Introduction
Gram-positive infections are associated with a high
incidence of morbidity and mortality in respiratory intensive care
units (ICUs) in China (1). The
mortality rate for methicillin-resistant Staphylococcus
aureus (MRSA) pneumonia has been reported to range from 33 to
55% in certain Asian countries, including Japan, Taiwan, Singapore,
China and Korea (2,3). In a number of studies, the rate of
thrombocytopenia identified in seriously ill patients, particularly
those in ICUs, has ranged from 19 to 63% (4–11).
Thrombocytopenia is strongly associated with poor outcome, illness
severity, the development of organ failure, the length of hospital
stay and mortality (5,7,10,12).
A common cause of acquired thrombocytopenia is the use of drugs,
including heparin, glycoprotein and antibiotics, in the ICU
(13). Linezolid, the first
oxazolidinone antibiotic agent, has demonstrated antibacterial
activity against gram-positive bacteria, including MRSA and
vancomycin-resistant enterococci. Although linezolid is safe, it
has been demonstrated to be associated with hematologic effects,
including thrombocytopenia, anemia and neutropenia.
Thrombocytopenia is the most frequent adverse effect associated
with linezolid (14,15). However, only a limited number of
well-designed studies have compared the incidence of
thrombocytopenia in patients treated with linezolid with that in
patients treated with other anti-gram-positive agents (16–19).
Since 2007, linezolid has been available in China
and thrombocytopenia associated with linezolid has been reported
(20). However, the incidence of
thrombocytopenia associated with linezolid varies in different
countries, such as Japan and United States (21–23).
The incidence of thrombocytopenia and time-to-event compared with
comparators in a large sample of Chinese patients in an ICU has, to
the best of our knowledge, not been reported to date.
The primary purpose of this investigation was to
compare the incidence of the thrombocytopenia associated with the
use of linezolid and glycopeptides in different age groups. The
secondary purpose was to conduct a time-to-event analysis between
patients receiving linezolid and glycopeptides. In order to assess
this, the mean number of platelets and the reduction in the number
of platelets were compared between patients receiving linezolid and
those receiving glycopeptides.
Patients and methods
Patients
This study was conducted on patients in the
respiratory ICU of the Chinese PLA General Hospital (Beijing,
China). Patients who received linezolid (Pfizer Inc., New York, NY,
USA; 1,200 mg as a 600-mg dose every 12 h), vancomycin (Eli Lilly
and Company, Indianapolis, IN, USA) or teicoplanin (Sanofi-Aventis
Ltd., Paris, France) via intravenous administration were included
in the study. The daily dosage of the vancomycin or teicoplanin was
according to the manufacturer’s instructions. The vancomycin dosage
was 15 mg/kg/day for patients with normal renal function and was
adjusted according to creatinine clearance. Teicoplanin was used at
a dose of 12 mg/kg/day for patients with normal renal function and
was also adjusted according to the creatinine clearance. The data
were collected from the electronic medical records in the
hospital’s central database of patients who were treated between
January 1, 2010 and June 30, 2013.
The inclusion criteria were as follows: i) Patients
with pneumonia who received linezolid or vancomycin or teicoplanin
for ≥5 days in the respiratory ICU for the treatment or prophylaxis
of pulmonary gram-positive infections; ii) the baseline number of
platelets was available; and iii) the platelets were examined every
day from the start to the end of the treatment.
The exclusion criteria were as follows: i) age
<18 years; ii) patients with a blood system or platelet number
reduction associated with an original disease, including leukemia,
myelodysplasia, aplastic anemia and tumors being treated using
chemotherapy; iii) patients who had experienced platelet
transfusion during the period when using linezolid or
glycopeptides; and iv) an abnormal platelet count
(<100×109/l or >400×109/l) prior to
therapy.
Data Extraction
Examination data and clinical notes were extracted
from electronic medical records. Medical charts/records without
patient names and/or identification numbers were stored in a
Microsoft Excel file. Data analyses performed by one doctor were
reviewed and confirmed by another doctor to ensure accuracy. As
this was a retrospective study, informed consent was waived.
Data included demographics, comorbidities,
hospitalization history, disease severity, microbiological data and
laboratory data.
Demographic characteristics included age, gender,
height, body weight, acute physiology and chronic health
evaluation-II (APACHE-II) score, length of hospital stay, treatment
duration, renal failure, outcome, mechanical ventilation and
infection type (hospital-acquired or community-acquired).
Comorbidities included heart failure, respiratory failure, renal
failure, chronic obstructive disease, diabetes mellitus,
hypertension, hepatic dysfunction, transplanted organ, active
malignancy and cancer. Laboratory data included creatinine levels,
baseline platelet count and blood urea nitrogen. Microbiological
data included the type of organism.
Definitions
Thrombocytopenia was defined as a platelet count of
<100×109/l. The reduction of the platelet count was
calculated as follows: Reduction in platelet count = platelet
countbaseline-platelet countvalue.
