Introduction
Patients with severe acute pancreatitis (SAP)
commonly present with acute lung injury (ALI) in the early stages;
the disease further develops into acute respiratory distress
syndrome (ARDS), the earliest concurrent disease with the highest
incidence rate amongst patients afflicted with SAP-induced multiple
organ system dysfunction (MODS) (1,2). A
current focus of SAP treatment is the quest for novel measures for
minimizing and preventing lung injury. Clinical studies and animal
experiments have shown that the apoptosis of alveolar type II
epithelial cells (AECs II) is important in ALI, but that the
pathway involved in AEC II apoptosis in severe pancreatitis-induced
ALI remains unclear (3–6).
The efficacy of the Chinese medicine, Qingyi
decoction (QYT), has been demonstrated by a number of years of
clinical practice and animal experiments; QYT is also an effective
prescription for the treatment of acute pancreatitis (7). QYT exhibits purgative functions,
promotes blood circulation, eliminates blood stasis and reduces
inflammation. It directly neutralize endotoxins and also protects
the intestinal barrier, reduces intestinal endotoxin generation and
absorption, inhibits excessive neutrophil activation, downregulates
NF-κB expression and minimizes the release of inflammatory
cytokines, including tumor necrosis factor-α (TNF-α) and nitric
oxide (NO). Therefore, the bioactivities of QYT include the
protection of the lung permeability barrier, prevention of
oxidative damage and improvement of the microcirculation (8). In our previous study on the role of
QYT in patients following acute pancreatitis, it was shown that QYT
administration reduced lung injury by decreasing the expression of
secretory type II phospholipase A2 at the
transcriptional level, thereby protecting pulmonary function
(8). Dexamethasone is a
glucocorticoid that exhibits significant functions, including
anti-inflammatory activity, microcirculation promotion, oxygen free
radical scavenging and NF-κB inhibition; it has recently been found
to inhibit lung inflammatory responses and excessive lung tissue
apoptosis (9). Moreover, QYT
combined with dexamethasone has been shown to exert synergistic
therapeutic effects on early systemic inflammatory response
syndrome associated with SAP (10). Verapamil, a Ca2+ channel
blocker, reduces the intracellular Ca2+ content, and
inhibits the production and release of inflammatory cytokines
(11). It also exerts protective
effects on rat lung injury caused by SAP. The combination of
Chinese and Western medicine is currently regarded as an effective
approach to curing pancreatitis.
The present study aimed to examine the role of AEC
II apoptosis in severe pancreatitis-induced ALI and the intervening
role of QYT. QYT, dexamethasone and verapamil were administered as
intervening therapies on a rat model of SAP-induced ALI. The
results were analyzed in order to clarify the mechanisms affecting
AECs in the course of ALI, and may serve as a basis for novel
formulations combining traditional Chinese medicine and Western
medicine for the therapeutic treatment of SAP-induced ALI.
Materials and methods
Animals and grouping
Sixty clean-grade healthy male Sprague-Dawley rats
(weight, 180–220 g; age, 8 weeks) were provided by the Experimental
Animal Center at Dalian Medical University (Dalian, China). The
animals were randomly divided into 5 groups (n=12 per group): The
model group (SAP), control group, QYT treatment group,
dexamethasone (Zhengzhou Ling Rui Pharmaceutical Co., Ltd.
Zhengzhou, China) treatment (DEX) group and verapamil (Shanghai
Harvest Pharmaceutical Co., Ltd., Shanghai, China) treatment group
(VER). This study was carried out in strict accordance with the
recommendations in the EU animal management practices (1986). The
animal use protocol was reviewed and approved by the Institutional
Animal Care and Use Committee of Dalian Medical University (Dalian,
China).
Model preparation
The SAP group: 10% chloral hydrate aldehyde (4 ml/kg
body weight) was intraperitoneally injected as an anesthetic. An
~2-cm long incision was made directly under the xiphoid midline.
Once the duodenum was located along the pylorus inside the abdomen,
the duodenal papilla opening was exposed, the cholangitic porta
hepatis was clipped with a small artery clamp, a 1-ml syringe
needle was placed (from the intestinal wall contralaterally to the
duodenal nipple) into the biliopancreatic duct through the duodenal
papillary opening on the cholo-pancreatic duct and 1.5% sodium
deoxycholate (Baier Di Biotechnology Co., Ltd., Beijing, China; 1
ml/kg body weight) was slowly infused retrogradely in 30 sec.
Following injection, an appropriate pressure was maintained by hand
across the gauze on the biliopancreatic duct through the duodenal
papillary for ~3 min, and changes to the pancreatic tissue (e.g.
congestion, edema and hemorrhage) were observed. The syringe and
arterial clamp were removed and the incision was sutured.
Postoperative feeding was conducted in the Research Center of
Dalian Medical University, the Central Laboratory of Dalian Central
Hospital and the Central Laboratory of First Affiliated Hospital,
Dalian Medical University.
The control group: An incision was made in the
abdomen of the animals and the pancreatic tissue was marginally
rotated several times prior to closing the abdomen.
The treatment groups: The model preparation method
was the same as that for the SAP group, but the QYT group was
orally treated with QYT (First Affiliated Hospital of Dalian
Medical University, Chinese Medicine Preparations Division; 10
ml/kg body weight/dose) 0.5 h prior to model preparation. The DEX
group was injected with dexamethasone via the tail vein (2 ml/kg
body weight/dose, slow bolus at a speed of 0.3 ml/min), and the VER
group was injected with verapamil via the tail vein (0.5 ml/kg body
weight/dose, diluted with saline to the volume used in the DEX
group, slow bolus at a speed of 0.3 ml/min). The administration was
repeated 6 and 12 h postoperatively. The remaining groups received
the same volume of saline by intragastric administration. The rats
were sacrificed by anesthetic overdose.
