Introduction
Diabetes is the fifth leading cause of mortality in
the United States and the world prevalence of diabetes is
increasing at an alarming rate with >1 million new patients per
year diagnosed in the United States (1,2).
Furthermore, diabetes results in substantial mortality and
morbidity (3). Although the
current methods of diagnosis and treatment of diabetes have
improved, the long-term prognosis remains poor (4). In addition, there are few effective
preventive measures against its development. Epidemiological data
suggested that disease prevalence is likely to continue to increase
globally without effective prevention and control (5).
Diabetic nephropathy (DN) is one of the most common
complications, which results in chronic kidney disease in diabetic
patients. It is also one of the causes of increased cardiovascular
mortality (4). However, a
considerable amount of significant diabetic renal structural injury
may occur in absolute clinical silence, which renders diagnosis
difficult. Currently, routine kidney biopsy for DN is not permitted
in clinical practice, as the procedure is invasive.
Microalbuminuria is considered as the best available, non-invasive
marker for DN risk, but certain studies have shown it to have
inadequate specificity and sensitivity (6,7).
Thus, additional studies into novel invasive risk markers are
required, and feasible measures for the diagnosis of DN prior to
advanced renal dysfunction are considered to be of clinical
importance with public health implications.
Vitamin D-binding protein (VDBP), also known as
gc-globulin, bonds and transports vitamin D throughout the body. It
also is important in the actin-scavenger system, ensuing immune
responses and inflammation processes (8). Clinically, it has been demonstrated
that exaggerated excretion of urinary VDBP is associated with
tubular dysfunction (9).
Therefore, it was hypothesized that the loss of urinary VDBP is
likely to be elevated in diabetic patients and particularly
accentuated in those patients with DN. In the present study, the
early detection and predictive value of urinary VDBP for DN was
assessed.
Materials and methods
Patient selection and urine specimen
collection
In this study, 105 Chinese Han individuals with
diabetes and 45 healthy volunteers (control group) were recruited
from the Traditional Chinese Medicine Hospital attached to Xinjiang
Medical University (Urumqi, China) from June 2012 to January 2013.
The patients were divided into three groups according to the value
of the urinary albumin:creatinine (Cr) ratio (UACR): DM group
without nephropathy and albuminuria (UACR<30 mg/g, n=35), early
DN group (DN1) with microalbuminuria (30≤UACR<300 mg/g, n=35)
and overt DN group (DN2) with macroalbuminuria (UACR≥300 mg/g,
n=35). All study subjects were >18 years old and blood pressure
was maintained at 140/90 mmHg by the use of angiotensin-receptor
blockers or angiotensin-converting enzyme inhibitors. Moreover,
blood glucose was controlled with human insulin. Within the 2 weeks
prior to urine collection, patients did not receive additional
medicine and the controls did not receive any medication. Patients
were excluded if they showed the following: Liver diseases,
autoimmune diseases, inflammatory diseases, pregnancy, urinary
system disorders, tumors, infections, decompensated heart failure,
cardiovascular events within 6 months, hematological diseases and
known renal diseases other than DN. Studies were approved by the
local ethics committee of the Traditional Chinese Medicine Hospital
attached to Xinjiang Medical University (Urumqi, China). All
patients were informed about the purpose of the study and gave
their written consent.
Urine specimen collection
The second voided clean-catch urine samples from all
patients were collected early in the morning. Each urine sample (20
ml) was directly collected into a sterile plastic tube and then
immediately centrifuged at 2,500 × g for 10 min at 4°C to remove
cell debris and particulate matter. The supernatant was stored at
−80°C for further analysis. Repeated freeze-thaw cycles were
avoided. Clinical data of all patients were also collected.
