Introduction
Hyperuricemia, characterized by high serum levels of
uric acid (UA), is frequently reported in mainland China (1). Several factors, including genetic
background, drug metabolism, kidney disease, and a high dietary
intake of purines and proteins, are associated with the
pathogenesis of hyperuricemia. These factors may induce aberrant
metabolism of purines resulting in excessive UA formation (2,3).
According to the literature, in ~10% of cases of hyperuricemia, UA
accumulates in the form of a sodium salt that is deposited in the
joints, soft tissues, cartilage and kidney, which may promote
inflammatory reactions and eventually lead to gout (4).
Febuxostat, a urate-lowering drug, has been
indicated for the treatment of hyperuricemia and chronic gout
(5,6). The drug acts a xanthine oxidase
inhibitor and is able to induce reductions in serum UA levels
(7). In the present study, single
and multiple doses of febuxostat were administered to healthy
Chinese subjects. The aim of the study was to investigate the
pharmacokinetic and pharmacodynamic parameters of febuxostat. In
addition, the concentration-time curve for febuxostat was
determined and analyzed, in order to obtain information that may be
helpful in the clinical management of patients with hyperuricemia
and gout.
Subjects and methods
Subjects
A total of 36 healthy subjects (male, 18; female,
18; aged 19–40 years old; BMI, 18–24) were enrolled in the study.
Key inclusion criteria were: normal ECG, negative urine pregnancy
test resukts, and values within the normal range for standard
laboratory tests of blood, urine and biochemical parameters.
Exclusion criteria included treatment with traditional Chinese or
Western medicine within 2 weeks prior to the investigation, and
blood donation or clinical tests for other medicines within the
previous 3 months. All subjects provided informed consent. This
study was approved by the Ethics Committee of the General Hospital
of Beijing Military Command (Beijing, China). The subjects were
randomized into three febuxostat dose groups, each containing 12
subjects (six male and six female) as follows: Group A, single oral
administration (40 mg); group B, single oral administration (80 mg)
followed by multiple oral doses for 7 days; and group C, single
oral administration (120 mg). All subjects received oral febuxostat
under fasting conditions. Subsequently, the subjects were observed
to identify any adverse effects or complications.
Treatment groups
In the single-dose group, 5 ml venous blood was
collected from each subject prior to febuxostat administration and
at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16 and 24 h after
febuxostat administration. In the multiple-dose group, 2 ml venous
blood was collected from each subject at 0, 5, 10 and 24 h on the
day prior to febuxostat administration (day -1). From day 1 to day
7, 5 ml venous blood was collected from each subject prior to
febuxostat administration. In addition, on day 7, 5 ml venous blood
was collected from each subject at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6,
8, 10, 12, 16 and 24 h after the administration of febuxostat to
determine drug level.
For each sample, 2 ml blood was used for assessment
of the UA concentration. The remaining 3 ml of blood was
centrifuged at 351 × g in a heparin tube, and the serum was stored
at −80°C for drug concentration testing.
Drug concentration testing
Drug concentration testing was performed according
to previously described methods (8,9). In
brief, 100 μl serum was mixed with the internal standard
(bezafibrate 50 μg/ml, 10 μl, purchased from the National Institute
for Drug and Food Control, Beijing, China). Acetonitrile (300 μl)
was added to the sample, which was centrifuged at 250 × g for 2 min
and then at 1,204 × g for 1 min. The supernatant (10 μl) was
collected for analysis.
Plasma concentrations of febuxostat were determined
using a validated high-performance liquid chromatography (HPLC)
method (8,9). The serum samples did not contain any
endogenous materials that interfered with the evaluation of
febuxostat or the internal standard. Calibration curves for
febuxostat were linear for concentrations ranging from 10 to 8,000
ng/ml (R2>0.995), which was consistent with the
required analytic sensitivity. Appropriate precision and accuracy
was achieved as the results of inter- and intra-laboratory
comparisons in high, middle and low concentrates were accepted with
a relative SD of <15% in the present study. The recovery rate of
the extractions was >70%. According to guidelines for the
research of chemical drug and clinical pharmacokinetics (3), after standing at room temperature for
4 h, the samples underwent three melt/freeze cycles at −80°C, were
stored at −80°C for 24 days, and then placed in a sampler after
sample treatment for 24 h.