Separation of AECs II
The lung tissues were quickly removed and sliced
into 1.0-mm3 pieces following lavage. Calf serum was
added to the sliced tissues to inactivate trypsin and then
phosphate-buffered saline (PBS) solution was added to a total
volume of 20 ml. The samples were then placed in a 37°C water bath
shaker for 5 min at 24.975 × g. Subsequently, the solution was
filtered and the cell filtrate was centrifuged at 352 × g for 10
min. The supernatant was removed and the precipitate was suspended
in Dulbecco’s modified Eagle’s medium (DMEM). The cell suspension
(10 ml) was placed in a large petri dish pre-coated with rat
immunoglobulin G (Beijing Boda Tektronix Biotechnology Co., Ltd.,
Beijing, China) and incubated for 1 h. The non-adherent cells were
gently removed from the centrifuge tube and the suspension was
centrifuged at 352 × g for 10 min. The precipitate was resuspended
with DMEM containing 10% fetal bovine serum and then the cells were
counted.
Pathological observations
Pathological observations were made under an optical
microscope (Leica DMIRB; Leica, Solms, Germany). The lower lobes of
the left lung and pancreatic tissue were cut, fixed with 4%
paraformaldehyde and dehydrated with gradient alcohol. The lobes
were then fixed with xylene and embedded with liquid paraffin to
obtain pathological sections for hematoxylin and eosin (H&E)
and immunohistochemical staining.
Detection of apoptosis
Following the instructions provided with the Annexin
V-FITC apoptosis detection kit (cat. no. 556547; BD Pharmingen,
Franklin Lakes, NJ, USA), AECs II were collected and the
concentration was adjusted to 1×106/ml. The cells were
incubated with Annexin V-FITC and propidium iodide dye in the dark
at room temperature. The Annexin-V binding buffer was then diluted
and analyzed with an Epics XL flow cytometer (Beckman Coulter,
Miami, FL, USA).
Detection of Ca2+
concentration
To the DMEM-resuspended AEC II suspension was added
Ca2+ microprobe Fluo-3/AM (500 μl, 10 μmol/l; cat. no.
sc-202612; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
cells were maintained at 37°C in the dark for 30–45 min. The cell
suspension was then placed in a dedicated petri dish, from which
eight views of each specimen were randomly selected. A laser
scanning confocal microscope (Leica TCS SP5) was used for
observation of the cells and image capture. The total number of
cells and the total fluorescence intensity (FI) per visual field
were calculated with Image-Pro Plus software, version 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA). The mean FI of individual
cells in each visual field was computed and expressed as the
intracellular free Ca2+ concentration.
Detection of TNF-α
A radioimmunoassay kit (cat. no. ABIN118027;
antibodies-online Inc., Atlanta, GA, USA) was used to determine the
serum TNF-α levels according to the manufacturer’s instructions.
Results are expressed in ng/ml.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from AECs II with TRIzol
solution (cat. no. 15596–018; Invitrogen Life Technologies,
Carlsbad, CA, USA) for reverse transcription. Following reverse
transcription, 10 μl cDNA was mixed with the PCR reaction system
(primer sequences shown in Table
I). The denaturation, annealing and extension temperatures were
94, 56 and 72°C, respectively, and the reaction times were 30, 30
and 1.5 min, respectively. For β-actin and Bax, 30 cycles were
conducted and for caspase-8, 35 cycles were conducted. The reaction
product (5 μl) was subjected to 2% agarose gel electrophoresis. A
gel scanning system [Protein Simple (Alphalmade HP), Santa Clara,
CA, USA] was used to scan and analyze the absorbance, provide
images and determine the integral optical density (IOD) value of
each band, with the results expressed as the IOD ratio of Bax and
caspase-8 to β-actin, as relative intensities of Bax and caspase-8
mRNA expression.
 | Table IPrimers and product sizes of
polymerase chain reaction. |
Table I
Primers and product sizes of
polymerase chain reaction.
| Product | 5′-Primer | 3′-Primer | Size (bp) |
|---|
| β-actin |
TCATGAAGTGTGTTGACATCCGTAAAG |
CCTAGAAGCATTTGCGGTGCACGATGGACG | 285 |
| Bax |
CTGAGCTGACCTTGGAGC |
GACTCCAGCCACAAAGATG | 413 |
| Caspase-8 |
TGATGAAGAGGCTCTGAGTAA |
TGGCAAAGTGACTGGATATA | 489 |
Immunohistochemistry
SP two-step staining was adopted, and the procedure
was performed according to the manufacturer’s instructions
(Streptavidin-Peroxidase Immunohistochemical staining kit;
Histostain-Plus Kit; Zymed Laboratories, San Francisco, CA, USA).
Paraffinized lung sections were dewaxed and hydrated, followed by
microwave antigen retrieval in citrate buffer, wherein 0.3%
H2O2 was used to remove endogenous peroxidase
and 5% sheep serum was used for lutation. The Bax and caspase-8
polyclonal antibodies (cat. no. sc-493 and sc-56070, respectively;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were added to the
sections, which were then incubated at 4°C overnight. After washing
with PBS, the sample was incubated with biotin-labeled secondary
antibody (cat. no. SAB3700835; Sigma-Aldrich, St. Louis, MO, USA)
for 1 h at room temperature, then stained with
3,3′-diaminobenzidine, re-stained with hematoxylin, dehydrated,
hyalinized and mounted. Dark staining detected by light microscopy
indicated a positive reaction and PBS was used in the negative
control group. Staining intensity was determined with optical
density (OD) values, and the concentration of Bax and caspase-8
proteins were expressed as the mean OD (total IOD/total area).
Statistical analysis
All data were statistically analyzed with SPSS
software, version 11.5 (SPSS, Inc., Chicago, IL, USA). The
experimental values are expressed as the mean ± standard deviation.