Western blot analysis
The stored supernatant was used for western blot
analysis after measuring the protein concentrations. The urine
samples were first thawed on ice, adding 1 mmol/l
phenylmethanesulfonyl fluoride (Sigma, St. Louis, MO, USA), and
then centrifuged using Centricon Plus-20, 10,000 MWCO devices
(Millipore, Bedford, MA, USA). Then the concentrated urinary
proteins were precipitated using a ReadyPrep 2-D clean up kit (GE
Healthcare, Piscataway, NJ, USA) to remove other interfering
components according to the manufacturer’s instructions The
concentrations of urinary proteins were determined by using the
Bradford protein assay kits (GE Healthcare). The samples examined
were from patients from the DM, DN1, DN2 and control groups (n=8
per group). A total of 20 μg prepared proteins isolated from the
urine samples were electrophoresed on a 12% sodium dodecyl
sulfate-polyacrylamide gel. The proteins were then transferred onto
polyvinylidene fluoride membranes (Immobilon P; Millipore,
Billerica, MA, USA). The membranes were blocked for 1 h at 37°C in
a solution of TBS containing 5% non-fat milk powder and 0.1%
Tween-20 (TBS-T) and then incubated overnight at 4°C with rabbit
monoclonal primary antibody against human VDBP (diluted 1:1,000;
Abcam, Cambridge, UK). Membranes were washed three times for 10 min
in TBS-T and then incubated with horseradish peroxidase
horseradish-coupled goat anti-rabbit IgG (Beijing Zhongshan
Biotechnology Co. Ltd., Beijing, China) at a 1:500 dilution at room
temperature for 1 h. The proteins were detected using an enhanced
chemiluminescence detection system (ECL-Direct systems RPN3000;
Pierce Biotechnology, Inc., Rockford, IL, USA). Triplicate gel
images of identical samples were used for analysis. It was
quantified by strip densitometry.
Enzyme-linked immunosorbent assay
(ELISA)
All the samples were centrifuged at 3,500 × g for 5
min at 4°C to remove interfering matter prior to ELISA analysis.
The concentrations of VDBP in the urine samples were measured with
a Human Vitamin D BP Quantikine ELISA kit (DVDBP0; R&D Systems,
Minneapolis, MN, USA). The assay was performed according to the
instructions recommended by the manufacturer. The standard curve
was created using the lyophilized human VDBP standard preparation
supplied with the assay. Following the colorimetric reaction, the
optical density (OD) readings were converted to concentrations in
ng/ml based on quantification of the OD at 450 nm using an
eight-channel spectrophotometer (Fast model; BD Biosciences,
Franklin Lakes, NJ, USA). Measured VDBP levels ranged from 0 to 250
ng/ml. Urine Cr levels were measured at the Department of Clinical
Laboratory, Traditional Chinese Medicine Hospital attached to
Xinjiang Medical University (Urumqi, China). The levels of VDBP
were normalized according to urine Cr concentrations so as to avoid
the influence of urine volume and presented as VDBP:Cr ratio
(VDBP-Cr; ng/mg of Cr) (10).
Every sample was tested in duplicate.
Statistical analysis
All data were collected and presented as the mean ±
standard deviation. The differences among groups were compared with
Student’s t-test between two groups or one-way analysis of variance
for three groups. Receiving operating curve (ROC) analyses were
used to explore the diagnostic performance of urinary VDBP:Cr over
a range of possible clinical results on the basis of estimating the
sensitivity versus its false-positive rate at optimal cut-offs
(10,11). The best statistical cut-off value
of VDBP:Cr was defined, which means the point at which the sum of
sensitivity and specificity is more than that at other points.
Pearson’s correlation coefficient analysis was employed to explore
the association between the VDBP:Cr and the clinical features of
patients. Multivariate logistical regression analysis was utilized
to determine the risk factors for DN. All statistical analyses were
performed with SPSS software, version 13.0 (SPSS, Chicago, IL,
USA). All tests were two tailed and P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical characteristics of patients
The clinical characteristics of all patients are
shown in Table I. Samples from 35
DM, 35 DN1 and 35 DN2 patients and 45 controls were collected for
ELISA analysis. Analysis of the patient data indicated that there
were no statistically significant differences in the majority of
clinical characteristics (e.g. age, gender, smoking and duration of
diabetes) among the four groups. However, significant differences
were identified in serum Cr, UACR and systolic blood pressure
between certain groups (serum-Cr: DN2 vs. control P=0.025, DN2 vs.