Statistical analysis
Statistical analysis was performed using
commercially available software (SPSS software, version 15.0, SPSS,
Inc., Chicago, IL, USA). The Student’s t-test (normal-distribution)
or Mann-Whitney U test (non-normal distribution) was used to
compare continuous variables. Categorical variables were compared
using χ2 test. The multi compression was performed by
one-way analysis of variance. For the analyses where the expected
cell frequency was low, Fisher’s exact test was utilized. P<0.05
was considered to indicate a statistically significant difference.
The hazard ratio with the 95% confidence interval was calculated
for each significant variable. Time-to-event analyses were
performed by computing Kaplan-Meier estimators (product-limit
method) for each case of thrombocytopenia under study. Survival
distributions were compared using the log-rank test.
Results
Patient data
Of 225 patients enrolled, 122 received linezolid and
103 received glycopeptides (vancomycin or teicoplanin) for ≥5 days.
The demographics and clinical characteristics of the study
population are displayed in Table
I. The demographic characteristics of the patients were similar
in the two treatment groups. However, the mean body weight of the
glycopeptide group was higher than that of the linezolid group
(62.4±13.2 kg for the linezolid group vs. 66.7±13.0 kg for the
glycopeptide group) (P=0.01). The incidence of renal failure in the
linezolid group (24.6%) was significantly higher than the incidence
of renal failure in the glycopeptide group (4.9%) (P<0.01).
 | Table IClinical and demographic
characteristics of patients receiving linezolid or glycopeptide
therapy. |
Table I
Clinical and demographic
characteristics of patients receiving linezolid or glycopeptide
therapy.
| Matching
variables | Linezolid group
(n=122) | Glycopeptide group