The differences between the treatment group and the control group
were compared via Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference. The correlation
between indicators was analyzed by Pearson product-moment
correlation analysis.
Results
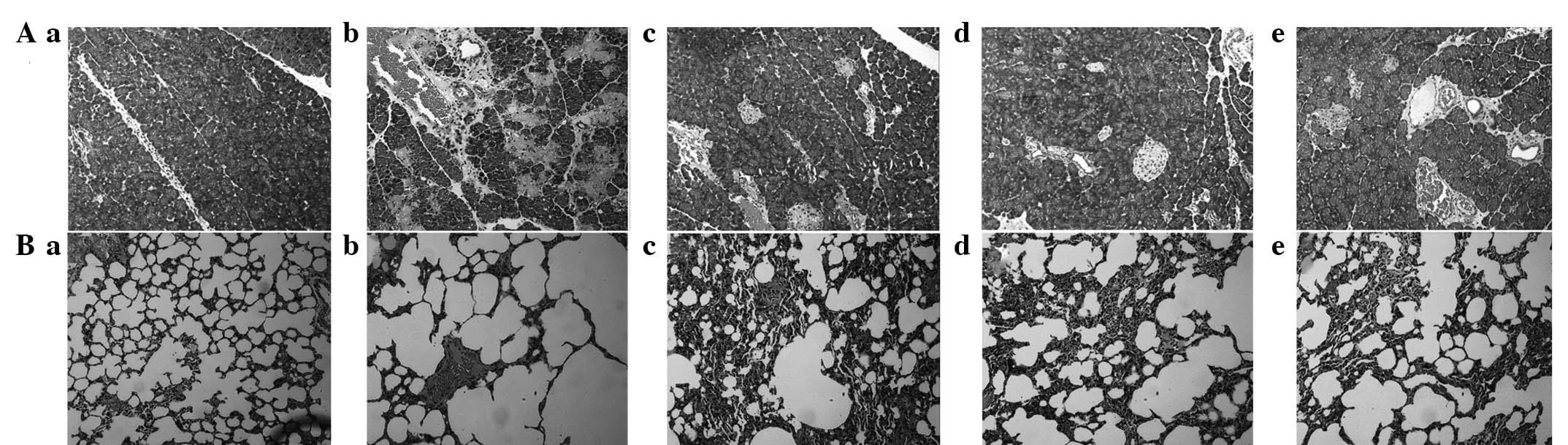
Pathological changes
To detect the effects of QYT, dexamethasone and
verapamil on the experimental animal models, the pathological
changes in the pancreas and lung tissues of different experimental
groups were observed with an optical microscope. The control group
showed normal pancreas (Fig. 1Aa)
and lung tissue morphology (Fig.
1Ba), whereas the SAP group exhibited pancreatic changes
indicated by acinar destruction, interstitial congestion, edema and
inflammatory cell infiltration (Fig.
1Ab). The changes in the lung tissues corresponded with ALI
changes, appearing as marked pulmonary edema and alveolar septum
damage, as well as a small concentration of white blood cells and a
large number of red blood cells in the alveolar cavity with the
retention and aggregation of neutrophils (Fig. 1Bb). The DEX (Fig. 1Ad and Bd) and VER groups (Fig. 1Ae and 1Be) showed significant
improvements compared with the SAP group, but pulmonary edema and
neutrophil infiltration remained. The effectiveness of treatment in
the QYT group (Fig. 1Ac and 1Bc)
was lower than that of the SAP group.
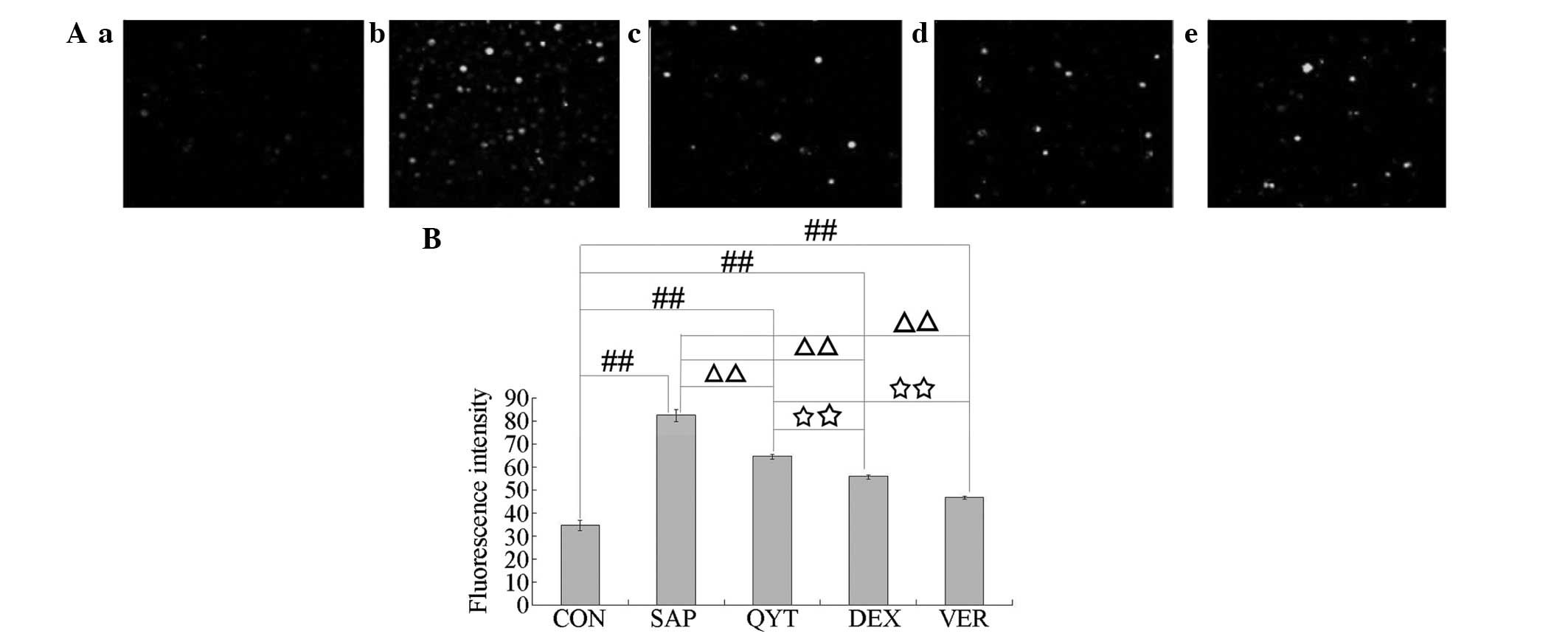
AEC II apoptosis
The AEC II apoptosis rates of the different
experimental groups were analyzed by flow cytometry, and the
results are shown in Fig. 2.