DM: P=0.012 DN2 vs. DN1: P=0.034, UACR-DN1 vs. control: P<0.001,
DN1 vs. DM: P<0.001; DN2 vs. control P<0.001, DN2 vs. DM:
P<0.001, DN2 vs. DN1: P<0.001; systolic blood pressure-DN1
vs. control: P=0.021, DN2 vs. control: P=0.014). Additionally, as
the patients in each experimental group had taken similar
medications, the effect of taking medicine on the results was
ignored.
 | Table IClinical and demographic data for the
patients subjected to ELISA analysis. |
Table I
Clinical and demographic data for the
patients subjected to ELISA analysis.
| Characteristics | Control | DM group | DN1 group | DN2 group |
|---|
| Cases (n) | 45 | 35 | 35 | 35 |
| Age (years) | 60.89±13.92 | 64.63±11.76 | 64.49±11.78 | 64.80±12.98 |
| Gender (n) |
| Male | 31 | 22 | 18 | 21 |
| Female | 14 | 13 | 17 | 4 |
| Smoking (n) |
| Yes | 20 | 14 | 17 | 19 |
| No | 25 | 21 | 18 | 16 |
| Diabetes (n) |
| Type 1 | 0 | 5 | 5 | 7 |
| Type 2 | 0 | 30 | 30 | 28 |
| Diabetic duration
(years) | 0 | 11.43±7.83 | 12.77±8.65 | 11.57±6.45 |
| Systolic BP
(mmHg) | 115±8.32 | 120±9.59 | 134±8.76* | 143±10.32* |
| Serum creatinine
(μmol/l) | 99.84±24.23 | 92.43±36.65 | 107.34±46.62 | 142.46±55.10*,**,*** |
| UACR (mg/g) | 10.52±2.78 | 13.08±4.11 | 134.66±47.2*,** |
1603.09±544.60*,**,*** |
| Taking ACEI (n) | 0 | 12 | 15 | 14 |
| Taking ARB (n) | 0 | 13 | 10 | 12 |
| Insulin therapy
(n) | 0 | 35 | 35 | 35 |
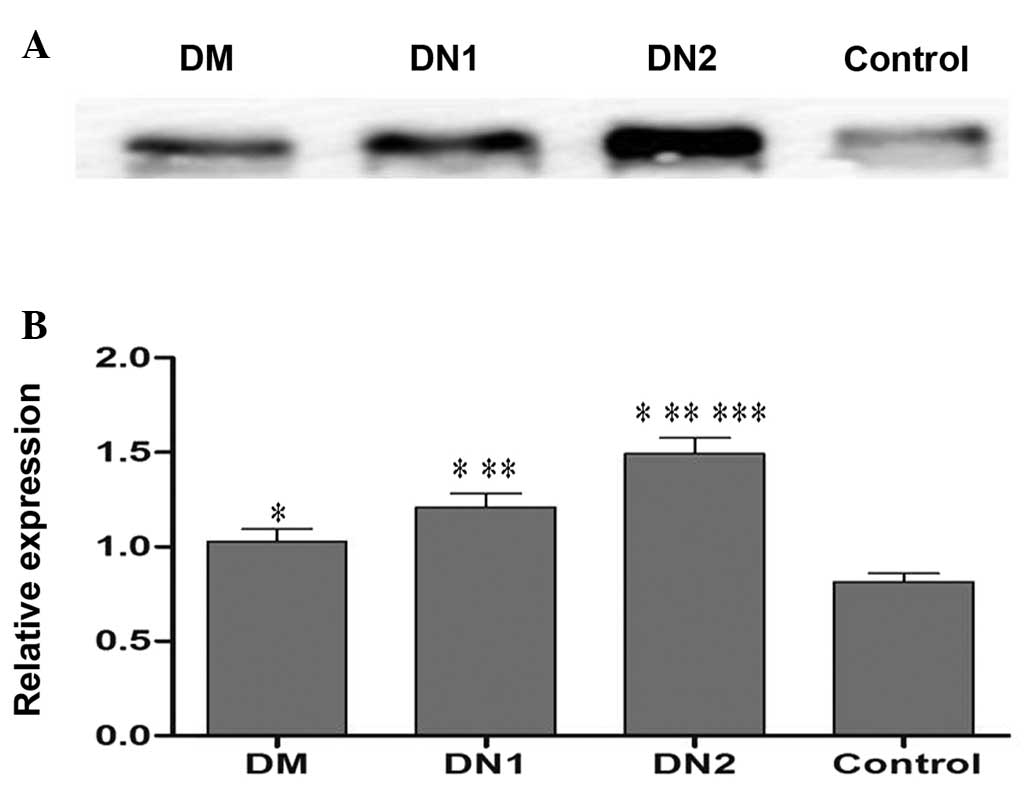
Analysis of urinary VDBP by western
blotting
To verify the expression of urinary VDBP in
individual urine samples by western blotting, 32 samples from the
DM, DN1, DN2 and control groups (n=8 per group) were randomly
selected. The results demonstrated that urinary VDBP levels were
significantly upregulated in the DM group compared with that of the
control group (Fig. 1).