(n=103) | P-value |
|---|
| Age (n,%) | 65.9±18.2 | 69.3±16.1 | 0.14 |
| <65 years | 44 (36.1) | 37 (35.9) | 0.98 |
| ≥65 years | 78 (63.9) | 66 (64.1) | |
| Gender |
| Male | 78 (63.9) | 69 (67.0) | 0.63 |
| Female | 44 (36.1) | 34 (33.0) | |
| Mean height (cm) | 167.3±7.2 | 167.6±7.3 | 0.73 |
| Mean body weight
(kg) | 62.4±13.2 | 66.7±13.0 | 0.01 |
| Treatment duration
(days) |
| Mean ± SD | 9.9±3.9 | 10.5±4.6 | 0.24 |
| Range | 5–28 | 5–28 | |
| Mean length of
hospital stay (days) | 27.7±17.2 | 30.6±20.0 | 0.26 |
| APACHE-II score | 17.6±7.2 | 15.7±6.7 | 0.06 |
| Baseline creatinine
level (μmol/l) | 95.6±110.9 | 98.6±124.4 | 0.85 |
| Baseline BUN
(μmol/l) | 8.4±6.5 | 8.7±7.1 | 0.69 |
| Mechanical
ventilation | 90 (73.8) | 81 (78.6) | 0.39 |
| Infection type |
|
Community-acquired | 15 (12.3) | 12 (11.7) | 0.88 |
|
Hospital-acquired | 107 (87.7) | 91 (88.3) | |
| Number of
comorbities | | | >0.05 |
| 0 | 17 (13.9) | 18 (17.5) | |
| 1 | 30 (24.6) | 26 (25.2) | |
| 2 | 34 (27.9) | 30 (29.1) | |
| 3 | 38 (31.1) | 35 (34.0) | |
| ≥4 | 33 (27.0) | 24 (23.3) | |
| Renal failure | 30 (24.6) | 5 (4.9) | <0.01 |
| Death | 30 (24.6) | 25 (24.3) | 0.96 |
| Organism |
| MRSA | 51 (41.8) | 45 (43.7) | 0.78 |
| MSSA | 22 (18.0) | 18 (17.5) | 0.91 |
|
Enterococcus spp. | 27 (22.1) | 22 (21.4) | 0.89 |
| Others | 22 (18.0) | 15 (14.6) | 0.48 |
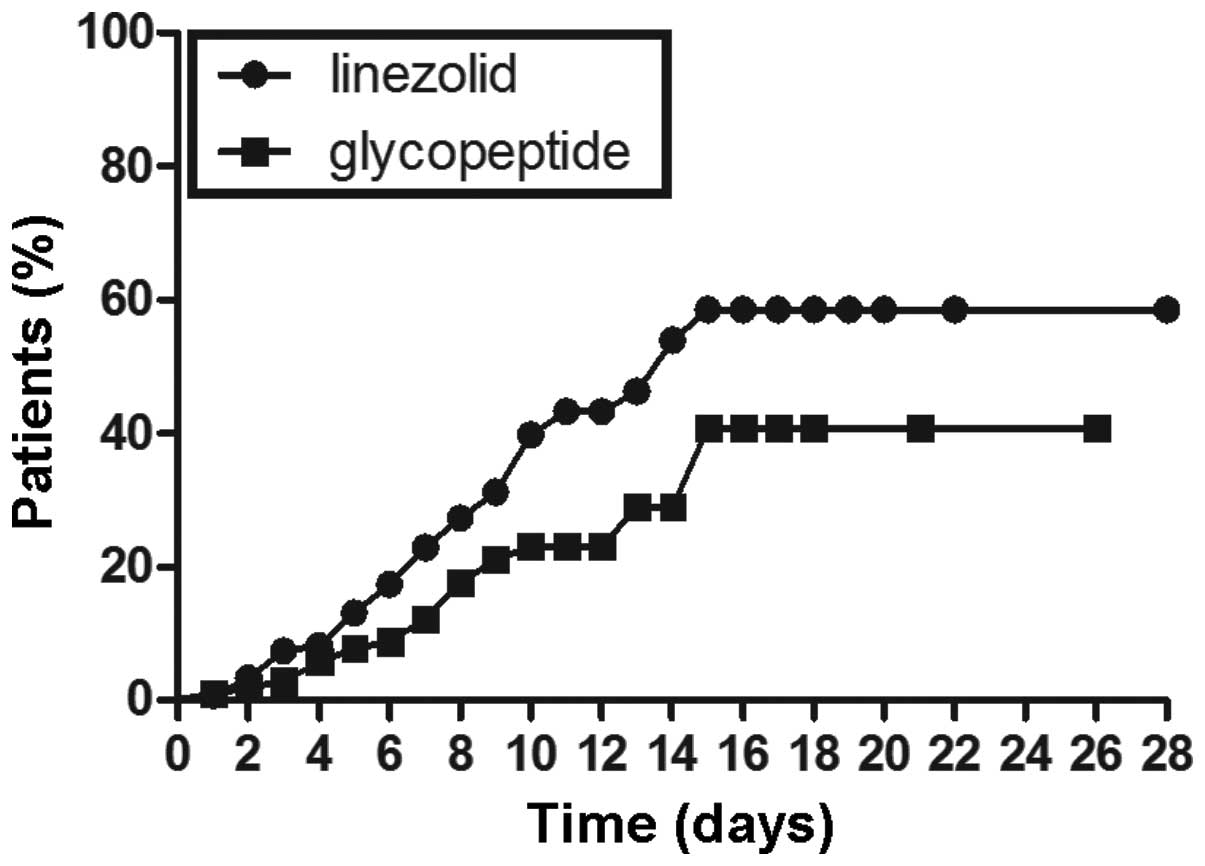
Kaplan-Meier plots of the percentage of patients
with thrombocytopenia are displayed in Figs. 1–3. The cumulative probability of
thrombocytopenia was significantly higher in the linezolid group
than in the glycopeptide group by the log rank test P<0.01
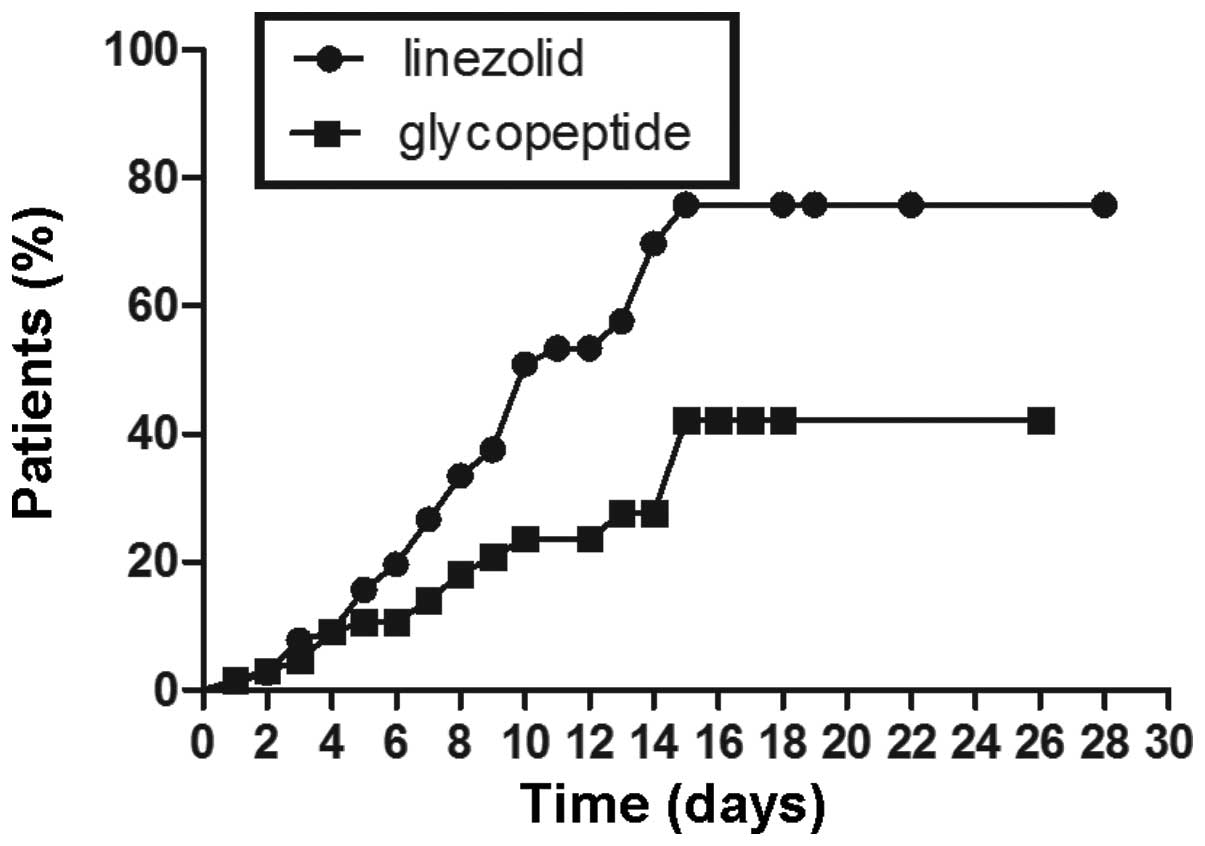
(Fig. 1). Further, a subgroup
analysis by age was conducted. In the age ≥65 years subgroup, a
similar result to the result of the whole group was obtained.