Compared with the AEC II apoptosis rate of the control group
(Fig. 2Aa), those of the SAP, QYT,
DEX and VER groups (Fig. 2Ab–e)
were significantly increased (P<0.01). The AEC II apoptosis
rates of the three treatment groups were significantly decreased
compared with that of the SAP group (P<0.01 Fig. 2B). The AEC II apoptosis rates of
the DEX and VER groups were significantly decreased compared with
that of the QYT group (P<0.01; Fig.
2B), and the AEC II apoptosis rate of the VER group was
significantly decreased compared with that of the DEX group
(P<0.01; Fig. 2B).
Intracellular free Ca2+
concentration
To investigate the role of Ca2+ in AEC II
apoptosis in SAP-induced lung injury, laser scanning confocal
microscopy was conducted to measure the intracellular free
Ca2+ concentration. The results are shown in Fig. 3. Compared with the intracellular FI
of the control group (Fig. 3Aa and
3B), those of the SAP group (Fig.
3Ab and 3B) and the QYT, DEX and VER treatment groups (Fig. 3Ac–e and 3B) were significantly
increased (P<0.01). The intracellular FIs of the QYT, DEX and
VER treatment groups were significantly decreased compared with
that of the SAP group (P<0.01). The intracellular FIs of the DEX
and VER groups were significantly decreased compared with that of
the QYT group (P<0.01), and the intracellular FI of the VER
group was significantly decreased compared with that of the DEX
group (P<0.01).
Serum TNF-α content and correlation
analysis
To elucidate the role of TNF-α and other
inflammatory cytokines in AEC II apoptosis caused by SAP-induced
lung injury, a radioimmunoassay was used to measure the serum TNF-α
levels. Table II shows that
compared with the TNF-α levels of the control group, those of the
SAP and the DEX, QYT and VER treatment groups were significantly
increased (P<0.01). The TNF-α levels of the three treatment
groups were significantly decreased compared that of the SAP group
(P<0.01). The TNF-α levels of the DEX and VER groups were
significantly decreased compared with that of the QYT group
(P<0.01), and the TNF-α level of the VER group was significantly
decreased compared with that of the DEX group (P<0.01).
Correlation analysis of the AEC II apoptosis index, intracellular
free Ca2+ concentration and serum TNF-α content
indicated that the apoptotic index was positively correlated with
the intracellular free Ca2+ concentration and serum
TNF-α content (Table III).
 | Table IISerum TNF-α levels of different
groups. |
Table II
Serum TNF-α levels of different
groups.
| Group (n=12) | TNF-α (ng/ml) |
|---|
| Control | 0.93±0.47 |
| SAP | 5.09±0.12a |
| QYT | 3.95±0.92a,b |
| DEX | 3.41±0.11a-c |
| VER | 2.04±0.72a-d |
 | Table IIICorrelation analysis of AEC II
apoptosis index with intracellular free Ca2+
concentration and serum TNF-α level. |
Table III
Correlation analysis of AEC II
apoptosis index with intracellular free Ca2+
concentration and serum TNF-α level.