Furthermore, the levels of urinary VDBP were significantly elevated
in the DN groups compared with those of the DM and control groups
(Fig. 1).
Detection of urinary VDBP expression
To explore the changes of urinary VDBP expression in
DN patients, ELISA analysis was conducted on the 150 urine samples
from the DM, DN1, DN2 and control groups.
The levels of urinary VDBP were significantly higher
in patients than in controls (961.41±542.77 versus 125.48±98.27
ng/mg, P<0.001; Fig. 2A). After
division of the patients into groups by UACR, it was indicated that
the expression levels of urinary VDBP were significantly higher in
the DN1 and DN2 groups than in the DM group (1,011.33±325.30 and
1,406.34±239.66 versus 466.54±213.63 ng/mg, respectively;
P<0.001). A significant difference was also observed between the
DN2 and DN1 groups (P<0.001; Fig.
2A and Table II).
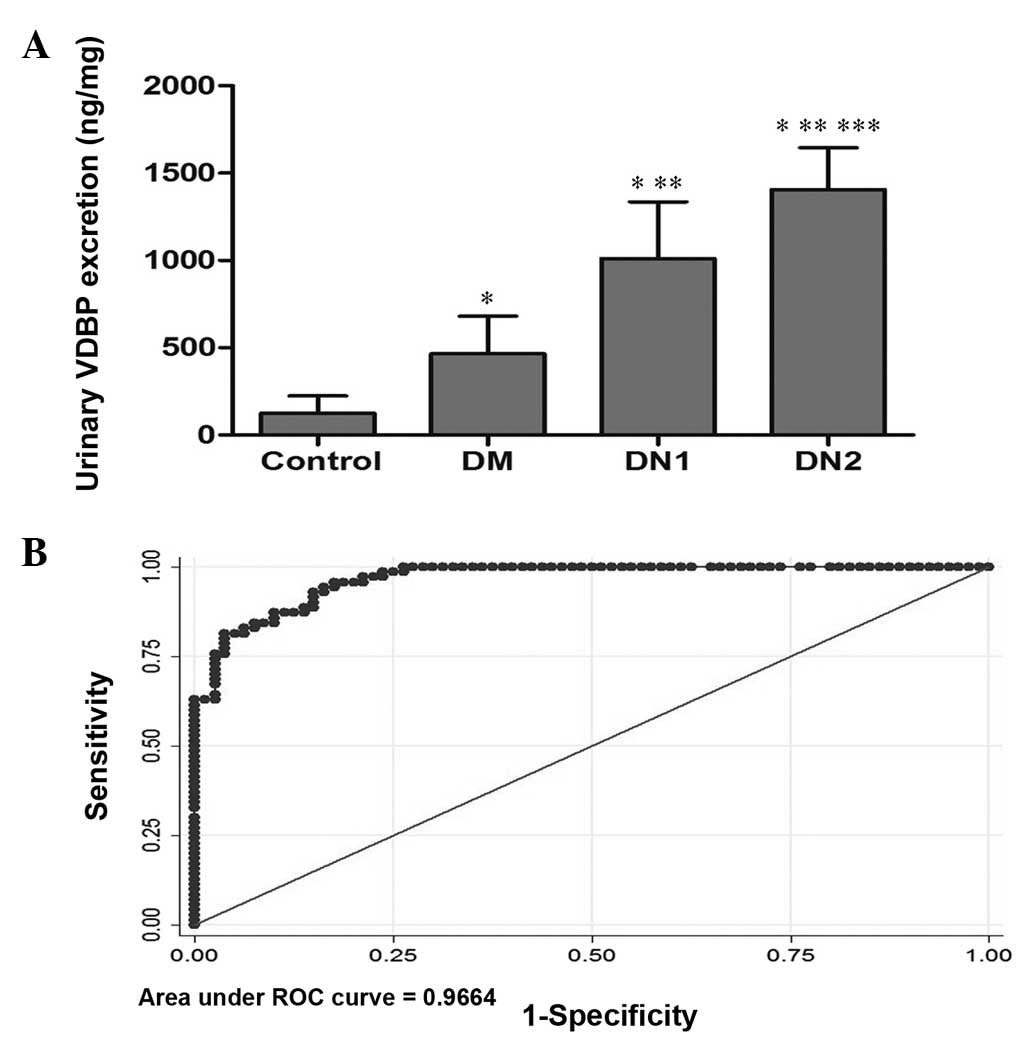 | Figure 2Evaluation of urinary VDBP levels as a
biomarker for DN. (A) ELISA quantification of VDBP levels in the
urine of DM, DN1, DN2 and control groups. Data are expressed as the
mean ± standard deviation. *P<0.05 compared with the
control group; **P<0.05 compared with the DM group;
and ***P<0.001 compared with the DN1 group. (B) ROC
curve of urinary VDBP as a biomarker of early detection and
prevention for DN was based on an optimum cut-off value of 552.243
ng/mg corresponding to 92.86% sensitivity and 85.00% specificity.
