Introduction
Endometriosis is a gynecological complication
characterized by extra-uterine localization of endometrial tissue,
mainly in pelvic organs. The disease affects 5–10% of females of
reproductive age (1). Clinical
presentations of endometriosis include persistent worsening of
pelvic adhesions, pain and infertility. Little is known about its
etiopathology. With the exception of the symptomatic treatment of
endometriosis-associated pain, only two main suboptimal therapeutic
approaches (hormonal and invasive surgery) are generally
recommended to patients (2). Thus,
it is important to identify a new specific target for the treatment
of endometriosis.
Shikonin is the main active component of
Lithopermum erythrorhizon, a perennial herb in the
Boraginaceae family. Shikonin is a naphthoquinone compound
(molecular formula, C16H16O5;
molecular weight, 288) that has anti-inflammatory, antitumor and
immunomodulatory bioactivities (3–5). In
a previous study in which the effects of shikonin against human
immunodeficiency virus were investigated, shikonin antagonized
chemokine function by downregulating the expression of chemokine
receptors, including CCR5, indicating that this compound is a
possible pan-chemokine inhibitor (6).
Previously, ectopic and normal endometrial tissues
were screened for 18 chemokine receptors (CCR1-10, CXCR1-6, XCR1
and CX3CR1) and it was found that CCR5 expression levels were
higher in the ectopic focus than in the eutopic endometrium in
endometriosis patients. Regulated upon activation normal T-cell
expressed and secreted (RANTES) is the ligand for CCR5, and it has
been confirmed that the chemokine and its receptors are bioactive
in endometriosis (7–9). These observations indicate that
shikonin may be a promising endometriosis treatment. In the present
study, the potential therapeutic effects and mechanism of shikonin
were assessed for the treatment of endometriosis.
Materials and methods
Reagents
Shikonin (purity, ≥97%) was provided by the National
Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China) and was dissolved in DMSO. Other reagents
included: triptorelin (Beaufour-Ipsen Pharmaceutical Co., Ltd.,
Tianjin, China); TRIzol reagent (Gibco-BRL, Gaithersburg, MD, USA);
Real Time polymerase chain reaction (PCR) Core kit (Takara Bio,
Inc., Dalian, China); carboxyfluorescein succinimidyl ester (CFSE)
and recombinant human RANTES (rhRANTES; R&D Systems, Wiesbaden,
Germany); mouse monocyte isolation buffer (Lengtonbio Biological
Co., Shanghai, China); immunostaining kits and keratin and vimentin
monoclonal antibodies (MaxVision™ HRP-Polymer anti-Mouse IHC kit;
Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, China);
and enzyme-linked immunosorbent assay (ELISA) kits (BioSource
International, Camarillo, CA, USA). All other chemicals were of
analytical reagent grade.
Human endometrial samples
Informed consent was obtained prior to endometrial
biopsy collection and the study was approved by the Nanjing Medical
University Ethics Committee (Nanjing, China). The ten donors were
premenopausal patients with endometriosis (aged 24–42 years), who
underwent laparoscopy and uterine curettage at the Nanjing Maternal
and Child Health Hospital Affiliated with Nanjing Medical
University. Fresh endometrial tissue was collected in Dulbecco’s
modified eagle medium (Gibco-BRL) and cut into 1–2-mm diameter
fragments under sterile conditions.
Animal endometriosis model
In total, 70 severe combined immunodeficiency (SCID)
female mice weighing 20–25 g, at 6–8 weeks-old [Beijing Vital River
Laboratory Animals Co., Beijing, China; certificate of quality no:
SCXK (Jing) 2007–0001], were housed under a 12 h light-dark cycle
with food and water available ad libitum and pathogen-free
conditions. All studies were approved by the experimental animal
committee of Nanjing Medical University. Human endometrial
fragments were implanted into the peritoneal cavity and mice were
intramuscularly injected with estradiol benzoate (30 μg/kg) one day
following implantation, in order to maintain the growth of the
implanted endometrium. After 14 days, 10 mice were euthanized by
cervical dislocation and the implanted endometrial tissue in the
peritoneal cavity was examined.
Groups and treatments
Model animals were randomly divided into the
following groups (n=12 per group): Negative control, oral DMSO
treatment; positive control, subcutaneous treatment with 30 μg/kg
tryptorelin (a gonadotropin-releasing hormone agonist); and low-,
intermediate- and high-shikonin dose groups that were orally
treated with shikonin at doses of 2.5, 5 and 10 mg/kg,
respectively. All animals were treated daily for 28 days. At the
end of the experiment, mice were sacrificed by cervical dislocation
and blood samples were collected. Implanted endometrial lesions
were dissected and frozen directly in liquid nitrogen for PCR or
fixed in 10% formalin and embedded in paraffin for
immunohistochemical analysis. Lesion volume (V) was calculated
using the formula: V (mm3) = 0.52 × length × width ×
height.
