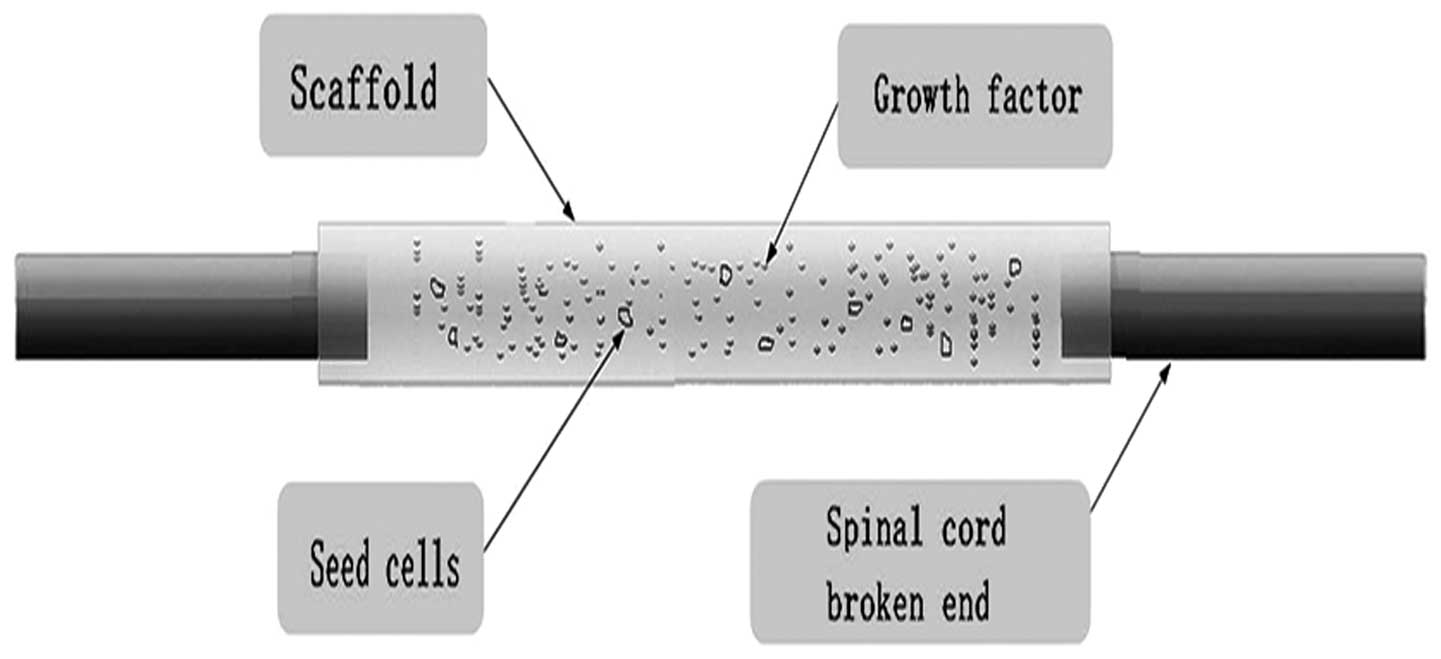
|
1
|
Findley PA, Banerjea R and Sambamoorthi U:
Excess mortality associated with mental illness and substance use
disorders among veteran clinic users with spinal cord injury.
Disabil Rehabil. 33:1608–1615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang M, Zhai P, Chen X, Schreyer DJ, Sun X
and Cui F: Bioengineered scaffolds for spinal cord repair. Tissue
Eng Part B Rev. 17:177–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hulsebosch CE: Recent advances in
pathophysiology and treatment of spinal cord injury. Adv Physiol
Educ. 26:238–255. 2002.PubMed/NCBI
|
|
4
|
Baumgaertner W, Spitzbarth I and Beineke
A: New knowledge on the pathology of spinal cord disease in dogs -
views on therapy. Praktische Tierarzt. 93:794–796. 2012.
|
|
5
|
Wasner G: Spinal cord injury pain - from
symptom to pathology. In: Proceedings of the 3rd International
Congress on Neuropathic Pain (NeuPSIG); Medimond, Bologna. pp.
107–111. 2010
|
|
6
|
Shimizu H, Kakita A and Takahashi H:
Spinal cord tau pathology in cervical spondylotic myelopathy. Acta
Neuropathol. 115:185–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Norenberg MD, Smith J and Marcillo A: The
pathology of human spinal cord injury: defining the problems. J
Neurotrauma. 21:429–440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Banerjea R, Findley PA, Smith B, Findley T
and Sambamoorthi U: Co-occurring medical and mental illness and
substance use disorders among veteran clinic users with spinal cord
injury patients with complexities. Spinal Cord. 47:789–795. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
May L, Day R and Warren S: Evaluation of
patient education in spinal cord injury rehabilitation: knowledge,
problem-solving and perceived importance. Disabil Rehabil.