Linezolid was associated with a higher incidence of
thrombocytopenia than glycopeptides were, with log-rank test
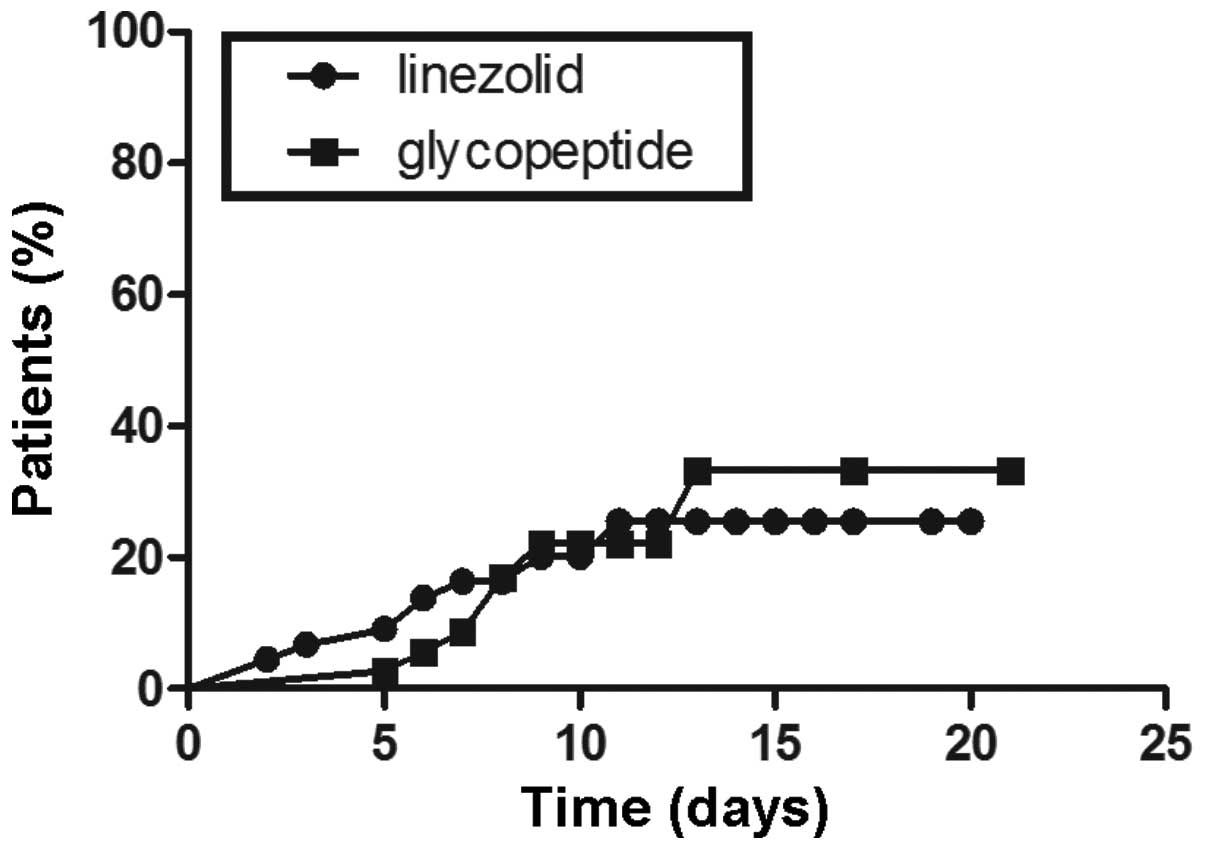
P<0.05 (Fig. 2). However, in
the patients aged <65 years subgroup, no significant differences
were identified in the probability of thrombocytopenia between the
two groups (P>0.05; Fig.
3).