| Statistic |
Ca2+ | TNF-α |
|---|
| R-value | 0.984 | 0.987 |
| P-value | <0.01 | <0.01 |
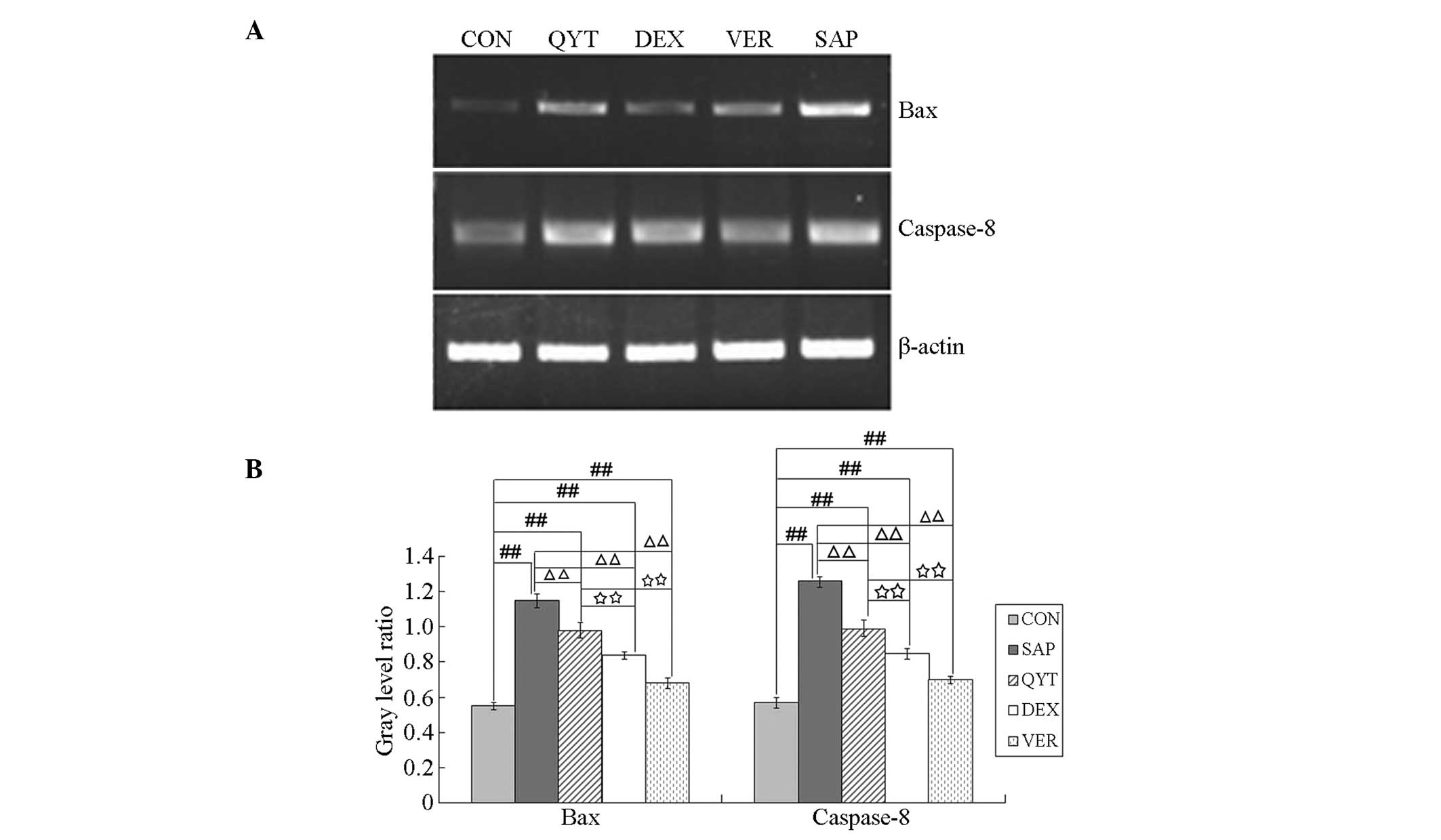
Bax and caspase-8 mRNA expression and
correlation analysis
To clarify the roles of Bax, caspase-8 and other
apoptosis-associated proteins in AEC II apoptosis caused by
SAP-induced lung injury, the expression levels of Bax and caspase-8
mRNA in the AECs II of the different experimental groups were
analyzed. The results are shown in Fig. 4. The gray level ratios for Bax and
caspase-8 mRNA expression in the control group were significantly
lower than those in the SAP, QYT, DEX and VER groups (P<0.01).
The gray level ratios in the SAP group were significantly higher
than those of the QYT, DEX and VER groups (P<0.01). The gray
level ratios in the QYT group were significantly higher than those
in the DEX and VER groups (P<0.01), and the gray level ratios in
the DEX group were significantly higher than those in the VER group
(P<0.01). Correlation analysis of the ACE II apoptosis index and
the expression levels of Bax and caspase-8 mRNA indicated that the
apoptotic index was positively correlated with the expression
levels of Bax and caspase-8 mRNA (Table IV).
 | Table IVCorrelation analysis of AEC II
apoptosis index with the expression levels of Bax and caspase-8
mRNA. |
Table IV
Correlation analysis of AEC II
apoptosis index with the expression levels of Bax and caspase-8
mRNA.
| Statistic | Bax | Caspase-8 |
|---|
| R-value | 0.979 | 0.97 |
| P-value | <0.01 | <0.01 |
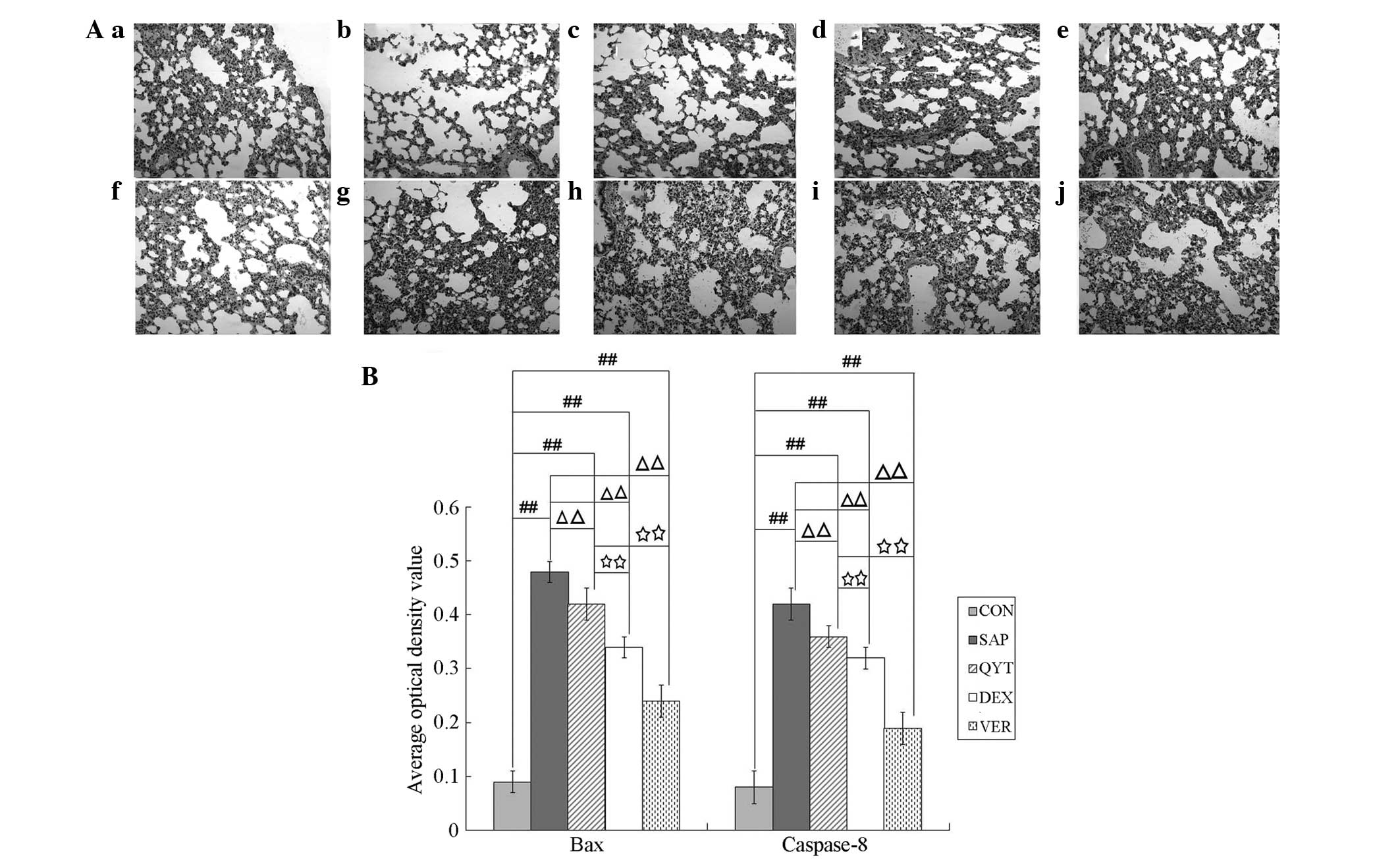
Expression of Bax and caspase-8
Immunohistochemical analysis was performed to detect
the expression of Bax and caspase-8 proteins in the lung cells of
the different experimental groups. The results demonstrated that
the mean OD values of Bax and caspase-8 protein expression in the
control group (Fig. 5a and f) were
significantly lower than those in the SAP (Fig. 5b and g), QYT (Fig. 5c and h), DEX (Fig. 5d and i) and VER (Fig. 5e and j) groups (P<0.01). The
mean OD values of Bax and caspase-8 protein expression in the SAP
group were significantly higher than those in the QYT, DEX and VER
groups (P<0.01). The mean OD values of Bax and caspase-8 protein
expression in the QYT (Fig. 5c and
h) group were significantly higher than those in the DEX
(Fig. 5d and i) and VER (Fig. 5e and j) groups (P<0.01). The
mean OD value of caspase-8 protein expression in the DEX (Fig. 5i) group showed no significant
difference (P>0.05), whereas that in the VER group (Fig. 5j) exhibited a significant
difference (P<0.01). The mean OD value of Bax and casepase-8
protein expression in the VER group (Fig. 5e and j) was significantly lower
than that of the DEX group (Fig. 5d
and i) (P<0.01).