28:405–413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cruz CD and Cruz F: Spinal cord injury and
bladder dysfunction: new ideas about an old problem.
ScientificWorldJournal. 11:214–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu WS, Tian DS, GUO ZB, et al: Inhibition
of EGFR/MAPK signaling reduces microglial inflammatory response and
the associated secondary damage in rats after spinal cord injury. J
Neuroinflammation. 9:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Madigan NN, McMahon S, O’Brien T,
Yaszemski MJ and Windebank AJ: Current tissue engineering and novel
therapeutic approaches to axonal regeneration following spinal cord
injury using polymer scaffolds. Respir Physiol Neurobiol.
169:183–199. 2009. View Article : Google Scholar
|
|
13
|
Hernandez J, Torres-Espín A and Navarro X:
Adult stem cell transplants for spinal cord injury repair: current
state in preclinical research. Curr Stem Cell Res Ther. 6:273–287.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He X, Fu W and Zheng J: Cell sources for
trachea tissue engineering: past, present and future. Regen Med.
7:851–863. 2012.PubMed/NCBI
|
|
15
|
Malik A and Khan W: Stem cell therapy and
tissue engineering applications for bone. Curr Stem Cell Res Ther.
8:183–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan WS and Malik A: Stem cell therapy and
tissue engineering applications for cartilage regeneration. Curr
Stem Cell Res Ther. 7:241–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salehi M, Pasbakhsh P, Soleimani M, et al:
Repair of spinal cord injury by co-transplantation of embryonic
stem cell-derived motor neuron and olfactory ensheathing cell. Iran
Biomed J. 13:125–135. 2009.
|
|
18
|
Wilcock AC, Swedlow JR and Storey KG:
Mitotic spindle orientation distinguishes stem cell and terminal
modes of neuron production in the early spinal cord. Development.
134:1943–1954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daadi MM, Grueter BA, Malenka RC, Redmond
DE Jr and Steinberg GK: Dopaminergic neurons from
midbrain-specified human embryonic stem cell-derived neural stem
cells engrafted in a monkey model of Parkinson’s disease. PLoS One.
7:e411202012.PubMed/NCBI
|
|
20
|
Meamar R, Dehghani L and Karamali F:
Toxicity effects of methamphetamine on embryonic stem cell-derived
neuron. J Res Med Sci. 17:470–474. 2012.PubMed/NCBI
|
|
21
|
Tavares I: Human neural stem cell
transplantation in spinal cord injury models: how far from clinical
application? Stem Cell Res Ther. 4:612013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deda H, Inci MC, Kurekci AE, et al:
Treatment of chronic spinal cord injured patients with autologous
bone marrow-derived hematopoietic stem cell transplantation: 1-year
follow-up. Cytotherapy. 10:565–574. 2008.PubMed/NCBI
|
|
23
|
Kim JW, Ha KY, Molon JN and Kim YH: Bone
marrow-derived mesenchymal stem cell transplantation for chronic
spinal cord injury in rats: comparative study between intralesional
and intravenous transplantation. Spine (Phila Pa 1976).
38:E1065–E1074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alessandri G, Pagano S, Bez A, et al:
Isolation and culture of human muscle-derived stem cells able to
differentiate into myogenic and neurogenic cell lineages. Lancet.
364:1872–1883. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lavasani M, Lu A, Thompson SD, Robbins PD,
Huard J and Niedernhofer LJ: Isolation of muscle-derived
stem/progenitor cells based on adhesion characteristics to
collagen-coated surfaces. Methods Mol Biol. 976:53–65. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu X, Wang S, Chen B and An X:
Muscle-derived stem cells: isolation, characterization,
differentiation, and application in cell and gene therapy. Cell
Tissue Res. 340:549–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Danisovic L, Varga I, Polák S, Bajciková
B, Adamkov M and Vojtassák J: Biological and morphological
characterization of in vitro expanded human muscle-derived stem
cells. Tsitologiia. 53:482–487. 2011.PubMed/NCBI
|
|
28
|
Danisovic L, Varga I, Polák S, Ulicna M,
Bohmer D and Vojtassák J: Morphology of in vitro expanded human
muscle-derived stem cells. Biomed Pap Med Fac Univ Palacky Olomouc
Czech Repub. 152:235–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Woo JC, Bae WJ, Kim SJ, et al:
Transplantation of muscle-derived stem cells into the corpus
cavernosum restores erectile function in a rat model of
cavernous nerve injury. Korean J Urol. 52:359–363. 2011.PubMed/NCBI
|
|
30
|
Stulpinas A, Imbrasaité A and Kalvelyté
AV: Daunorubicin induces cell death via activation of apoptotic
signalling pathway and inactivation of survival pathway in
muscle-derived stem cells. Cell Biol Toxicol. 28:103–114. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shibuya M, Miura T, Fukagawa Y, et al:
Tongue muscle-derived stem cells express connexin 43 and improve
cardiac remodeling and survival after myocardial infarction in
mice. Circ J. 74:1219–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwon EB and Lee JY, Piao S, Kim IG, Ra JC
and Lee JY: Comparison of human muscle-derived stem cells and human
adipose-derived stem cells in neurogenic trans-differentiation.