The incidence of thrombocytopenia in the linezolid
and glycopeptide groups is displayed in Table II. No significant differences were
identified in the incidence of thrombocytopenia between patients
receiving linezolid or glycopeptides in first six days of usage of
the drug. On day seven, the incidence of thrombocytopenia began to
differ, with a significant difference between the two groups and a
95% CI of 1.03–4.51 (P=0.04). On days 7–14, the incidence of
thrombocytopenia in patients treated with linezolid remained higher
than that in the patients treated with glycopeptides
(P<0.05).
 | Table IIRisk of adverse platelet outcome
(platelet count <100×109/l) between the linezolid and
glycopeptide groups at different time-points. |
Table II
Risk of adverse platelet outcome
(platelet count <100×109/l) between the linezolid and
glycopeptide groups at different time-points.
| Time (days) | Linezolid
group | Glycopeptide
group | Risk ratio | 95% CI | P-value |
|---|
| Overall | 46 (37.7) | 23 (22.3) | 2.10 | 1.17–3.81 | 0.01 |
| 1 | 1 (0.8) | 1 (1.0) | 0.84 | 0.05–13.65 | 1.00 |
| 2 | 4 (3.3) | 2 (1.9) | 1.71 | 0.31–9.54 | 0.69 |
| 3 | 9 (7.4) | 3 (2.9) | 2.65 | 0.70–10.08 | 0.23 |
| 4 | 10 (8.2) | 6 (5.8) | 1.43 | 0.51–4.12 | 0.49 |
| 5 | 16 (13.1) | 8 (7.8) | 1.79 | 0.73–4.37 | 0.19 |
| 6 | 21 (17.2) | 9 (8.7) | 2.17 | 0.95–4.97 | 0.06 |
| 7 | 27 (22.1) | 12 (11.7) | 2.16 | 1.03–4.51 | 0.04 |
| 8 | 31 (25.4) | 14 (13.6) | 2.12 | 1.05–4.25 | 0.03 |
| 9 | 34 (27.9) | 15 (14.6) | 2.18 | 1.11–4.31 | 0.02 |
| 10 | 40 (32.8) | 19 (18.4) | 2.16 | 1.15–4.03 | 0.02 |
| 11 | 40 (32.8) | 19 (18.4) | 2.16 | 1.15–4.03 | 0.02 |
| 12 | 42 (34.4) | 19 (18.4) | 2.32 | 1.25–4.33 | 0.01 |
The mean number of platelets and the mean reduction
in the number of platelets was also analyzed between the two
groups. After 12 days, the number of patients in the linezolid
group was <30; therefore, the mean number of platelets and
reduction in the number of platelets was not compared after 12 days
of treatment. The mean numbers of platelets in the linezolid and
glycopeptide groups at different time-points are displayed in
Table III. The mean platelet
counts were similar between patients who received linezolid or
glycopeptides during the treatment (P>0.05). Furthermore, the
mean reductions in the numbers of platelets were calculated and
analyzed. The reductions in the numbers of platelets at different
time-points are displayed in Table
IV. No significant differences were identified in the mean
reductions in the number of platelets between the two groups on
days 1–6 (P>0.05). On days 7–12, the mean reductions in the
numbers of platelets were significantly higher in the linezolid
group than in the glycopeptide group (P<0.05).
 | Table IIIMean platelet counts at different
time-points. |
Table III
Mean platelet counts at different
time-points.
| Time (days) | Patients (n) | Linezolid group
(mean ± SD) | Patients (n) | Glycopeptide group
(mean ± SD) | P-value |
|---|
| Baseline | 122 | 233.5±7.4 | 103 | 230.2±8.1 | 0.77 |
| 1 | 122 | 222.4±7.3 | 103 | 223.0±8.1 | 0.90 |
| 2 | 122 | 217.1±7.6 | 103 | 218.0±8.0 | 0.90 |
| 3 | 122 | 213.7±8.1 | 103 | 215.0±8.0 | 0.90 |
| 4 | 122 | 210.0±8.5 | 103 | 210.6±8.3 | 0.96 |
| 5 | 122 | 203.4±8.6 | 103 | 208.2±8.2 | 0.69 |
| 6 | 115 | 191.5±9.0 | 97 | 203.9±8.7 | 0.32 |
| 7 | 102 | 184.0±10.7 | 92 | 197.0±9.1 | 0.32 |
| 8 | 81 | 183.7±20.0 | 73 | 199.3±11.1 | 0.31 |
| 9 | 64 | 178.0±11.9 | 54 | 199.4±13.2 | 0.23 |
| 10 | 58 | 176.1±12.9 | 47 | 208.4±15.8 | 0.11 |
| 11 | 45 | 181.5±15.4 | 37 | 207.9±18.6 | 0.27 |
| 12 | 32 | 183.7±20.0 | 36 | 200.9±19.1 | 0.53 |
 | Table IVMean platelet count reductions at
different time-points. |
Table IV
Mean platelet count reductions at
different time-points.