The area under the ROC curve was 0.966 (95% CI, 0.924–0.989). VDBP,
vitamin D binding protein; DN, diabetes nephropathy; DM, UACR<30
mg/g; DN, diabetic nephropathy; DN1, with microalbuminuria
30<UACR<300 mg/g; DN2, with microalbuminuria UACR>300
mg/g; UACR, urinary albumin:creatinine; ROC, receiver operating
characteristics. |
 | Table IICorrelation between levels of urinary
VDBP and clinical features of diabetic patients. |
Table II
Correlation between levels of urinary
VDBP and clinical features of diabetic patients.
| Clinical
features | No. | Urinary VDBP levels
(ng/mg) | P-value |
|---|
| Age |
| >65 years | 52 | 1009.52±533.27 | 0.37 |
| ≤65 years | 53 | 914.20±552.91 | |
| Gender |
| Female | 44 | 1001.44±491.33 | 0.52 |
| Male | 61 | 932.53±579.32 | |
| Hypertension |
| With | 36 | 1050.86±426.16 | 0.22 |
| Without | 69 | 914.74±592.15 | |
| Smoking |
| Yes | 50 | 1043.62±618.68 | 0.14 |
| No | 55 | 886.66±456.19 | |
| Renal
dysfunction |
| With | 38 | 1263.15±531.33 | <0.001 |
| Without | 67 | 790.27±473.08 | |
| Diabetes |
| Type 1 | 18 | 1083.46±582.21 | 0.30 |
| Type 2 | 87 | 936.15±534.31 | |
| Diabetic
duration |
| >12 years | 43 | 975.74±543.32 | 0.82 |
| ≤12 years | 62 | 951.46±546.60 | |
| DM group | 35 | 466.54±213.63 | <0.001 |
| DN1 group | 35 | 1011.33±325.30 | |
| DN2 group | 35 | 1406.34±539.66 | |
Correlation and multivariate logistical
regression analyses
Additional subgroup analyses were performed to
clarify the correlation between urinary VDBP levels and clinical
characteristics of the patients (Table II). No significant association was
observed between the urinary loss of VDBP and the clinical features
of DM patients, such as age, gender, smoking, duration of DM and
hypertension, with the exception of DN and renal dysfunction. The
enhanced excretion levels of urinary VDBP were significantly higher
in patients with renal dysfunction than in the patients without
dysfunction (1,263.15±531.33 versus 790.27±473.08 ng/mg;
P<0.001). Furthermore, higher urinary VDBP concentrations were
detected in the DN1 and DN2 groups than in the DM group
(P<0.001). The Spearman rank correlation was 0.707, which
indicated that the levels of urinary VDBP had a strong positive
correlation with the development of DN. Also, multivariate
logistical regression analysis was conducted to assess the
independent risk factors for DN. The results suggested that serum
Cr may be an independent predictor of DN (OR=1.29, 95% confidence
interval, 1.02–1.57; P=0.04).
Evaluation of urinary VDBP as a biomarker
for DN
Following quantitative analysis of 150 urine samples
by ELISA, ROC curves were used to assess the potential utility of
urinary VDBP detection in patients with DN. The area under the ROC
curve of urine VDBP levels for the diagnosis of DN was 0.966 (95%
CI, 0.924–0.989). The analysis rendered an optimum cut-off value of
552.243 ng/mg corresponding to 92.86% sensitivity and 85.00%
specificity (Fig. 2B).