Korean J Urol. 52:852–857. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kwon JS, Kim GH, Kim da Y, et al:
Chitosan-based hydrogels to induce neuronal differentiation of rat
muscle-derived stem cells. Int J Biol Macromol. 51:974–979. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zuk PA: The adipose-derived stem cell:
looking back and looking ahead. Mol Biol Cell. 21:1783–1787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilson B, Liotta LA and Petricoin EF:
Dynamic protein pathway activation mapping of adipose-derived stem
cell differentiation implicates novel regulators of adipocyte
differentiation. Mol Cell Proteomics. 12:2522–2535. 2013.
View Article : Google Scholar
|
|
36
|
Su SJ, Chang KL, Su SH, Yeh YT, Shyu HW
and Chen KM: Caffeine regulates osteogenic differentiation and
mineralization of primary adipose-derived stem cells and a bone
marrow stromal cell line. Int J Food Sci Nutr. 64:429–436. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Merceron C, Portron S, Masson M, et al:
The effect of two- and three-dimensional cell culture on the
chondrogenic potential of human adipose-derived mesenchymal stem
cells after subcutaneous transplantation with an injectable
hydrogel. Cell Transplant. 20:1575–1588. 2011. View Article : Google Scholar
|
|
38
|
Liqing Y, Jia G, Jiqing C, et al: Directed
differentiation of motor neuron cell-like cells from human
adipose-derived stem cells in vitro. Neuroreport. 22:370–373. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Valenzuela CD, Allori AC, Reformat DD, et
al: Characterization of adipose-derived mesenchymal stem cell
combinations for vascularized bone engineering. Tissue Eng Part A.
19:1373–1385. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kleintjes WG: Treatment of basal cell
carcinoma with autogenous growth factors and adipose-derived stem
cells. Plast Reconstr Surg. 126:312e–313e. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sterodimas A, de Faria J, Nicaretta B and
Pitanguy I: Tissue engineering with adipose-derived stem cells
(ADSCs): current and future applications. J Plast Reconstr Aesthet
Surg. 63:1886–1892. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oh JS, Park IS, Kim KN, Yoon do H, Kim SH
and Ha Y: Transplantation of an adipose stem cell cluster in a
spinal cord injury. Neuroreport. 23:277–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Barriga A, Medrano M, De-Juan J and Burgos
J: Intravenous infusion of adult adipose tissue stem cells for
repairing spinal cord ischaemic lesions. An experimental study on
animals. Rev Esp Cir Ortop Traumatol. 57:89–94. 2013.(In
Spanish).
|
|
44
|
Ferrero-Gutierrez A, Menendez-Menendez Y,
Alvarez-Viejo M, Meana A and Otero J: New serum-derived albumin
scaffold seeded with adipose-derived stem cells and olfactory
ensheathing cells used to treat spinal cord injured rats. Histol
Histopathol. 28:89–100. 2013.
|
|
45
|
Chung JY, Kim W, Im W, et al:
Neuroprotective effects of adipose-derived stem cells against
ischemic neuronal damage in the rabbit spinal cord. J Neurol Sci.
317:40–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barry DS, Pakan JM, O’Keeffe GW and
McDermott KW: The spatial and temporal arrangement of the radial
glial scaffold suggests a role in axon tract formation in the
developing spinal cord. J Anat. 222:203–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Roberts A, Li WC and Soffe SR: A
functional scaffold of CNS neurons for the vertebrates: the
developing Xenopus laevis spinal cord. Dev Neurobiol.
72:575–584. 2012. View Article : Google Scholar
|
|
48
|
Yoshii S, Ito S, Shima M, Taniguchi A and
Akagi M: Functional restoration of rabbit spinal cord using
collagen-filament scaffold. J Tissue Eng Regen Med. 3:19–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ellis-Behnke RG and Schneider GE: Peptide
amphiphiles and porous biodegradable scaffolds for tissue
regeneration in the brain and spinal cord. Methods Mol Biol.