| Time (days) | Patients (n) | Linezolid group
(mean ±SD) | Patients (n) | Glycopeptide group
(mean ±SD) | P-value |
|---|
| 1 | 122 | 11.1±2.5 | 103 | 6.4±2.0 | 0.16 |
| 2 | 122 | 16.4±3.4 | 103 | 11.7±3.4 | 0.34 |
| 3 | 122 | 19.8±4.4 | 103 | 15.2±4.1 | 0.46 |
| 4 | 122 | 23.4±5.3 | 103 | 19.6±4.8 | 0.59 |
| 5 | 122 | 30.0±5.5 | 103 | 22.0±4.9 | 0.29 |
| 6 | 115 | 42.0±6.5 | 97 | 26.1±5.7 | 0.07 |
| 7 | 102 | 51.9±7.0 | 92 | 31.9±6.1 | 0.03 |
| 8 | 81 | 54.2±8.2 | 73 | 30.3±7.8 | 0.04 |
| 9 | 64 | 65.6±10.6 | 54 | 26.9±9.8 | 0.01 |
| 10 | 58 | 65.3±11.2 | 47 | 23.9±11.7 | 0.01 |
| 11 | 45 | 70.4±13.1 | 37 | 13.0±14.1 | 0.00 |
| 12 | 32 | 77.9±17.7 | 36 | 20.1±12.3 | 0.00 |
Discussion
Thrombocytopenia is a common adverse event
associated with the administration of linezolid. This retrospective
study analyzed the thrombocytopenia induced by the use of linezolid
or glycopeptides in critically ill patients with documented or
suspected gram-positive bacterial infections. With regard to the
renal toxicity of glycopeptides, a previous study identified that
the incidence of renal failure was higher in patients treated with
linezolid than in those treated with glycopeptides (24). Currently, there are no uniform
diagnostic criteria for linezolid-induced thrombocytopenia.
Thrombocytopenia maybe defined as a platelet count
<100×109/l (criterion 1) or a 25% reduction from
baseline count (criterion 2) (21). For patients with a normal baseline
number of platelets, thrombocytopenia is usually defined as a
platelet count of <100×109/l (21). Criterion 2 is used for small
reductions in platelet counts, usually in patients with baseline
numbers of platelets that are less than the lower limit of the
normal range (15). In the present
study, patients with abnormal baseline numbers of platelets were
excluded, so criterion 1 was used as the definition of
thrombocytopenia. The Kaplan-Meier curves suggest that linezolid
was associated with thrombocytopenia more frequently than
glycopeptide therapy was (P<0.05). Age and long periods of drug
administration are known independent risk factors for
thrombocytopenia in adult Chinese patients (25). The cutoff point for risk factors
has been determined by well-known human pathologic classifications,
such as an age of ≥65 years (elderly group), rather than the
analysis of clinical data. In the present study, a subgroup
analysis was conducted regarding the cumulative probability of
thrombocytopenia in the age <65 years and age ≥65 years
subgroups. In the age ≥65 years subgroup, the result was consistent
with the result of the whole group. The cumulative incidence of
thrombocytopenia in patients receiving linezolid was significantly
higher than that of the patients receiving glycopeptides in the
subgroup age ≥65 years (P<0.05). However, in the subgroup age
<65 years, no significant differences in the cumulative
incidence of thrombocytopenia were identified between the patients
receiving linezolid and those receiving glycopeptides (P>0.05).
These findings are consistent with those of previous studies
(26,27). Falagas et al conducted a
meta-analysis which included 12 randomized controlled trials (RCTs)
involving 6,093 patients and identified that thrombocytopenia was
recorded more commonly in patients receiving linezolid [odds
ratio=11.72 (95% CI, 3.66–37.57)] than in patients receiving
glycopeptide or β-lactams (26).
Kalil et al conducted a meta-analysis including nine RCTs
and demonstrated that the risks of thrombocytopenia (95% CI,
1.30–2.87; p=0.001) were higher with the use of linezolid than with
the use of glycopeptides (27).
However, certain studies have observed no significant difference in
the incidence of thrombocytopenia between patients who received
linezolid and those who received glycopeptides (19,28).
An et al conducted a meta-analysis of 12 RCTs and the
results indicated that there were no significant differences in the
incidence of thrombocytopenia between linezolid- and
glycopeptide-treated groups (29).
In an evaluation of thrombocytopenia risk (defined as ≤150,000
platelets/mm3) in 686 patients with nosocomial pneumonia
enrolled in two randomized trials, no statistically significant
differences were identified in the incidence of thrombocytopenia
between linezolid recipients (6.4%) and vancomycin recipients
(7.7%) (22). None of these
studies conducted a subgroup analysis by age; to the best of our
knowledge, the present study is the first trial that has compared
the cumulative incidence of thrombocytopenia between patients
treated with linezolid and glycopeptides in different age groups.
Age has been shown to be an independent risk factor of
linezolid-induced thrombocytopenia (22). The functions of organs degenerate
and metabolism slows in elderly patients; therefore, drugs more
readily accumulate in elderly patients. Hiraki et al
demonstrated a positive correlation between the incidence of
thrombocytopenia and the plasma concentration of linezolid
(30). The slowing of the
metabolism may be one of the mechanisms responsible for the
increased frequency of thrombocytopenia in elderly patients.