Assessment of toxicity and adverse
effects
All animals were closely monitored for abnormal
behavioral changes and symptoms, including marked temperature
change, diarrhea, weight loss, fur discoloration, lethargy,
irritation and convulsion during treatment. All observations were
performed by investigators blinded to the treatment group
assignments.
Blood samples were collected following the sacrifice
of the mice. Serum levels of alanine aminotransferase (ALT),
aspartate aminotransferase (AST), blood urea nitrogen (BUN),
creatinine (Cr), lactate dehydrogenase (LDH), creatine kinase (CK)
and total protein, albumin and globulin were tested by auto
analyzer (HITACHI 7020; Hitachi, Tokyo, Japan).
Cell culture and co-culture
To study the potential role of shikonin on RANTES
expression in a pelvic environment of endometriosis, a cell
co-culture system [U937 cells-human peritoneal mesothelial cells
(HPMCs)-endometrial stromal cell (ESCs)] was constructed. The ESCs,
HMrSV5 (HPMCs) and U937 human monocyte cell line (Cell Bank,
Chinese Academy of Sciences, Shanghai, China) were cultured
according to previously described methods (7).
The HPMCs were cultured in 12-well plates at a
concentration of 1×105 cells/well until they adhered to
the plastic. The media were removed and the ESCs and U937 cells
were spread over the HPMC monolayer at the same concentration.
HPMC, ESC and U937 cells cultured alone in the same media were used
as controls.
Cell treatment
Cells were seeded on 12-well plates
(1×105/well) and the medium was changed to 2.5% fetal
bovine serum (FBS) after 24 h. Cells were treated with various
shikonin concentrations (range, 0.5–50 μM) for 24 h. Untreated
cells were used as controls. Supernatants were harvested at the end
of each experiment.
Hematoxylin and eosin (H&E) and
immunostaining
Fixed and embedded histological material was cut
into 4-μm sections that were deparaffinized in xylene, rehydrated
with graded alcohol and stained with H&E. Keratin and vimentin
immunostaining was performed using an
avidin-biotin-immunoperoxidase technique with the immunostaining
kit, according to the manufacturer’s instructions.
Quantitative PCR (qPCR)
Total RNA was isolated from the implanted
endometrium using TRIzol reagent and, following the digestion of
genomic DNA, was reverse-transcribed with the Real Time PCR Core
kit. qPCR was performed on a Rotor-Gene 3000 real-time DNA analysis
system (Corbett Research, Sydney, Australia) with the following
primers: RANTES sense, 5′-ACCAGTGGCAAGTGCTCCAAC-3′ and anti-sense,
5′-CTCCCAAGCTAGGACAAGAGCAAG-3′; and GAPDH sense,
5′-GCACCGTCAAGGCTGAGAAC-3′ and anti-sense,
5′-TGGTGAAGACGCCAGTGGA-3′. PCR conditions were 95°C for 90 sec,
95°C for 5 sec, 60°C for 30 sec, 95°C for 1 min, 55°C for 1 min and
55°C for 10 sec, for 30 cycles. Relative quantification was
performed using the cycle threshold (CT) 2−ΔΔCT method,
with relative quantification of the target calculated as: ΔΔCT =
(CTTarget-CTGAPDH)X -
(CTTarget-CTGAPDH)0, where X is
the treated group and 0 is the control group. Assays were conducted
in triplicate for each sample.
ELISA
The rhRANTES or recombinant mouse RANTES (rmRANTES;
R&D Systems) levels in the peritoneal fluid or cell
supernatants were determined using the previously mentioned ELISA
kit, according to the manufacturer’s instructions. Peritoneal fluid
was collected by irrigating the abdominal cavity with 1 ml cold
phosphate-buffered saline (PBS) plus 0.02%
ethylenediamine-tetraacetic acid. The samples were each centrifuged
to remove cellular debris and then stored at −170°C.
In vivo chemotaxis assay
Three mice were treated with 5 μM shikonin for 24 h
and 2×107 U937 cells were labeled with CFSE for 5 min
prior to injection. The CFSE-labeled cells in 200 μl PBS were
injected intravenously into the tail vein, while 10 μg rhRANTES in
200 μl PBS was injected intraperitoneally. Following 24 h, the
peritoneal fluid was collected by irrigating the abdominal cavity
with 7 ml cold PBS. Cells were harvested by centrifugation and
CFSE-labeled cells were counted using a fluorescence microscope
(IX71 Olympus; Tokyo, Japan).
In vitro chemotaxis assay
Blood was obtained from the SCID mice by cardiac
puncture and mixed with Hank’s solution in a 1:1 ratio. The mixture
was resuspended in 2 ml mouse monocyte isolation buffer and
centrifuged at 1,500 × g for 15 min to layer the mixture. The layer
containing enriched monocytes was carefully removed and washed with
Hank’s solution, followed by centrifugation at 2,000 × g for 10 min
to collect the monocytes. Monocyte purity was >95% as determined
by anti-CD14 staining in flow cytometric experiments.