726:259–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Stokols S and Tuszynski MH: Freeze-dried
agarose scaffolds with uniaxial channels stimulate and guide linear
axonal growth following spinal cord injury. Biomaterials.
27:443–451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guo SZ, Ren XJ, Wu B and Jiang T:
Preparation of the acellular scaffold of the spinal cord and the
study of biocompatibility. Spinal Cord. 48:576–581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Silva NA, Salgado AJ, Sousa RA, et al:
Development and characterization of a novel hybrid tissue
engineering-based scaffold for spinal cord injury repair. Tissue
Eng Part A. 16:45–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chung TW and Chang YL: Silk
fibroin/chitosan-hyaluronic acid versus silk fibroin scaffolds for
tissue engineering: promoting cell proliferations in vitro. J Mater
Sci Mater Med. 21:1343–1351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hilmi AB, Halim AS, Hassan A, Lim CK,
Noorsal K and Zainol I: In vitro characterization of a chitosan
skin regenerating template as a scaffold for cells cultivation.
Springerplus. 2:792013. View Article : Google Scholar
|
|
55
|
Guan L, Tian P, Ge H, et al:
Chitosan-functionalized silk fibroin 3D scaffold for keratocyte
culture. J Mol Histol. May 1–2013.(Epub ahead of print).
|
|
56
|
She Z, Liu W and Feng Q: Self-assembly
model, hepatocytes attachment and inflammatory response for silk
fibroin/chitosan scaffolds. Biomed Mater. 4:0450142009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
She Z, Jin C, Huang Z, Zhang B, Feng Q and
Xu Y: Silk fibroin/chitosan scaffold: preparation,
characterization, and culture with HepG2 cell. J Mater Sci Mater
Med. 19:3545–3553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cigognini D, Satta A, Colleoni B, et al:
Evaluation of early and late effects into the acute spinal cord
injury of an injectable functionalized self-assembling scaffold.
PLoS One. 6:e197822011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Comolli N, Neuhuber B, Fischer I and
Lowman A: In vitro analysis of PNIPAAm-PEG, a novel, injectable
scaffold for spinal cord repair. Acta Biomater. 5:1046–1055. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kubinová S, Horák D, Hejčl A, et al:
SIKVAV-modified highly superporous PHEMA scaffolds with oriented
pores for spinal cord injury repair. J Tissue Eng Regen Med.
February 11–2013.(Epub ahead of print).
|
|
61
|
Kang KN, Lee JY, Kim da Y, et al:
Regeneration of completely transected spinal cord using scaffold of
poly(D,L-lactide-coglycolide)/small intestinal submucosa seeded
with rat bone marrow stem cells. Tissue Eng Part A. 17:2143–2152.
2011. View Article : Google Scholar
|
|
62
|
Liu T, Houle JD, Xu J, Chan BP and Chew
SY: Nanofibrous collagen nerve conduits for spinal cord repair.
Tissue Eng Part A. 18:1057–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhu Y, Wang A, Shen W, et al: Nanofibrous
patches for spinal cord regeneration. Adv Funct Mater.
20:1433–1440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Teng YD, Lavik EB, Qu X, et al: Functional
recovery following traumatic spinal cord injury mediated by a
unique polymer scaffold seeded with neural stem cells. Proc Natl
Acad Sci USA. 99:3024–3029. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Du BL, Zeng CG, Zhang W, Quan DP, Ling EA
and Zeng YS: A comparative study of gelatin sponge scaffolds and
PLGA scaffolds transplanted to completely transected spinal cord of
rat. J Biomed Mater Res A. June 15–2013.(Epub ahead of print).
|
|
66
|
McCall J, Weidner N and Blesch A:
Neurotrophic factors in combinatorial approaches for spinal cord
regeneration. Cell Tissue Res. 349:27–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
de Leon RD: Could neurotrophins replace
treadmill training as locomotor therapy following spinal cord
injury? Focus on ‘neurotrophic factors promote and enhance
locomotor recovery in untrained spinalized cats’. J Neurophysiol.
98:1845–1846. 2007.PubMed/NCBI
|
|
68
|
Sharma HS: Neurotrophic factors in
combination: a possible new therapeutic strategy to influence
pathophysiology of spinal cord injury and repair mechanisms. Curr
Pharm Des. 13:1841–1874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sharma HS and Sharma A: Rodent spinal cord
injury model and application of neurotrophic factors for
neuroprotection. Methods Mol Biol. 846:393–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Berry A, Bindocci E and Alleva E: NGF,
brain and behavioral plasticity. Neural Plast.