Discussion
Improving the early prediction and detection of DN
remains a great challenge in disease management (13,14).
Improving the predictive ability of testing would greatly benefit
the treatment of patients with DN and facilitate the monitoring of
the condition. To explore whether urine VDBP levels may be a novel
non-invasive biomarker for DN, the results of the present study
demonstrated that the expression level of urinary VDBP was highly
upregulated in patients with DN. Furthermore, the levels of VDBP
were measured in the urine samples from the control, DM and DN
groups.
VDBP is a 58-kDa glycoprotein and is present in the
serum at a concentration of 300–600 mg/ml (15). It serves as the main carrier
protein for vitamin D in the bloodstream. VDBP is important in the
bioavailability of active 1,25-dihydroxyvitamin D
(1,25(OH)2D) and its precursor 25-hydroxyvitamin D
(25OHD) (16,17). The transportation of vitamin D by
VDBP is important for the function of a wide variety of tissues and
changes in VDBP activity result in the development of a number of
diseases (8).
In addition to its transport function, VDBP is the
parent molecule of VDBP-maf (macrophage activating factor).
VDBP-maf is the product of the selectively deglycosylated form of
VDBP and has been demonstrated to be a potent antiangiogenic and
antitumorigenic molecule (18).
Such functions would greatly benefit the regulation of the growth
of cancer cells and protection against certain immune disorders
(19). These important and diverse
properties of VDBP have been suggested in previous studies with
regard to a number of tumor types (20,21).
Moreover, VDBP is important in the actin scavenger system and
inflammation processes (8).
Studies concerning the actions of VDBP in the kidney have received
increased attention and have suggested that VDBP is vital in the
endocrine biosynthetic process of 1,25(OH)2D within
renal proximal tubules; in this process, 25OHD binds to VDBP and
the complex is actively recovered from glomerular filtrate through
megalin-mediated receptor endocytosis (22,23).
In the present study, following quantitative
measurements of 150 urine samples with ELISA, it was identified
that the VDBP expression levels were significantly higher in the
urine samples from patients with DN than in the urine from the DM
and control groups. Furthermore, a strong positive correlation was
observed between the urinary VDBP levels and the development of DN.
ROC analysis rendered that an optimum cut-off value of urinary VDBP
of 552.243 ng/mg corresponding to 92.86% sensitivity and 85.00%
specificity is appropriate for detecting DN. On the basis of these
analyses, urinary VDBP was indicated to be a potential biomarker
for the early detection and prevention of DN.
The reasons underlying the enhanced excretion of
urinary VDBP in patients with DN remain unclear. One possible
explanation is that elevated urinary VDBP levels may be associated
with renal tubular damage in DN patients (24,25).
Renal tubular epithelial cell damage becomes increasingly severe as
DN develops. In a previous study, increased excretion of urinary
VDBP was observed following long-term cadmium exposure, and it was
suggested that the marked loss of VDBP in the urine may be linked
to renal tubular dysfunction and bone lesions in the inhabitants of
cadmium-polluted areas (26). In
addition, it has been demonstrated that the presence of vitamin D
deficiency or insufficiency in patients with diabetes is
independently associated with the development of DN. Moreover,
exaggerated urinary excretion of VDBP was observed in patients with
Type I diabetes, which contributed mechanistically to vitamin D
deficiency in this disease (9,27,28).
Therefore, a further possibility for the elevated urinary VDBP
levels identified in the present study may be associated with the
relatively lower serum vitamin D levels. Further studies are
required to clarify the role of VDBP in the pathogenesis of DN.
Clinically, there have been studies on aspects of
other diseases, which demonstrated the increased urinary excretion
of VDBP. Recently, Mirković et al indicated that the urinary
excretion of VDBP may be a novel urinary biomarker of
tubulointerstitial damage (29).
Cho et al demonstrated that the urinary VDBP loss was
significantly elevated in patients with endometriosis than those
without. However, the authors suggested that urinary VDBP has
limited value as a potential early diagnostic biomarker for
endometriosis (10). A study by
Zoidakis et al identified that the reduction of VDBP levels
in the urine of patients with invasive bladder cancer was
significant (30), which is
consistent with the findings by Li et al(8). Moreover, Li et al also
demonstrated that the expression levels of urinary VDBP were
positively associated with the pathological classification of
bladder cancer (9). Their results
suggested that urinary VDBP may be a potential non-invasive
biomarker for the early diagnosis and effective surveillance of
bladder cancer (8). In the present
study, to the best of our knowledge, it was demonstrated for the
first time that increased urinary VDBP levels occurred in patients
with DN, and there was a strong positive association between
urinary VDBP levels and the development of DN.