Chemotaxis of the mouse monocytes in response to
rmRANTES was assayed using 12-well chemotactic chambers (pore size,
5 μm; Costar, Cambridge, MA, USA). The upper chamber compartment
was loaded with 100 μl mouse monocyte suspension (2×106
cells/l). The lower compartment was filled with 600 μl RPMI-1640
with 2.5% FBS containing peritoneal fluid from the various groups
of SCID mice, with or without rat anti-mouse RANTES (10 ng/ml;
R&D Systems). Following 3 h at 37°C, the filters were stained
with Giesma stain and the number of cells that had migrated to the
lower surface of the filter was counted microscopically and was
determined as the mean of 20 random high-power fields per well.
Each assay was performed at least three times. The chemotactic
index was the ratio of the number of cells that had migrated in
response to chemokine, divided by the total number of cells.
Statistical analysis
All data are expressed as mean ± SEM and were
analyzed with the Statistical Package SPSS, version 12.0 for
Windows (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was used to determine statistically significant
differences among groups and the means of pairs of groups were
compared using a Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
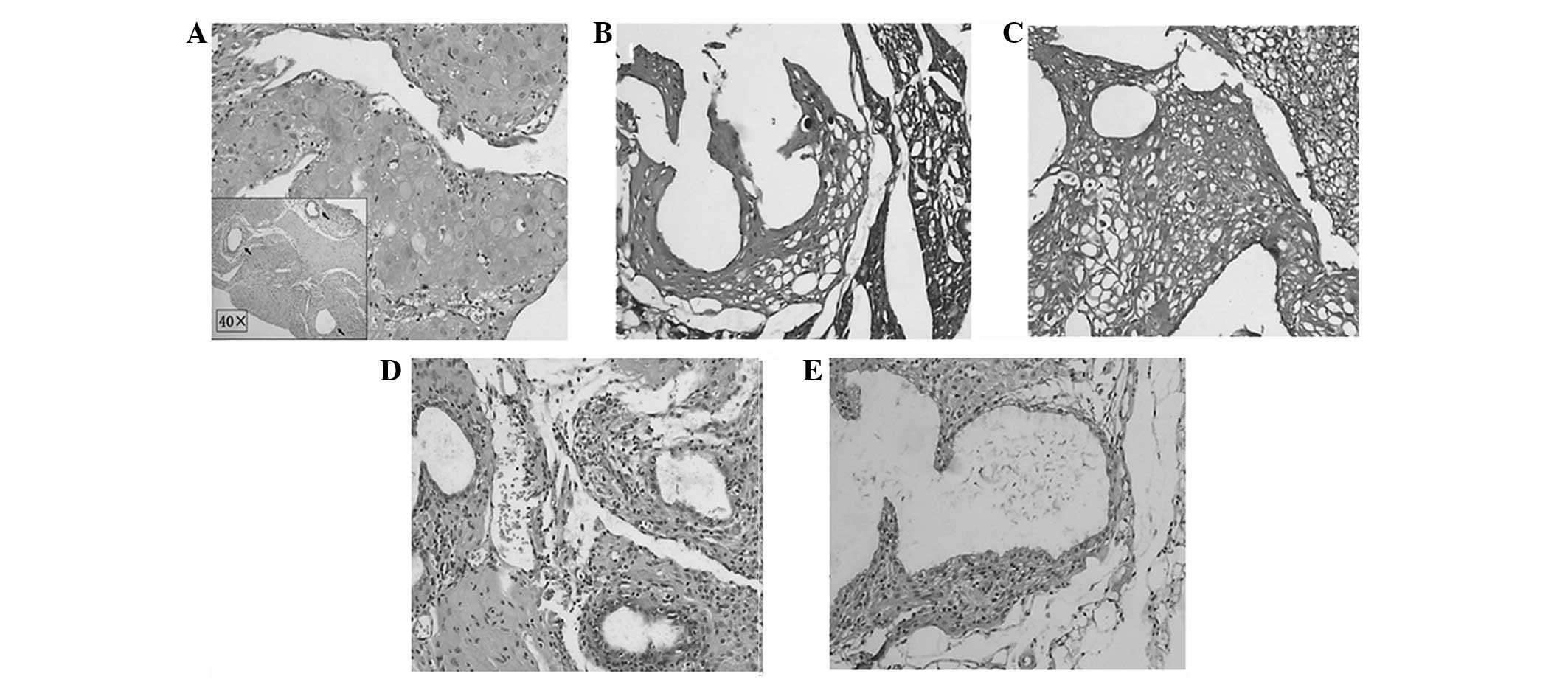
Shikonin causes lesion regression
Endometrial lesions in the model animals were
verified by H&E staining and immunostaining, which showed
endometrial acinar glands on a background of stromal cells.