Discussion
SAP-induced ALI is a component of systemic
inflammatory response syndrome and systemic anti-inflammatory
response syndrome in acute pancreatitis. It is a complication in
MODS, which is associated with SAP occurring at the earliest stage
and associated with the highest mortality rate (1,2)
The ALI/ARDS-induced explosive inflammatory response
of the alveolar wall is closely associated with neutrophils
(12), alveolar macrophages
(13), airway epithelial cells
(14) and pulmonary vascular
endothelial cells (15). These
cells produce numerous inflammatory factors that are closely
associated with apoptosis.
Recent studies have shown that AECs, particularly
AECs II, as progenitor cells play a critical role in apoptosis in
the pathological process of ALI (16). In the study of apoptosis-regulating
genes, the bcl-2 family, Fas-FasL and the caspase family are
commonly investigated (17).
Bcl-2/Bax is embedded in the mitochondrial membrane, and regulates
the release of apoptosis-inducing proteins and mitochondrial
functions (18). Unphosphorylated
Bcl-2-associated death promoter (Bad) directly causes the
haplomerization of Bax, thereby forming the mitochondrial permeable
exchange channel, which results in mitochondrial Ca2+
influx and cytochrome c outflow. These phenomena reduce
membrane potential, result in insufficient ATP formation and
caspase activation, activate a series of downstream apoptotic genes
and induce apoptosis. The present study aimed to identify the role
of AEC II apoptosis in severe pancreatitis-induced ALI, as well as
the intervening role of QYT. Bax expression was observed to be
minimal in normal lung tissues, significantly enhanced in ALI lung
tissues and positively correlated with the alveolar cell apoptosis
index, consistent with previous studies (19,20).
Caspase family members are the key effector molecules of apoptotic
signal transduction and molecular performers of apoptosis; their
activation is the key biochemical event for apoptotic effects
(5). As a crucial caspase,
caspase-8 activates caspase-3, as well as stimulating its
hydrolysis and activating polymerase, thereby inducing cell death
(21). The results suggest that,
as the key promoter in the Fas-FasL-mediated apoptotic pathway and
mitochondrial-mediated apoptotic pathway, caspase-8 mRNA and
protein expression levels significantly increase with increasing
AEC apoptosis in ALI.
Controlling the intensity of inflammation in SAP is
essential for the body to maintain a steady state. Recent studies
have shown that glucocorticoids exert protective effects against
lipopolysaccharide-induced rat ALI, which may be associated with
inhibition of the expression of inflammatory cytokines, such as
TNF-α and IL-1β (22).