An important limitation of the present study
regarding the specificity of this biomarker should be considered
when urinary VDBP detection is used for early prevention of DN. It
has been demonstrated that urinary VDBP levels are closely
associated with renal dysfunction. In the present study, urine
samples were collected from patients with DN only, but not from
patients with additional nephropathies. This may have caused an
overestimation of the specificity of VDBP as a biomarker for the
detection of DN. Therefore, further studies including a larger
sample and analyses from patients with various types of
non-diabetic nephropathy are required to clarify this issue.
In conclusion, the current study demonstrated that
urinary VDBP levels were significantly elevated in patients with
DN. Moreover, a strong positive correlation was observed between
the expression level of urinary VDBP and the development of DN.
Thus, the findings indicate that urinary VDBP levels are a
potential biomarker for the early detection and prevention of DN.
Further studies are warranted to examine the pathogenic mechanisms
of elevated VDBP and its role in the diagnosis of DN.
References
|
1
|
Hu FB, Manson JE, Stampfer MJ, Colditz G,
Liu S, Solomon CG and Willett WC: Diet, lifestyle, and the risk of
type 2 diabetes mellitus in women. N Engl J Med. 345:790–797. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pittas AG, Lau J, Hu FB and Dawson-Hughes
B: The role of vitamin D and calcium in type 2 diabetes. A
systematic review and meta-analysis. J Clin Endocrinol Metab.
92:2017–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iso H: Changes in coronary heart disease
risk among Japanese. Circulation. 118:2725–2729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valmadrid CT, Klein R, Moss SE and Klein
BE: The risk of cardiovascular disease mortality associated with
microalbuminuria and gross proteinuria in persons with older-onset
diabetes mellitus. Arch Intern Med. 160:1093–1100. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053.
2004.PubMed/NCBI
|
|
6
|
Tabaei BP, Al-Kassab AS, Ilag LL, Zawacki
CM and Herman WH: Does microalbuminuria predict diabetic
nephropathy? Diabetes Care. 24:1560–1566. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang H, Guan G, Zhang R, Liu G, Cheng J,
Hou X and Cui Y: Identification of urinary soluble E-cadherin as a
novel biomarker for diabetic nephropathy. Diabetes Metab Res Rev.
25:232–241. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li F, Chen DN, He CW, Zhou Y, Olkkonen VM,
He N, Chen W, Wan P, Chen SS, Zhu YT, Lan KJ and Tan WL:
Identification of urinary Gc-globulin as a novel biomarker for
bladder cancer by two-dimensional fluorescent differential gel
electrophoresis (2D-DIGE). J Proteomics. 77:225–236. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thrailkill KM, Jo CH, Cockrell GE, Moreau
CS and Fowlkes JL: Enhanced excretion of vitamin D binding protein
in type 1 diabetes: a role in vitamin D deficiency? J Clin
Endocrinol Metab. 96:142–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cho S, Choi YS, Yim SY, Yang HI, Jeon YE,
Lee KE, Kim H, Seo SK and Lee BS: Urinary vitamin D-binding protein
is elevated in patients with endometriosis. Hum Reprod. 27:515–522.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hanley JA and McNeil BJ: The meaning and
use of the area under a receiver operating characteristic (ROC)
curve. Radiology. 143:29–36. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang H, Guan G, Zhang R, Liu G, Liu H,
Hou X and Cheng J: Increased urinary excretion of orosomucoid is a
risk predictor of diabetic nephropathy. Nephrology (Carlton).
14:332–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dihazi H and Müller GA: Urinary
proteomics: a tool to discover biomarkers of kidney diseases.
Expert Rev Proteomics. 4:39–50. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Varghese SA, Powell TB, Budisavljevic MN,
Oates JC, Raymond JR, Almeida JS and Arthur JM: Urine biomarkers
predict the cause of glomerular disease. J Am Soc Nephrol.
18:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cooke NE and David EV: Serum vitamin
D-binding protein is a third member of the albumin and alpha
fetoprotein gene family. J Clin Invest. 76:2420–2424. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chun RF, Peercy BE, Adams JS and Hewison
M: Vitamin D binding protein and monocyte response to
25-hydroxyvitamin D and 1,25-dihydroxyvitamin D: analysis by
mathematical modeling. PLoS One. 7:e307732012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Christakos S, Ajibade DV, Dhawan P,
Fechner AJ and Mady LJ: Vitamin D: metabolism. Endocrinol Metab
Clin North Am. 39:243–253. 2010. View Article : Google Scholar
|
|
18
|
Yamamoto N and Naraparaju VR: Vitamin
D3-binding protein as a precursor for macrophage activating factor
in the inflammation-primed macrophage activation cascade in rats.
Cell Immunol. 170:161–167. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gregory KJ, Zhao B, Bielenberg DR, Dridi
S, Wu J, Jiang W, Huang B, Pirie-Shepherd S and Fannon M: Vitamin D
binding protein-macrophage activating factor directly inhibits
proliferation, migration, and uPAR expression of prostate cancer
cells. PLoS One. 5:e134282010. View Article : Google Scholar
|
|
20
|
Yamamoto N, Naraparaju VR and Asbell SO:
Deglycosylation of serum vitamin D3-binding protein leads to
immunosuppression in cancer patients. Cancer Res. 56:2827–2831.
1996.PubMed/NCBI
|
|
21
|
Yamamoto N, Suyama H, Nakazato H, Yamamoto
N and Koga Y: Immunotherapy of metastatic colorectal cancer with
vitamin D-binding protein-derived macrophage-activating factor,
GcMAF. Cancer Immunol Immunother. 57:1007–1016. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nykjaer A, Dragun D, Walther D, Vorum H,
Jacobsen C, Herz J, Melsen F, Christensen EI and Willnow TE: An
endocytic pathway essential for renal uptake and activation of the
steroid 25-(OH) vitamin D3. Cell. 96:507–515. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zehnder D, Bland R, Williams MC, McNinch
RW, Howie AJ, Stewart PM and Hewison M: Extrarenal expression of
25-hydroxyvitamin D3-1α-hydroxylase. J Clin Endocrinol
Metab. 86:888–894. 2001.PubMed/NCBI
|
|
24
|
Colston K, Williams NJ and Cleeve HJ:
Studies on vitamin D binding protein in the nephrotic syndrome.
Clin Chem. 31:718–721. 1985.PubMed/NCBI
|
|
25
|
Vilasi A, Cutillas PR, Maher AD, Zirah SF,
Capasso G, Norden AW, Holmes E, Nicholson JK and Unwin RJ: Combined
proteomic and metabonomic studies in three genetic forms of the
renal Fanconi syndrome. Am J Physiol Renal Physiol. 293:F456–F467.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uchida M, Teranishi H, Aoshima K, Katoh T,
Kasuya M and Inadera H: Elevated urinary levels of vitamin
D-binding protein in the inhabitants of a cadmium polluted area,
Jinzu River basin, Japan. Tohoku J Exp Med. 211:269–274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Svoren BM, Volkening LK, Wood JR and
Laffel LM: Significant vitamin D deficiency in youth with type 1
diabetes mellitus. J Pediatr. 154:132–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tahrani AA, Ball A, Shepherd L, Rahim A,
Jones AF and Bates A: The prevalence of vitamin D abnormalities in
South Asians with type 2 diabetes mellitus in the UK. Int J Clin
Pract. 64:351–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mirković K, Doorenbos CR, Dam WA, Lambers
Heerspink HJ, Slagman MC, Nauta FL, Kramer AB, Gansevoort RT, van
den Born J, Navis G and de Borst MH: Urinary vitamin D binding
protein: a potential novel marker of renal interstitial
inflammation and fibrosis. PLoS One. 8:e558872013.PubMed/NCBI
|
|
30
|
Zoidakis J, Makridakis M, Zerefos PG,
Bitsika V, Esteban S, Frantzi M, Stravodimos K, Anagnou NP,
Roubelakis MG, Sanchez-Carbayo M and Vlahou A: Profilin 1 is a
potential biomarker for bladder cancer aggressiveness. Mol Cell
Proteomics. 11:M111.0094492012. View Article : Google Scholar
|