Analysis of UA concentration
UA is considered to be a pharmacodynamic marker of
febuxostat as the therapeutic effect of febuxostat depends on
reductions in UA concentrations. For each sample, 2 ml blood was
examined using a Roche Modular P800 Automatic Biochemistry Analyzer
(Roche Hong Kong Ltd., Hong Kong, China) to measure the UA
concentration.
Statistical analysis
Data are expressed as the mean ±SD. Noncompartmental
pharmacokinetic analysis was performed using the Drug and
Statistics software (DAS 2.0) as described previously (10). P<0.05 was considered to indicate
a statistically significant difference.
Actual measured UA values were adopted for
pharmacodynamic analysis. Quantitative descriptions and statistics
of pharmacodynamic parameters are displayed using mean, SD,
geometric mean, statistical charts and diagrams. The correlation
between dose and pharmacodynamic parameters was evaluated using
Microsoft Office Excel 2003.
Results
Pharmacokinetic parameters and
concentration-time curve of febuxostat
With regard to pharmacokinetic parameters, no
significant difference was noted between genders in the single-dose
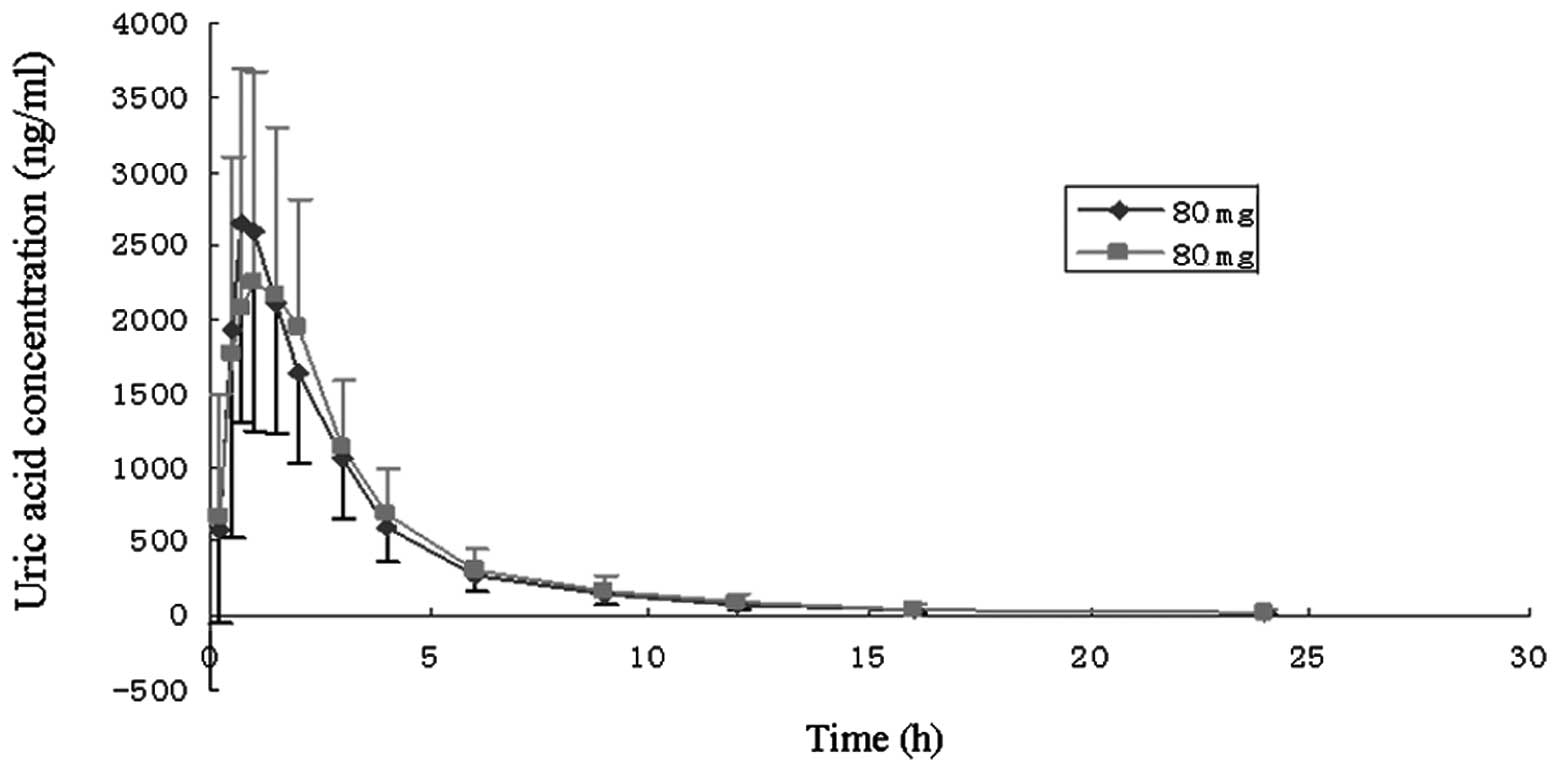
groups. In the multiple-dose group (80 mg, once-daily for 7 days),
no statistical difference was identified in the drug absorption
degree and rate. There was no evidence of drug accumulation. The
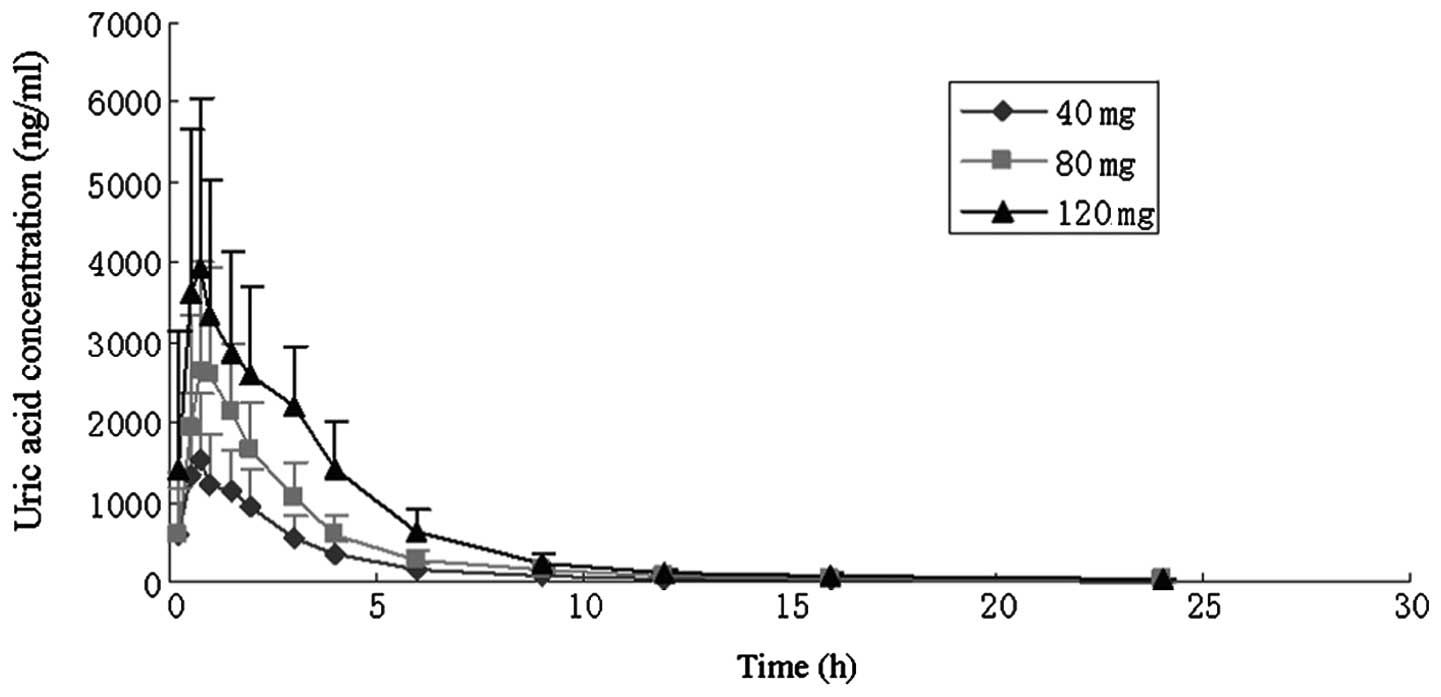
main pharmacokinetic parameters are shown in Table I, and the mean concentration-time
curves for each dose group are illustrated in Figs. 1 and 2.