Dexamethasone has been shown to inhibit Fas antibody and
interferon-γ, and mitigate AEC apoptosis. A previous study
indicated that methylprednisolone is able to inhibit the pulmonary
inflammatory response and excessive apoptosis of lung tissues,
confirming that glucocorticoids regulate lung tissue apoptosis
(23). As a glucocorticoid,
dexamethasone has significant functions, such as anti-inflammatory,
microcirculation-improving, oxygen free radical-scavenging and
NF-κB-inhibiting roles. The present study demonstrated that
dexamethasone significantly decreased the TNF-α and serum amylase
levels compared with those in rats with SAP. Pancreatic and lung
injury significantly decreased and the AEC II apoptosis rate
significantly decreased in the dexamethasone-treated rats.
Moreover, the expression of apoptotic Bax and caspase-8 at the mRNA
and proteins levels significantly decreased. Even though
dexamethasone is a strong immunosuppressant, long-term and
substantial administration causes severe body immunosuppression,
thereby increasing infection and other complications, as well as
mortality due to sepsis. These effects have resulted in
considerable controversy with regard to its application. However,
from the perspective of the effects on SAP lung injury,
dexamethasone is able to inhibit inflammation, stabilize lysosomal
enzymes, promote the secretion of pulmonary surfactants and protect
AECs, exerting a certain therapeutic effect in ARDS patients.
However, the timing of application, dosage and course of treatment
require further exploration.
Calcium channel blockers have been shown to exert
protective effects against SAP-induced lung injury and such effects
may be associated with the following factors: i) Verapamil, a
calcium channel blocker, inhibits the increase in intracellular
Ca2+, thereby preventing the production and release of
inflammatory cytokines (24,25).
ii) Verapamil also inhibits exocrine secretion by the pancreas and
pancreatic enzyme activities; iii) it may reduce thromboxane
content, stabilize the ratio of thromboxane/prostaglandin I
(26), inhibit platelet
aggregation and attenuate the microcirculation disturbance of the
pancreas, thereby reducing pathological lesions in the pancreas.
iv) Verapamil may reduce intracellular calcium overload and reduce
calcium overload-induced apoptosis (27,28).
The results of the present study suggest that compared with the
serum TNF-α levels and serum amylase of the SAP group, those of the
VER group significantly decreased. Lung and pancreas damage, AEC II
apoptosis and the expression of Bax and caspase-8 mRNA also
significantly decreased. The 5 h postoperative situation of the VER
group was not as good as that of the DEX group after modeling 5 h,
a result that may be associated with the side-effects of calcium
channel agents. The appropriate application and dosage require
further study.
Approximately 1,700 years ago, Zhang Zhongjing
stated in ‘Medical Treasures of the Golden Chamber’ that pressing
‘would make the heart full of pain and the sudden death should be
applied Da Chaihu Tang’. This phrase describes conditions that are
highly similar to the main symptoms of acute pancreatitis. The
Chinese prescription, QYT, is effective against acute pancreatitis
as indicated by years of clinical and experimental (animal)
application. It may exhibit purgative functions, promote blood
circulation, eliminate blood stasis and reduces inflammation. It
not only directly neutralizes endotoxins, but also protects the
intestinal barrier, reduces the generation and absorption of
intestinal endotoxins, inhibits the excessive activation of
neutrophils, downregulates NF-κB expression and minimizes the
release of inflammatory cytokines, such as TNF-α and NO. Therefore,
its bioactivities protect the lung permeability barrier, prevent
oxidative damage and improve microcirculation. The results of the
present study showed that using QYT significantly improved the
symptoms of intestinal obstruction in SAP rats. Compared with the
serum TNF-α and amylase levels of the SAP group, those of the
remaining treatment groups significantly decreased. Lung and
pancreatic damage, AEC II apoptosis and the expression levels of
Bax and caspase-8 mRNA also significantly decreased (29).
In conclusion, the results of the present study
demonstrated that AEC II apoptosis participates in SAP-induced ALI,
and that the mitochondrial pathway and death receptor pathway have
regulatory roles in AEC II apoptosis. The application of QYT,
dexamethasone and verapamil significantly reduced the extent of
lung injury. These results suggest that QYT is beneficial for the
treatment of SAP-induced ALI. Further studies are required to
investigate whether a synergistic effect occurs when traditional
Chinese medicine and Western medicines are combined.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81173452 and 81273919).
References
|
1
|
Akbarshahi H, Rosendahl AH,
Westergren-Thorsson G and Andersson R: Acute lung injury in acute
pancreatitis - awaiting the big leap. Respir Med. 106:1199–1210.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Surbatović M, Jovanović K, Radaković S and
Filipović N: Pathophysiological aspects of severe acute
pancreatitis-associated lung injury. Srp Arh Celok Lek. 133:76–81.
2005.(In Serbian).
|
|
3
|
Kreuz S, Siegmund D, Rumpf JJ, et al:
NFkappaB activation by Fas is mediated through FADD, caspase-8, and
RIP and is inhibited by FLIP. J Cell Biol. 166:369–380. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donepudi M, Mac Sweeney A, Briand C and
Grütter MG: Insights into the regulatory mechanism for caspase-8
activation. Mol Cell. 11:543–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gogvadze V, Robertson JD, Zhivotovsky B
and Orrenius S: Cytochrome c release occurs via
Ca2+-dependent and Ca2+-independent
mechanisms that are regulated by Bax. J Biol Chem. 276:19066–19071.