Glandular epithelial cells were cuboidal with clear cytoplasms and
distinct vacuoles. The coexpression of keratin and vimentin, shown
by immunostaining, indicated that endometriosis lesions had been
established in the mice (Fig. 1).
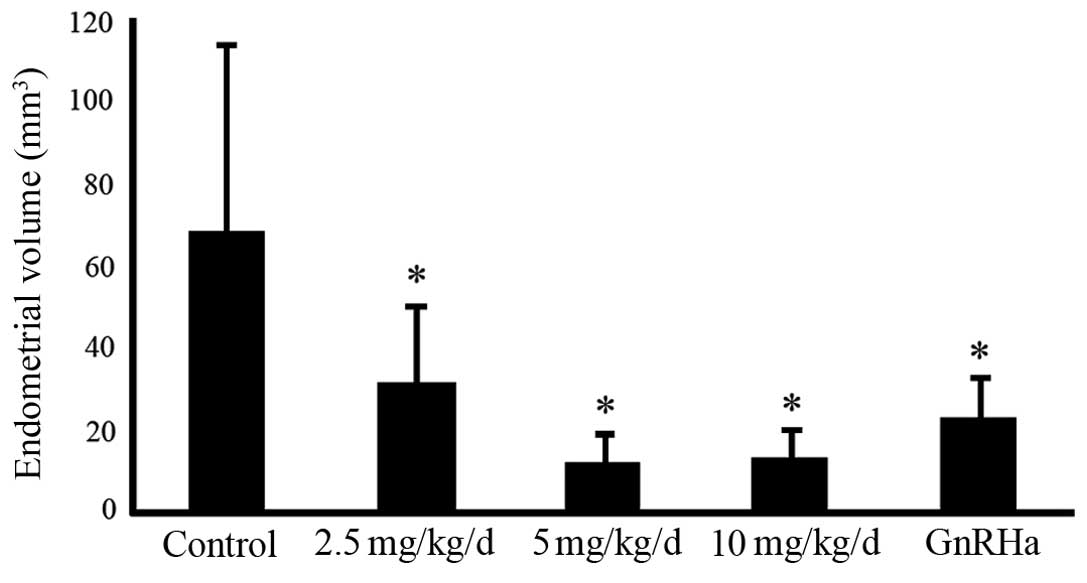
Compared with the lesions in the controls, those in the shikonin-
and triptorelin-treated mice were significantly decreased in size.
Although the high and intermediate doses of shikonin caused greater
regression, no significant differences were observed between these
two treatment groups (Fig. 2).
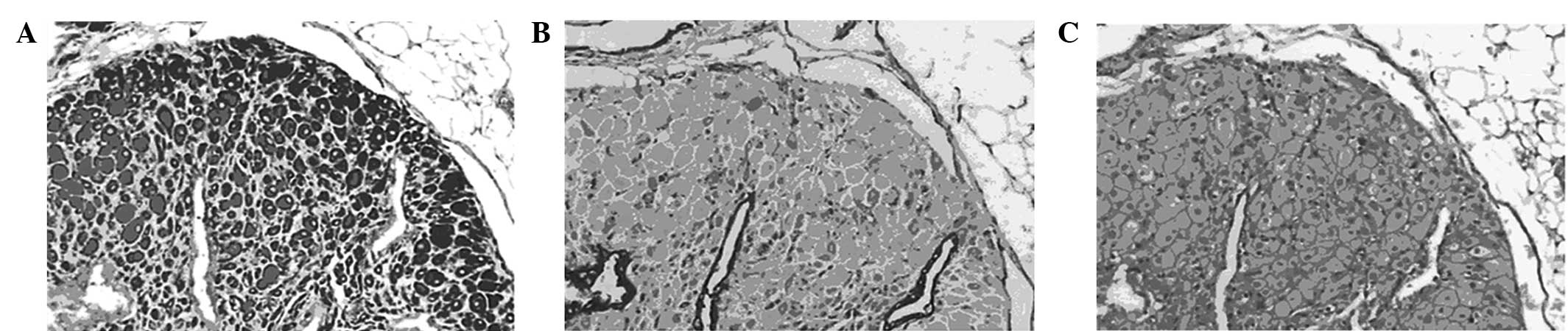
In the groups treated with high and intermediate
doses of shikonin, H&E staining showed marked atrophy of the
endometrial acinar glands, scattered glandular epithelial cells,
vacuolar degeneration of stromal cells, incomplete acinar
structures and angionecrosis of adjacent vessels with glassy
degeneration. Lesions in the triptorelin-treated group showed
similar morphological changes. However in the low-dose shikonin
group, endometrial tissues were a mixture of normal, atrophied and
half-atrophied morphologies (Fig.
3).