 | Table IPharmacokinetic parameters of
febuxostat after single and multiple oral doses under fasting
conditions in healthy subjects |
Table I
Pharmacokinetic parameters of
febuxostat after single and multiple oral doses under fasting
conditions in healthy subjects
| Dose level |
|---|
|
|
|---|
| Parameters | 40 mg | 80 mg | 120 mg | 80 mg/qd/7d |
|---|
| AUC0–24h
(ng·h/ml) | 4536.6±1382.3 | 8216.0±2873.2 | 14404.1±4132.7 | - |
| T1/2
(h) | 3.81±1.77 | 5.02±1.23 | 3.91±1.52 | - |
| Tmax
(h) | 1.02±0.72 | 0.96±0.35 | 1.21±0.95 | - |
| CL/F (l/h) | 9.41±2.87 | 10.92±4.66 | 8.97±2.81 | - |
| Vd/F (l) | 48.80±19.49 | 77.90±32.35 | 49.01±21.63 | - |
| Cmax
(ng/ml) | 1911.95±678.40 | 2966.70±1176.13 | 4868.61±1792.14 | - |
| Cmax ss
(ng/ml) | - | - | - | 2957.07±1290.74 |
| Cmin ss
(ng/ml) | - | - | - | 25.39±12.52 |
| Cav ss
(ng/ml) | - | - | - | 360.29±149.62 |
| DF | - | - | - | 8.23±2.11 |
| Rac | - | - | - | 1.14±0.54 |
Pharmacodynamic parameters of
febuxostat
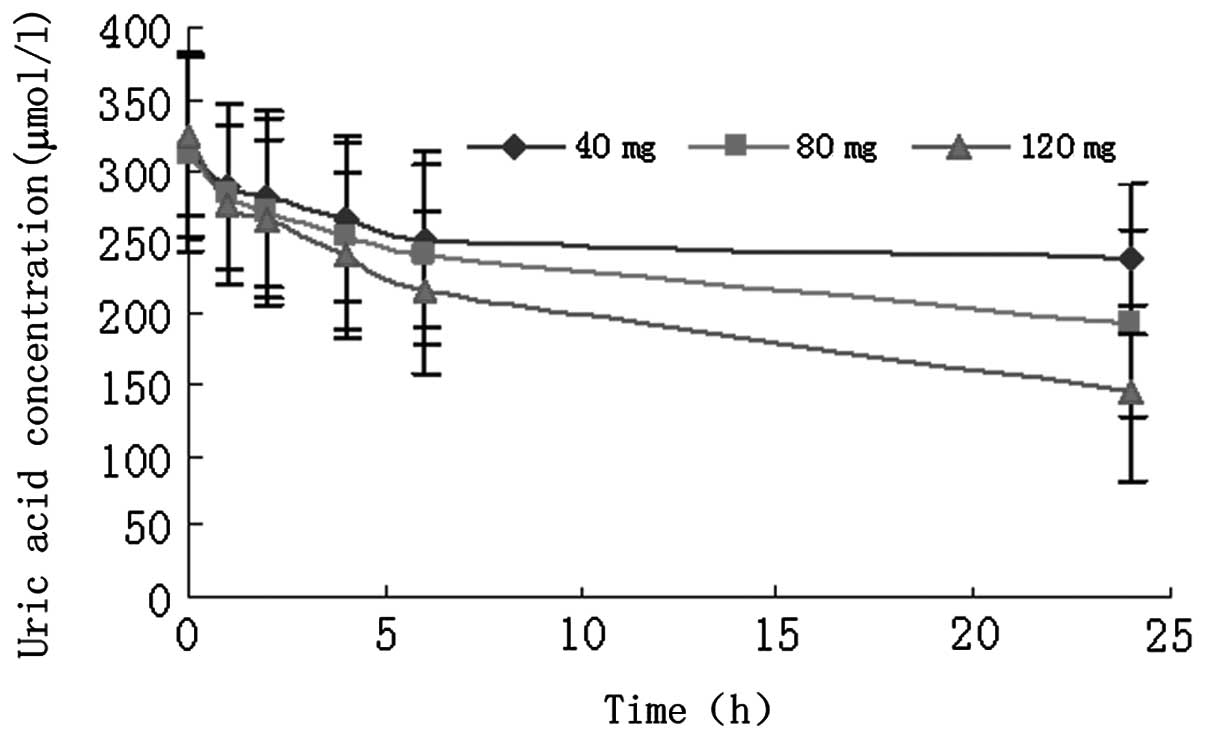
In all three single-dose groups, decreased UA
activity was observed 1 h after febuxostat administration (Fig. 3). Regression analyses and Student’s
t-test were used to determine the regression coefficient. The
linear relationship between febuxostat dose and 24-h UA
concentration (UA24) was expressed as follows: y = −1.16× + 283.95
(R2=0.9994). ANOVA evaluation of UA24/dose revealed
significant differences between dose groups (P<0.05). In the
multiple-dose group, compared with baseline, reductions in UA
concentrations of 38.13, 46.67, 51.89, 52.93, 55.77 and 52.69% were
noted on days 2, 3, 4, 5, 6 and 7, respectively. After 4
consecutive days of treatment with febuxostat, the UA levels in the
subjects were low (Fig. 4).