Furthermore, in the present study a time-to-event
analysis of the incidence of thrombocytopenia was conducted. The
risk of thrombocytopenia increases over time, so it is important to
determine a mean time at which the cumulative percentage of
patients with low platelet counts begins to diverge between
linezolid and a comparator. Rubinstein et al analyzed seven
comparator-controlled clinical trials and identified that with
longer treatment durations (>14 days) the cumulative percentage
of patients with thrombocytopenia was slightly higher in the
linezolid group than in the comparator group (18). However Orrick et al proposed
that the risk for thrombocytopenia may occur earlier than the
14-day frame and demonstrated that the median duration of therapy
for patients who developed thrombocytopenia was 12 days (31). These two studies did not include
critically ill patients in respiratory ICUs. The present study
identified that the mean duration of therapy when the incidence of
thrombocytopenia and the mean reduction in the number of platelets
were higher for the linezolid group was 7 days in the critically
ill patients in the respiratory ICU. The median duration of therapy
for patients who developed thrombocytopenia in the present study
was shorter than that in previous studies (18,31).
Respiratory tract infections, the severity of the illness and
APACHE-II scores are reported to be independent risk factors of
thrombocytopenia induced by linezolid (23). The patients enrolled in the present
study were critically ill in a respiratory ICU with pneumonia and
had high APACHE-II scores, which may have increased the incidence
of thrombocytopenia and shortened the mean treatment duration prior
to the occurrence of thrombocytopenia.
In contrast with other studies, the design of the
present study and its setting provided several strengths. The
patients included in the study were all from a respiratory ICU and
had respiratory infections. A subgroup analysis was conducted
between the linezolid and glycopeptide-treated patients by the age
of 65 years. All the platelet counts were recorded from the start
to the end of the therapy and the patients with missed platelet
counts were excluded from the study. Based on the initial data, it
was possible to calculate the mean platelet count and the reduction
in the number of platelets for every day until the end of the
therapy.
Several considerations should be noted when
interpreting the results: i) This was a retrospective analysis so
there were no randomizations between the two groups; ii) this study
focused on patients receiving therapy at the ICU of the Chinese PLA
General Hospital and the choice of study population may limit the
ability to generalize these results to other populations; iii) low
pretreatment platelet count, low body weight, low serum albumin
concentration, long-term drug administration, advanced age and
renal insufficiency are all reported to be risk factors for
linezolid-induced thrombocytopenia (32), however, only a subgroup analysis by
age was conducted and the risk of thrombocytopenia according to
other risk factors was not compared.
Overall, the present study concluded that linezolid
treatment was associated with a higher incidence of
thrombocytopenia compared with glycopeptide treatment, mainly in
elderly patients aged ≥65 years or with a treatment duration of ≥7
days. Based on the data, linezolid should be used more carefully in
patients whose age is ≥65 years or treatment duration is ≥7 days
and the platelet count should be monitored during the treatment.
However, a stricter prospective study is required to support these
findings.
References
|
1
|
Liu YN, Cao B, Wang H, et al: Adult
hospital acquired pneumonia: a multicenter study on microbiology
and clinical characteristics of patients from 9 Chinese cities.
Zhonghua Jie He He Hu Xi Za Zhi. 35:739–746. 2012.(In Chinese).
|
|
2
|
Diekema DJ, Pfaller MA, Schmitz FJ, et al;
SENTRY Partcipants Group. Survey of infections due to
Staphylococcus species: frequency of occurrence and
antimicrobial susceptibility of isolates collected in the United
States, Canada, Latin America, Europe, and the Western Pacific
region for the SENTRY Antimicrobial Surveillance Program,
1997–1999. Clin Infect Dis. 32(Suppl 2): S114–S132. 2001.PubMed/NCBI
|
|
3
|
Kim HB, Park WB, Lee KD, et al: Nationwide
surveillance for Staphylococcus aureus with reduced
susceptibility to vancomycin in Korea. J Clin Microbiol.
41:2279–2281. 2003.
|
|
4
|
Riska EB, Böstman O, von Bonsdorff H, et
al: Outcome of closed injuries exceeding 20-unit blood transfusion
need. Injury. 19:273–276. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stéphan F, Hollande J, Richard O, Cheffi
A, Maier-Redelsperger M and Flahault A: Thrombocytopenia in a
surgical ICU. Chest. 115:1363–1370. 1999.
|
|
6
|
Baughman RP, Lower EE, Flessa HC and
Tollerud DJ: Thrombocytopenia in the intensive care unit. Chest.
104:1243–1247. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shalansky SJ, Verma AK, Levine M, Spinelli
JJ and Dodek PM: Risk markers for thrombocytopenia in critically
ill patients: a prospective analysis. Pharmacotherapy. 22:803–813.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strauss R, Wehler M, Mehler K, Kreutzer D,
Koebnick C and Hahn EG: Thrombocytopenia in patients in the medical
intensive care unit: bleeding prevalence, transfusion requirements,
and outcome. Crit Care Med. 30:1765–1771. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vanderschueren S, De Weerdt A, Malbrain M,
et al: Thrombocytopenia and prognosis in intensive care. Crit Care
Med. 28:1871–1876. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cawley MJ, Wittbrodt ET, Boyce EG and
Skaar DJ: Potential risk factors associated with thrombocytopenia
in a surgical intensive care unit. Pharmacotherapy. 19:108–113.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akca S, Haji-Michael P, de Mendonça A,
Suter P, Levi M and Vincent JL: Time course of platelet counts in
critically ill patients. Crit Care Med. 30:753–756. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang HL, Aguilera C, Knopf KB, Chen TM,
Maslove DM and Kuschner WG: Thrombocytopenia in the Intensive Care
Unit. J Intensive Care Med. 28:268–280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patnode NM and Gandhi PJ: Drug-induced
thrombocytopenia in the coronary care unit. J Thromb Thrombolysis.