Effects of shikonin on RANTES
expression
The level of mRANTES secretion in the peritoneal
fluid of the animal endometriosis model was markedly higher than
that in normal SCID mice. However, this was not the case for
hRANTES secretion. Following shikonin treatment, mRANTES secretion
in the peritoneal fluid of the animal endometriosis model was
decreased markedly in a dose-dependent manner (Fig. 4A). Human endometrium implanted in
SCID mice also expressed low hRANTES at the transcriptional
level.
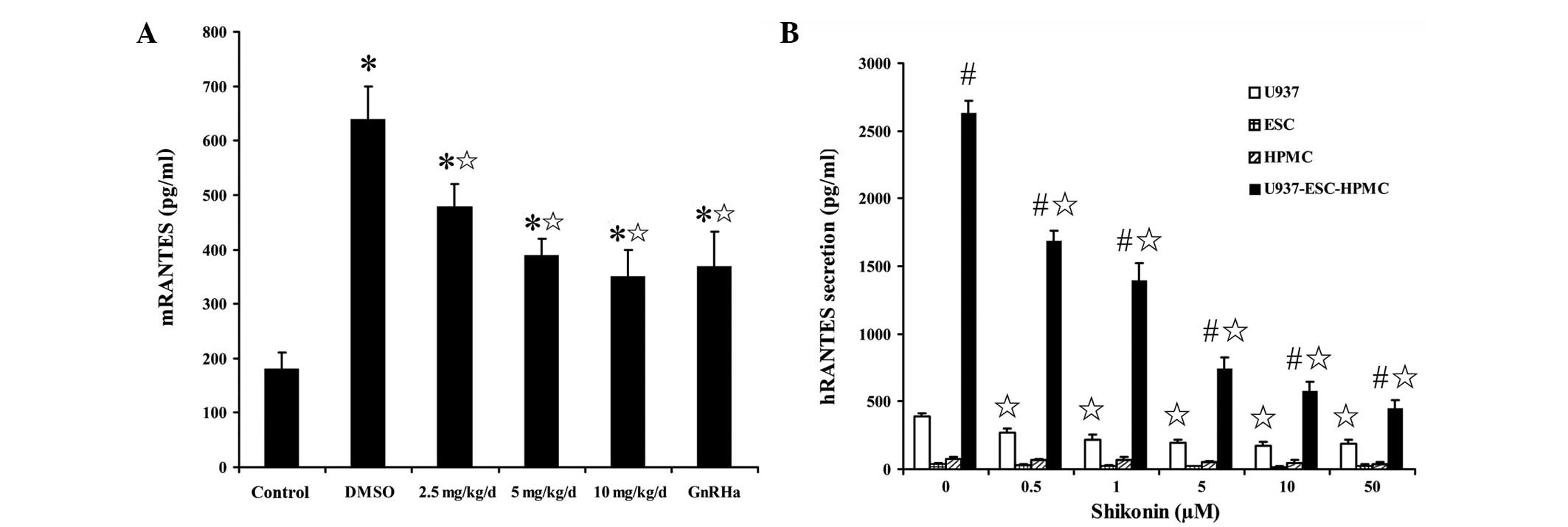 | Figure 4Effect of shikonin on RANTES
expression. (A) mRANTES secretion in the peritoneal fluid of SCID
mice following shikonin treatment at the indicated dose or GnRHa
(triptorelin) at 30 μg/kg. (B) hRANTES secretion in U937, ESCs and
HPMCs cultured alone or co-cultured, following shikonin treatment
at the indicated dose, detected by ELISA. Data are presented as
mean ± SEM. *P<0.05, vs. normal/naïve control;
¶P<0.05, vs. DMSO groups; #P<0.05, vs.
groups cultured alone. RANTES, regulated upon activation normal
T-cell expressed and secreted; SCID, severe combined
immunodeficiency; ESC, endometrial stromal cells; HPMC, human
peritoneal mesothelial cells; ELISA, enzyme-linked immunosorbent
assay; GnRHa, gonadotropin-releasing hormone agonist. |
Under normal conditions, U937 cells secreted more
RANTES than HPMCs and ESCs and the co-culture of HPMCs with ESCs
and U937 cells promoted significant RANTES secretion. However,
following shikonin treatment, RANTES secretion by U937 cells and
HPMC-ESC-U937 co-cultured cells was also significantly decreased
compared with that in the controls (Fig. 4B). These observations indicate that
shikonin may inhibit RANTES expression in the peritoneal cavity of
females with endometriosis.