2012:7840402012.PubMed/NCBI
|
|
71
|
Allen SJ, Watson JJ, Shoemark DK, Barua NU
and Patel NK: GDNF, NGF and BDNF as therapeutic options for
neurodegeneration. Pharmacol Ther. 138:155–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Weishaupt N, Blesch A and Fouad K: BDNF:
the career of a multifaceted neurotrophin in spinal cord injury.
Exp Neurol. 238:254–264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Weishaupt N, Li S, Di Pardo A, Sipione S
and Fouad K: Synergistic effects of BDNF and rehabilitative
training on recovery after cervical spinal cord injury. Behav Brain
Res. 239:31–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mantilla CB, Gransee HM, Zhan WZ and Sieck
GC: Motoneuron BDNF/TrkB signaling enhances functional recovery
after cervical spinal cord injury. Exp Neurol. 247:101–109. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Stokols S, Sakamoto J, Breckon C, Holt T,
Weiss J and Tuszynski MH: Templated agarose scaffolds support
linear axonal regeneration. Tissue Eng. 12:2777–2787. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shang AJ, Hong SQ, Xu Q, et al:
NT-3-secreting human umbilical cord mesenchymal stromal cell
transplantation for the treatment of acute spinal cord injury in
rats. Brain Res. 1391:102–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Shumsky JS, Tobias CA, Tumolo M, Long WD,
Giszter SF and Murray M: Delayed transplantation of fibroblasts
genetically modified to secrete BDNF and NT-3 into a spinal cord
injury site is associated with limited recovery of function. Exp
Neurol. 184:114–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen Q, Smith GM and Shine HD: Immune
activation is required for NT-3-induced axonal plasticity in
chronic spinal cord injury. Exp Neurol. 209:497–509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Guo JS, Zeng YS, Li HB, et al:
Cotransplant of neural stem cells and NT-3 gene modified Schwann
cells promote the recovery of transected spinal cord injury. Spinal
Cord. 45:15–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wang X, Li Y, Gao Y, et al: Combined use
of spinal cord-mimicking partition type scaffold architecture and
neurotrophin-3 for surgical repair of completely transected spinal
cord in rats. J Biomater Sci Polym Ed. 24:927–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Hara T, Fukumitsu H, Soumiya H, Furukawa Y
and Furukawa S: Injury-induced accumulation of glial cell
line-derived neurotrophic factor in the rostral part of the injured
rat spinal cord. Int J Mol Sci. 13:13484–13500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Lin S, Wang Y, Zhang C and Xu J:
Modification of the neurotrophin-3 gene promotes cholinergic
neuronal differentiation and survival of neural stem cells derived
from rat embryonic spinal cord in vitro and in vivo. J Int Med Res.
40:1449–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yao L, Yao S, Daly W, Hendry W, Windebank
A and Pandit A: Non-viral gene therapy for spinal cord
regeneration. Drug Discov Today. 17:998–1005. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bo X, Wu D, Yeh J and Zhang Y: Gene
therapy approaches for neuroprotection and axonal regeneration
after spinal cord and spinal root injury. Curr Gene Ther.
11:101–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Blesch A, Fischer I and Tuszynski MH: Gene
therapy, neurotrophic factors and spinal cord regeneration. Handb
Clin Neurol. 109:563–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Foust KD, Flotte TR, Reier PJ and Mandel
RJ: Recombinant adeno-associated virus-mediated global anterograde
delivery of glial cell line-derived neurotrophic factor to the
spinal cord: comparison of rubrospinal and corticospinal tracts in
the rat. Hum Gene Ther. 19:71–82. 2008. View Article : Google Scholar
|
|
87
|
Koda M, Hashimoto M, Murakami M, et al:
Adenovirus vector-mediated in vivo gene transfer of brain-derived
neurotrophic factor (BDNF) promotes rubrospinal axonal regeneration
and functional recovery after complete transection of the adult rat
spinal cord. J Neurotrauma. 21:329–337. 2004. View Article : Google Scholar
|
|
88
|
Blits B, Kitay BM, Farahvar A, Caperton
CV, Dietrich WD and Bunge MB: Lentiviral vector-mediated
transduction of neural progenitor cells before implantation into
injured spinal cord and brain to detect their migration, deliver
neurotrophic factors and repair tissue. Restor Neurol Neurosci.
23:313–324. 2005.
|
|
89
|
Morizono K and Chen IS: Targeted gene
delivery by intravenous injection of retroviral vectors. Cell
Cycle. 4:854–856. 2005. View Article : Google Scholar : PubMed/NCBI
|