10:155–167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soriano A, Ortega M, García S, et al:
Comparative study of the effects of pyridoxine, rifampin, and renal
function on hematological adverse events induced by linezolid.
Antimicrob Agents Chemother. 51:2559–2563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerson SL, Kaplan SL, Bruss JB, et al:
Hematologic effects of linezolid: summary of clinical experience.
Antimicrob Agents Chemother. 46:2723–2726. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kohno S, Yamaguchi K, Aikawa N, et al:
Linezolid versus vancomycin for the treatment of infections caused
by methicillin-resistant Staphylococcus aureus in Japan. J
Antimicrob Chemother. 60:1361–1369. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stevens DL, Herr D, Lampiris H, Hunt JL,
Batts DH and Hafkin B: Linezolid versus vancomycin for the
treatment of methicillin-resistant Staphylococcus aureus
infections. Clin Infect Dis. 34:1481–1490. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rubinstein E, Isturiz R, Standiford HC, et
al: Worldwide assessment of linezolid’s clinical safety and
tolerability: comparator-controlled phase III studies. Antimicrob
Agents Chemother. 47:1824–1831. 2003.
|
|
19
|
Nasraway SA, Shorr AF, Kuter DJ, O’Grady
N, Le VH and Cammarata SK: Linezolid does not increase the risk of
thrombocytopenia in patients with nosocomial pneumonia: comparative
analysis of linezolid and vancomycin use. Clin Infect Dis.
37:1609–1616. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu HB, Jiang RH, Li L and Xiao HP:
Linezolid in the treatment of MDR-TB: a retrospective clinical
study. Int J Tuberc Lung Dis. 16:358–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Attassi K, Hershberger E, Alam R and
Zervos MJ: Thrombocytopenia associated with linezolid therapy. Clin
Infect Dis. 34:695–698. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niwa T, Suzuki A, Sakakibara S, et al:
Retrospective cohort chart review study of factors associated with
the development of thrombocytopenia in adult Japanese patients who
received intravenous linezolid therapy. Clin Ther. 31:2126–2133.
2009. View Article : Google Scholar
|
|
23
|
Takahashi Y, Takesue Y, Nakajima K, et al:
Risk factors associated with the development of thrombocytopenia in
patients who received linezolid therapy. J Infect Chemother.
17:382–387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Le Moyec L, Racine S, Le Toumelin P, et
al: Aminoglycoside and glycopeptide renal toxicity in intensive
care patients studied by proton magnetic resonance spectroscopy of
urine. Crit Care Med. 30:1242–1245. 2002.PubMed/NCBI
|
|
25
|
Xie L, He S, Fu L, et al: The prevalence
and risk factors of thrombocytopenia after living-related renal
transplantation in Chinese adult recipients. Transplant Proc.
45:197–199. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Falagas ME, Siempos II and Vardakas KZ:
Linezolid versus glycopeptide or beta-lactam for treatment of
Gram-positive bacterial infections: meta-analysis of randomised
controlled trials. Lancet Infect Dis. 8:53–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalil AC, Murthy MH, Hermsen ED, Neto FK,
Sun J and Rupp ME: Linezolid versus vancomycin or teicoplanin for
nosocomial pneumonia: a systematic review and meta-analysis. Crit
Care Med. 38:1802–1808. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patel N, VanDeWall H, Tristani L, et al: A
comparative evaluation of adverse platelet outcomes among Veterans’
Affairs patients receiving linezolid or vancomycin. J Antimicrob
Chemother. 67:727–735. 2012.PubMed/NCBI
|
|
29
|
An MM, Shen H, Zhang JD, Xu GT and Jiang
YY: Linezolid versus vancomycin for meticillin-resistant
Staphylococcus aureus infection: a meta-analysis of
randomised controlled trials. Int J Antimicrob Agents. 41:426–433.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiraki Y, Tsuji Y, Hiraike M, et al:
Correlation between serum linezolid concentration and the
development of thrombocytopenia. Scand J Infect Dis. 44:60–64.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orrick JJ, Johns T, Janelle J and Ramphal
R: Thrombocytopenia secondary to linezolid administration: what is
the risk? Clin Infect Dis. 35:348–349. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen C, Guo D, Cao X, et al: Risk factors
for thrombocytopenia in adult chinese patients receiving linezolid
therapy. Curr Ther Res Clin Exp. 73:195–206. 2012. View Article : Google Scholar : PubMed/NCBI
|