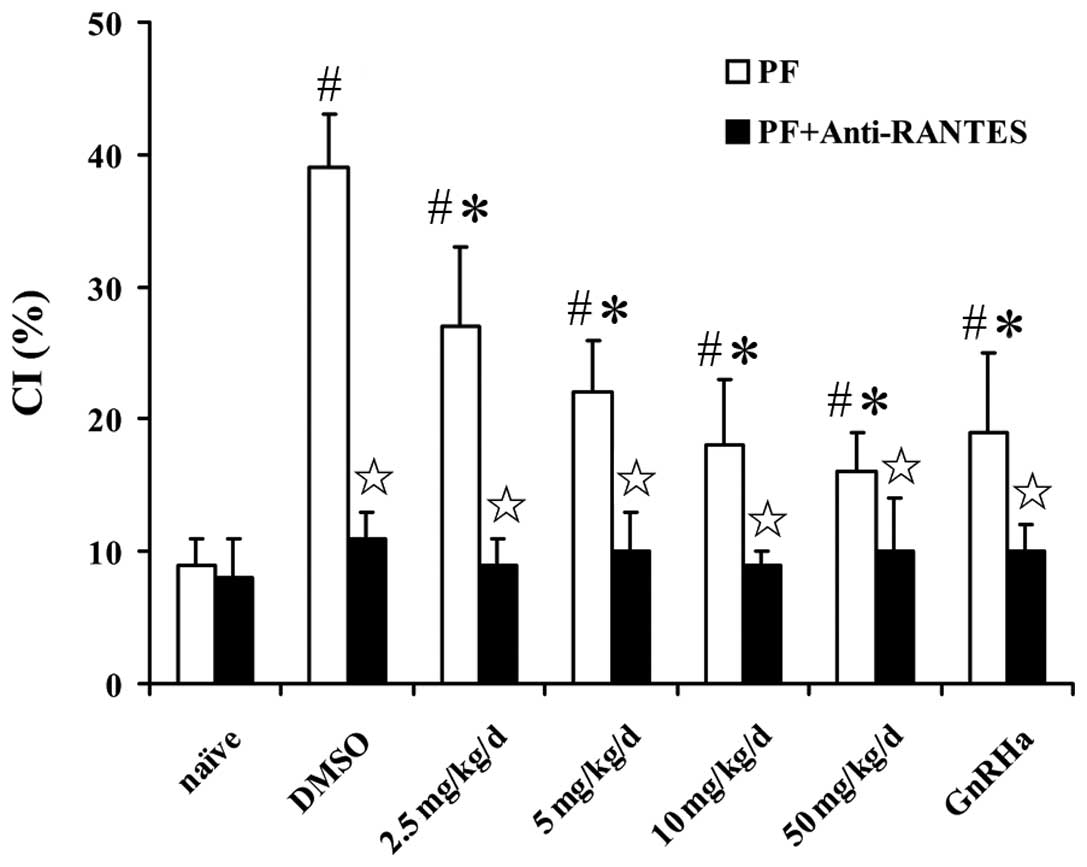
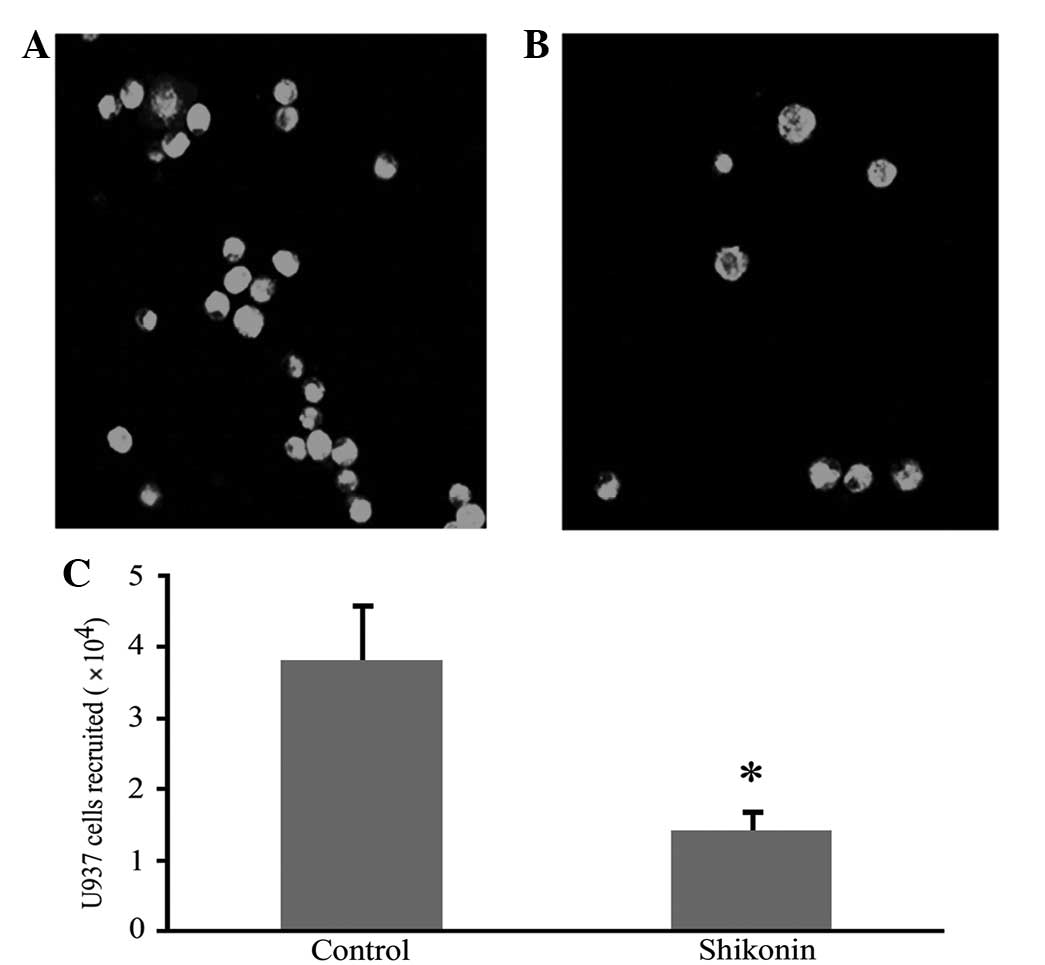
Shikonin inhibits U937 cell chemotaxis in
response to RANTES
An in vitro chemotaxis assay showed that the
peritoneal fluid from SCID mice with endometriosis promoted
monocyte chemotaxis more than that of naïve mice. The chemotactic
response was inhibited by shikonin in a concentration-dependent
manner and a maximal inhibitory concentration of shikonin ranged
between 10 and 50 ng/ml. The inhibitory effect was eradicated by
adding mRANTES antibody to the peritoneal fluid (Fig. 5). Shikonin also significantly
inhibited the migration of CFSE-labeled U937 cells to the
peritoneal cavity in vivo (Fig.
6).
Adverse effects and toxicity
No abnormal behavioral changes or clinical signs
were observed in the mice at any of the doses of shikonin. In
addition, no abnormalities were observed in the results of the
serum biochemistry assays: ALT, AST, BUN, Cr, LDH, total protein,
albumin and globulin data were within the normal ranges and no
significant differences were identified between the results of the
shikonin- and vehicle-treated groups.
Discussion
To investigate the effects of shikonin on
endometriosis, human/mouse chimeric models were established by
injecting human endometrial tissue into the peritoneal cavity of
SCID mice, which are widely used to study sensitivity to
pharmacological therapy (10).
H&E staining and immunohistochemistry assays demonstrated that
ectopic implantation of human endometrium into the mice was
accompanied by the formation of extensive adhesions of local
peritoneal tissues. This further demonstrated that peritoneal
inflammation was a significant characteristic of endometriosis
(11).
In addition, the effects of shikonin on human/mouse
chimeric SCID endometriosis were studied. It was shown that
shikonin was capable of suppressing the growth of the implanted
endometrium, decreasing the inflammatory responses induced by the
graft and improving perilesional adhesions. In addition, shikonin
exhibited significant inhibitory effects following administration
at intermediate and high doses. Specific changes in H&E-stained