2001.PubMed/NCBI
|
|
6
|
Schild L, Keilhoff G, Augustin W, Reiser G
and Striggow F: Distinct Ca2+ thresholds determine
cytochrome c release or permeability transition pore opening in
brain mitochondria. FASEB J. 15:565–567. 2001.PubMed/NCBI
|
|
7
|
Wang G, Chen HL, Ren F, Li J and Li YQ:
Expression of Cav-1, AQP1 and AQP5 in lung of acute
pancreatitis-associated lung injury rats and the therapeutic role
of Qingyitang. Zhonghua Yi Xue Za Zhi. 90:2564–2569. 2010.(In
Chinese).
|
|
8
|
Zhang XM, Chen HL and Wang ZH: Expression
of secretory type II phospholipase A2in acute lung
injury following acute pancreatitis and interventional effect of
Qingyi decoction on it. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
22:518–521. 2010.(In Chinese).
|
|
9
|
Cornélio Favarin D, Martins Teixeira M,
Lemos de Andrade E, et al: Anti-inflammatory effects of ellagic
acid on acute lung injury induced by acid in mice. Mediators
Inflamm. 2013:1642022013.PubMed/NCBI
|
|
10
|
Bi XD, Zhao J and Fu XG: Experimental
study of the effects QingYi decoction combination with
Dexamethasone on early systemic inflammatory response syndrome
(SIRS) of severe acute pancreatitis (SAP). China Journal of Modern
Medicine. 20:2760–2766. 2010.(In Chinese).
|
|
11
|
Mirghazanfari SM, Keshavarz M, Nabavizadeh
F, Soltani N and Kamalinejad M: The effect of ‘Teucrium
polium L.’ extracts on insulin release from in situ isolated
perfused rat pancreas in a newly modified isolation method:the role
of Ca2+ and K+ channels. Iran Biomed J.
14:178–185. 2010.
|
|
12
|
Rzepka JP, Haick AK and Miura TA:
Virus-infected alveolar epithelial cells direct neutrophil
chemotaxis and inhibit their apoptosis. Am J Respir Cell Mol Biol.
46:833–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Domínguez-Fandos D, Peinado VI, Puig-Pey
R, et al: Pulmonary inflammatory reaction and structural changes
induced by cigarette smoke exposure in the Guinea pig. COPD.
9:473–484. 2012.PubMed/NCBI
|
|
14
|
Stewart JP, Kipar A, Cox H, Payne C,
Vasiliou S and Quinn JP: Induction of tachykinin production in
airway epithelia in response to viral infection. PLoS One.
3:e16732008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lahm T, Crisostomo PR, Markel TA, Wang M,
Lillemoe KD and Meldrum DR: The critical role of vascular
endothelial growth factor in pulmonary vascular remodeling after
lung injury. Shock. 28:4–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Taneja R, Wang W, et al: Human
alveolar epithelial cells attenuate pulmonary microvascular
endothelial cell permeability under septic conditions. PLoS One.
8:e553112013. View Article : Google Scholar
|
|
17
|
Han F, Luo Y, Li Y, et al: Seawater
induces apoptosis in alveolar epithelial cells via the
Fas/FasL-mediated pathway. Respir Physiol Neurobiol. 182:71–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsukahara S, Yamamoto S, Tin-Tin-Win-Shwe,
et al: Inhalation of low-level formaldehyde increases the Bcl-2/Bax
expression ratio in the hippocampus of immunologically sensitized
mice. Neuroimmunomodulation. 13:63–68. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leung PO, Lee HH, Kung YC, Tsai MF and
Chou TC: Therapeutic effect of C-phycocyanin extracted from blue
green algae in a rat model of acute lung injury induced by
lipopolysaccharide. Evid Based Complement Alternat Med.
2013:9165902013.PubMed/NCBI
|
|
20
|
Li L, Wu W, Huang W, Hu G, Yuan W and Li
W: NF-κB RNAi decreases the Bax/Bcl-2 ratio and inhibits
TNF-α-induced apoptosis in human alveolar epithelial cells. Inflamm
Res. 62:387–397. 2013.
|
|
21
|
Aguirre A, Shoji KF, Sáez JC, Henríquez M
and Quest AF: FasL-triggered death of Jurkat cells requires caspase
8-induced, ATP-dependent. J Cell Physiol. 228:458–493. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HA, Park JH, Lee S, Choi JS, Rhim T
and Lee M: Combined delivery of dexamethasone and plasmid DNA in an
animal model of LPS-induced acute lung injury. J Control Release.
156:60–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nutt LK, Chandra J, Pataer A, et al:
Bax-mediated Ca2+ mobilization promotes cytochrome
c release during apoptosis. J Biol Chem. 277:20301–20308.
2002.PubMed/NCBI
|
|
24
|
Shan HL, Wang Y, Wu JW, et al: Verapamil
reverses cardiac iron overload in streptozocin-induced diabetic
rats. Naunyn Schmiedebergs Arch Pharmacol. 386:645–650. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu G, Chen J, Jing G and Shalev A:
Preventing β-cell loss and diabetes with calcium channel blockers.
Diabetes. 61:848–856. 2012.
|
|
26
|
Suzuki K, Saito SY and Ishikawa T:
Involvement of phosphatidylcholine-specific phospholipase C in
thromboxane A2 receptor-mediated extracellular
Ca2+ influx in rat aorta. Eur J Pharmacol. 677:123–130.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Long S, Wilson M, Bengtén E, Clem LW,
Miller NW and Chinchar VG: Identification and characterization of a
FasL-like protein and cDNAs encoding the channel catfish
death-inducing signaling complex. Immunogenetics. 56:518–530. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nomura J, Matsumoto K, Iguchi-Ariga SM and
Ariga H: Mitochondria-independent induction of Fas-mediated
apoptosis by MSSP. Oncol Rep. 14:1305–1309. 2005.PubMed/NCBI
|
|
29
|
Jiang S: Rhubarb Gardenia Treated 28 cases
of acute edematous pancreatitis. Chinese Journal of Integrated
Traditional and Western Medicine. 3:1831999.(In Chinese).
|