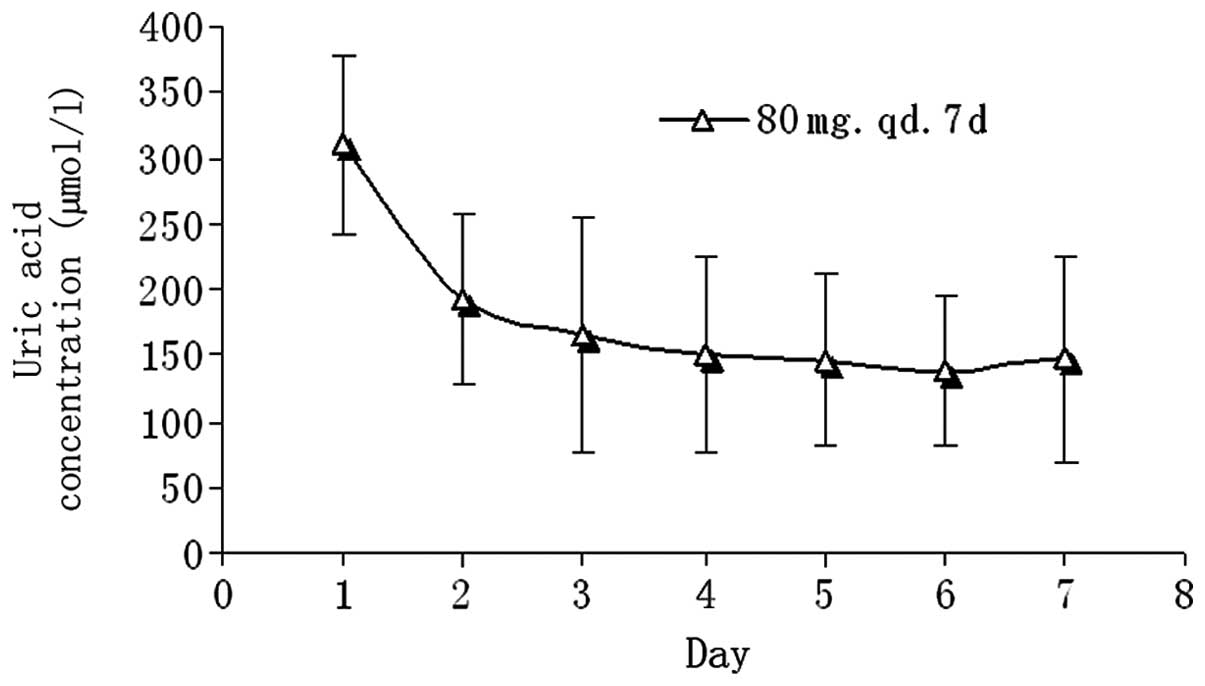
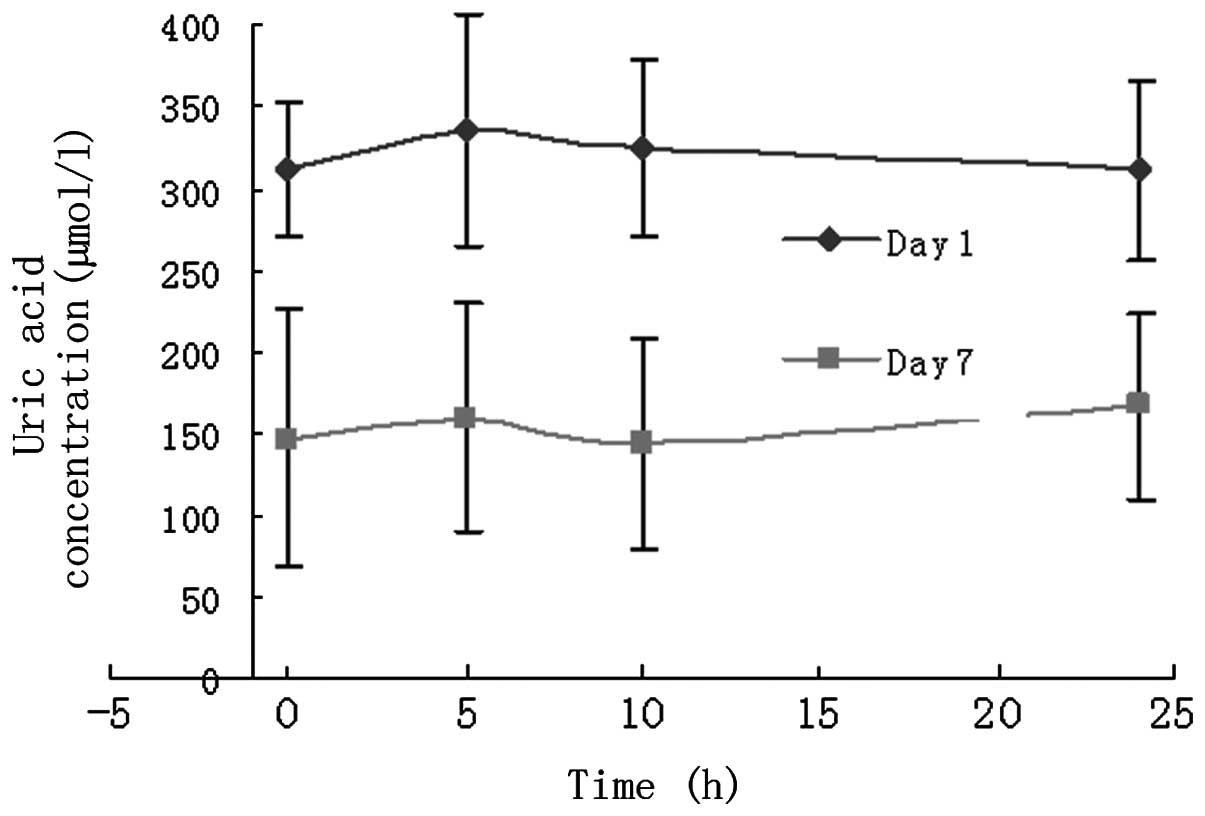
Comparison of UA concentrations prior to
and following febuxostat administration
On day -1 and 7 days after febuxostat
administration, the concentration of UA was measured at 0, 5, 10
and 24 h, respectively. The reduction of the UA concentration on
day 7 compared with that on day -1 (baseline) suggests that
febuxostat has a therapeutic effect (Fig. 5). The mean reduction in the UA
concentration on day 7 relative to that on day -1 was
51.83±7.00%.
Safety evaluation
Among the 36 healthy subjects, leukocytes were
detected in the urine of two male subjects who received a single
40-mg dose of febuxostat. In addition, one female subject in the
multiple-dose group reported dizziness and drowsiness, which
resolved spontaneously after a meal. These symptoms may have had no
association with febuxostat usage. No drug-related side-effects
and/or severe adverse events were observed in the subjects in this
study.
For all 36 healthy subjects, serum UA concentrations
were re-assessed 48 h after febuxostat was discontinued. The
results indicated that the UA concentrations had returned to the
normal range (mean, 301.56±13.34 μmol/l; normal, 119–416
μmol/l).
Discussion
Febuxostat, a highly selective inhibitor of xanthine
oxidase, is a novel drug for the management of hyperuricemia plus
gout. The therapeutic effect of febuxostat mainly depends on its
ability to lower UA concentrations. In the present study,
febuxostat reduced UA24 in a dose-dependent manner in healthy
subjects, which was consistent with a previous study (11). In the 40-, 80- and 120-mg
single-dose groups, the mean reduction in UA24 was 25.38, 38.13 and
55.69%, respectively.
In the present study, linear absorption of
febuxostat was observed across the dose range after single and
multiple doses, which was supported by the fact that the increasing
dose levels and administration of multiple doses had no effect on
the Tmax or the dose-normalised Cmax of
febuxostat. Moreover, our study was consistent with a previous
study (11), which indicated that
the volume of distribution of febuxostat was not affected by
increases in dose level and the administration of multiple doses.