specimens from intermediate- and high-dose treatment groups were
observed, including significant atrophy of ectopic endometrial
glands, sparseness of glandular epithelial cells (some of which had
no integral structure), vacuolar degeneration of stromal cells and
necrosis and atrophy of peripheral vessels accompanied by hyaline
degeneration. However, no significant differences were found
between the two groups. In the low-dose group, normal, atrophic and
semi-atrophic states in the endometrial glands were all observed,
with an even distribution of peripheral stromal cells, indicating a
disparity in the sensitivity of glands to shikonin.
Endometriosis is an immuno-inflammatory disease
characterized by peritoneal inflammation (11). Patients with endometriosis have a
significantly increased number of macrophages compared with that in
females without endometriosis, and their peritoneal fluid reveals
abnormal expression of proinflammatory cytokines (12). As monocytes and HPMCs were obtained
from mice and human-derived endometrium in the present experimental
animal study, it was possible to detect the RANTES secretion from
mice and humans. The results showed that the mRANTES secretion in
the peritoneal fluid of the animal endometriosis model was higher
than that in normal SCID mice and decreased markedly in a
dose-dependent manner following shikonin treatment. By contrast,
the expression of hRANTES by implanted human endometrial tissue was
low at the transcriptional and translational levels.
As is commonly understood, the endometriotic tissue
is composed mainly of the ectopic endometrium, peritoneal
mesothelial cells, macrophages and extracellular matrix. It has
been shown that there are increased levels of macrophages in the
peritoneal cavity of females with endometriosis. These infiltrated
macrophages are harbored in ectopic tissues and peritoneum
(13). SCID mice, with combined
deficiencies in T- and B-lymphocyte function, have a mononuclear
macrophage system. Once human endometrium, as an exogenous antigen,
is injected into SCID mice, it promotes the mononuclear macrophage
system to induce immunological inflammation.
