human pancreatic ductal cancer tissues. Int J Oncol. 23:593–598.
2003.PubMed/NCBI
|
|
23
|
Gong Q, Davis M, Chipitsyna G, et al:
Blocking angiotensin II type 1 receptor triggers apoptotic cell
death in human pancreatic cancer cells. Pancreas. 39:581–594. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uemura H, Ishiguro H, Nakaigawa N, et al:
Angiotensin II receptor blocker shows antiproliferative activity in
prostate cancer cells: a possibility of tyrosine kinase inhibitor
of growth factor. Mol Cancer Ther. 2:1139–1147. 2003.
|
|
25
|
Guimond MO, Battista MC, Nikjouitavabi F,
et al: Expression and role of the angiotensin II AT2 receptor in
human prostate tissue: in search of a new therapeutic option for
prostate cancer. Prostate. 73:1057–1068. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hoshino K, Ishiguro H, Teranishi J, et al:
Regulation of androgen receptor expression through angiotensin II
type 1 receptor in prostate cancer cells. Prostate. 71:964–975.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kosaka T, Miyajima A, Shirotake S, et al:
Phosphorylated Akt up-regulates angiotensin II type-1 receptor
expression in castration resistant prostate cancer. Prostate.
71:1510–1517. 2011.PubMed/NCBI
|
|
28
|
Di MJ, Wang WX, Lan MY, et al: Expression
and significance of angiotensin II typeI receptor in human
hepatocellular carcinoma. Medical Journal of Wuhan University.
26:235–237. 2005.(In Chinese).
|
|
29
|
Wu Y, Cahill PA and Sitzmann JV: Decreased
angiotensin II receptors mediate decreased vascular response in
hepatocellular cancer. Ann Surg. 223:225–231. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: a study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Capece D, Fischietti M, Verzella D, et al:
The inflammatory microenvironment in hepatocellular carcinoma: a
pivotal role for tumor-associated macrophages. Biomed Res Int.
2013:1872042013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holash J, Maisonpierre PC, Compton D, et
al: Vessel cooption, regression, and growth in tumors mediated by
angiopoietins and VEGF. Science. 284:1994–1998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li XM, Tang ZY, Qin LX, et al: Serum
vascular endothelial growth factor is a predictor of invasion and
metastasis in hepatocellular carcinoma. J Exp Clin Cancer Res.
18:511–517. 1999.PubMed/NCBI
|
|
34
|
Fujiyama S, Matsubara H, Nozawa Y, et al:
Angiotensin AT(1) and AT(2) receptors differentially regulate
angiopoietin-2 and vascular endothelial growth factor expression
and angiogenesis by modulating heparin binding-epidermal growth
factor (EGF)-mediated EGF receptor transactivation. Circ Res.
88:22–29. 2001. View Article : Google Scholar
|
|
35
|
Takeda H and Kondo S: Differences between
squamous cell carcinoma and keratoacanthoma in angiotensin type-1
receptor expression. Am J Pathol. 158:1633–1637. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
De Paepe B, Verstraeten VL, De Potter CR,
et al: Growth stimulatory angiotensin II type-1 receptor is
upregulated in breast hyperplasia and in situ carcinoma but not in
invasive carcinoma. Histochem Cell Biol. 116:247–254.
2001.PubMed/NCBI
|