Based on this finding, it may be speculated that the dose level and
schedule of febuxostat administration did not affect tissue
distribution in vivo.
According to UA testing, the renal clearance of
febuxostat had no significant role in the elimination of the drug
in vivo as only a small proportion of the drug remained
unchanged. Under different drug concentrations, the increase of AUC
was partly due to the increase of the concentration. This
phenomenon was most likely due to the potential increase in renal
clearance.
For the majority of drugs, pharmacodynamic effects
appear later than would be predicted by plasma concentration levels
because serum is not their site of action. In the present study,
the serum UA concentration was considered to be a therapeutic
marker mainly to facilitate sampling and testing. It was also
confirmed that there was a time delay between peak plasma drug
concentrations and the therapeutic effects of febuxostat.
Notably, gout attacks may become more frequent in
patients with hyperuricemia plus gout when febuxostat is taken at
an early stage (5). It has been
postulated that this may be due to the mobilization of urate from
tissue deposits as the serum UA concentration begins to fall.
Therefore, it is to be emphasized that our conclusions are limited
to healthy subjects.
In this study, febuxostat was orally administered to
healthy subjects and changes in UA concentrations were measured,
based on which the pharmacological activity of febuxostat was
investigated. Our results demonstrate that single doses of
febuxostat are associated with dose-dependent reductions in UA
concentrations. After 3 days of continuous febuxostat
administration, UA levels remained low (145.17±10.31 μmol/l). The
mean reduction in the UA concentration on day 7 relative to that on
day -1 was 51.83±7.00%. The concentrations of UA returned to normal
48 h after febuxostat was discontinued.
References
|
1
|
Liu B, Wang T, Zhao H, Yue W, Yu H, Liu C,
Yin J, Jia R and Nie H: The prevalence of hyperuricemia in China: a
meta-analysis. BMC Public Health. 11:8322011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nyhan WL: Disorders of purine and
pyrimidine metabolism. Mol Genet Metab. 86(1–2): 25–33. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guidelines for the research of chemical
drug and clinical pharmacokinetics. China Food and Drug
Administration; 2005, (In Chinese).
|
|
4
|
Chen JH, Pan WH, Hsu CC, Yeh WT, Chuang
SY, Chen PY, Chen HC, Chang CT and Huang WL: Impact of obesity and
hypertriglyceridemia on gout development with or without
hyperuricemia: a prospective study. Arthritis Care Res (Hoboken).
65:133–140
|
|
5
|
Reinders MK and Jansen TL: Management of
hyperuricemia in gout: focus on febuxostat. Clin Interv Aging.
5:7–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wallace KL, Riedel AA, Joseph-Ridge N and
Wortmann R: Increasing prevalence of gout and hyperuricemia over 10
years among older adults in a managed care population. J Rheumatol.
31:1582–1587. 2004.PubMed/NCBI
|
|
7
|
Bruce SP: Febuxostat: a selective xanthine
oxidase inhibitor for the treatment of hyperuricemia and gout. Ann
Pharmacother. 40:2187–2194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Szasz T, Davis RP, Garver HS, Burnett RJ,
Fink GD and Watts SW: Long-term inhibition of xanthine oxidase by
febuxostat does not decrease blood pressure in deoxycorticosterone
acetate (DOCA)-salt hypertensive rats. PloS One. 8:e56046
|
|
9
|
Khosravan R, Grabowski B, Wu JT,
Joseph-Ridge N and Vernillet L: Effect of food or antacid on
pharmacokinetics and pharmacodynamics of febuxostat in healthy
subjects. Br J Clin Pharmacol. 65:355–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang X, Li J, Sun R, Cheng Z and Zheng Q:
Drug and Statistics Software (DAS), version 2.0. Mathematical
Pharmacology Professional Committee of China. Am J Pharm Educ.
69:397–398. 2005.
|
|
11
|
Khosravan R, Grabowski BA, Wu JT,
Joseph-Ridge N and Vernillet L: Pharmacokinetics, pharmacodynamics
and safety of febuxostat, a non-purine selective inhibitor of
xanthine oxidase, in a dose escalation study in healthy subjects.
Clin Pharmacokinet. 45:821–841. 2006. View Article : Google Scholar : PubMed/NCBI
|