To further study the potential effect of shikonin on
RANTES expression in a pelvic environment of endometriosis, a cell
co-culture system was constructed. In vitro analysis of the
expression of RANTES in U937 cells cultured alone or co-cultured
with HPMCs and ESCs was performed. The results showed that U937
cells secrete more RANTES than HPMCs and ESCs. The co-culture of
ESC-HPMC-U937 cells significantly promoted RANTES production and
release. Shikonin showed a concentration-related inhibitory effect
on the secretion of RANTES in U937 cells and ESC-HPMC-U937 cells.
The results were also consistent with a previous study, which
showed extremely low secretion levels of RANTES by in vitro
cultured endometrial cells, whereas mononuclear macrophages
cultured alone or co-cultured with HPMC were a significant source
of RANTES secretion (9).
It is well known that RANTES is a potent chemokine
for mononuclear macrophages (9).
To further prove the potential role of shikonin on decreasing
inflammatory responses, chemotaxis assays were performed. In
vitro and in vivo chemotaxis assays demonstrated that
shikonin had a significant effect on decreasing the sensitivity of
monocytes to RANTES chemotactic signals.
In conclusion, the present study showed the
potential ability of shikonin to inhibit the growth of ectopic
endometrial tissue in human/mouse chimeric models. The mechanism of
the therapeutic effect of shikonin may involve the inhibition of
monocyte recruitment, and the downregulation of RANTES expression
in the peritoneal cavity of females with endometriosis, followed by
the alleviation of peritoneal inflammation. Further investigations
of this compound may provide the basis for the development of new
drugs for use in the treatment of endometriosis.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81100405 and 81160469),
the Key Program of Nanjing Medical Science and Technology
Development Foundation, Nanjing Department of Health (nos. ZKX10019
and QRX11109) and Nanjing Medical University (no. 2002NJMU212). The
authors are grateful to Professor XR Guo and DJ Li for useful
comments and discussions.
References
|
1
|
Giudice LC: Clinical practice.
Endometriosis N Engl J Med. 362:2389–2398. 2010.PubMed/NCBI
|
|
2
|
Khoufache K, Bazin S, Girard K,
Guillemette J, Roy MC, Verreault JP, et al: Macrophage migration
inhibitory factor antagonist blocks the development of
endometriosis in vivo. PLoS One. 7:e372642012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pietrosiuk A, Skopińska-Rózewska E,
Furmanowa M, Wiedenfeld H, Sommer E, Sokolnicka I, et al:
Immunomodulatory effect of shikonin derivatives isolated from
Lithospermum canescens on cellular and humoral immunity in
Balb/c mice. Pharmazie. 59:640–642. 2004.
|
|
4
|
Lu L, Qin A, Huang H, Zhou P, Zhang C, Liu
N, et al: Shikonin extracted from medicinal Chinese herbs exerts
anti-inflammatory effect via proteasome inhibition. Eur J
Pharmacol. 658:242–247. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu MY, Wang RB, Zhou W and Li SS:
Antitumor effect research progress of shikonin and its derivatives.
Yao Xue Xue Bao. 47:588–593. 2012.(In Chinese).
|
|
6
|
Chen X, Yang L, Zhang N, Turpin JA,
Buckheit RW, Osterling C, et al: Shikonin, a component of chinese
herbal medicine, inhibits chemokine receptor function and
suppresses human immunodeficiency virus type 1. Antimicrob Agents
Chemother. 47:2810–2816. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi YL, Luo XZ, Zhu XY, Hua KQ, Zhu Y and
Li DJ: Effects of combined 17beta-estradiol with TCDD on secretion
of chemokine IL-8 and expression of its receptor CXCR1 in
endometriotic focus-associated cells in co-culture. Hum Reprod.
21:870–879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi YL, Luo XZ, Zhu XY and Li DJ:
Combination of 17beta-estradiol with the environmental pollutant
TCDD is involved in pathogenesis of endometriosis via up-regulating
the chemokine I-309-CCR8. Fertil Steril. 88:317–325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y,
Wang L and Li DJ: The high level of RANTES in the ectopic milieu
recruits macrophages and induces their tolerance in progression of
endometriosis. J Mol Endocrinol. 45:291–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Awwad JT, Sayegh RA, Tao XJ, Hassan T,
Awwad ST and Isaacson K: The SCID mouse: an experimental model for
endometriosis. Hum Reprod. 14:3107–3011. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lousse JC, Van Langendonckt A, Defrere S,
Ramos RG, Colette S and Donnez J: Peritoneal endometriosis is an
inflammatory disease. Front Biosci (Elite Ed). 4:23–40. 2012.
View Article : Google Scholar
|
|
12
|
Agic A, Xu H, Finas D, Banz C, Diedrich K
and Hornung D: Is endometriosis associated with systemic
subclinical inflammation? Gynecol Obstet Invest. 62:139–147. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan KN, Masuzaki H, Fujishita A, Kitajima
M, Sekine I and Ishimaru T: Differential macrophage infiltration in
early and advanced endometriosis and adjacent peritoneum. Fertil
Steril. 81:652–661. 2004. View Article : Google Scholar : PubMed/NCBI
|