Introduction
Despite the common occurrence of cutaneous melanoma
and squamous cell carcinoma (SCC), only a few cases of a solitary
skin tumor with an admixture of the features of melanoma and SCC
have been reported. This tumor was first described by Rosen et
al(1) and named as a
squamomelanocytic tumor (SMT) by Pool et al(2). Boyd and Rapini (3), who reviewed 69 cutaneous collision
tumor epidemiological studies, identified no cases of SMT. There
are only eighteen reported cases of SMT in the literature. The most
common anatomical locations are on the face and neck. Our case, the
second report of oculocutaneous SMT, is a 63-year-old female, with
solar keratosis and solar lentigo in the periphery. This is the
first case of such a specific growth pattern. In a number of cases,
it has been reported that the histogenesis of the SMT may be
associated with burn scars and solar damage. However, in reference
to our case, we consider that a long-term history of solar
keratosis is correlated with the SMT, which may be the last stage
of the solar keratosis process. Other cutaneous neoplasms,
including a mixture of malignant melanoma (MM) with
keratoacanthoma, SCC, pigmented squamous cell carcinoma, pigmented
keratinizing basal cell carcinoma and pseudoepitheliomatous, are
further discussed. A review of the literature and a case analysis
for the potential histogenesis of SMT and histological differential
diagnoses are discussed. This study was conducted in accordance
with the Declaration of Helsinki and with approval from the Ethics
Committee of the First Affiliated Hospital, Medical College of
Xi’an Jiaotong University. Written informed consent was obtained
from the participant.
Case report
Clinical presentation
A previously healthy 63-year-old Chinese female
presented with a brown-coloured spot located on the left lateral
canthus. The spot had been 2 mm in diameter for 20 years; however,
it progressively expanded over six months to a dimension of 12x10x5
mm. One month later, a clinical diagnosis of MM or a pigmented
basal cell carcinoma was considered; therefore, the lesion was
completely excised. Due to the unusual epithelioid features of the
tumor, the case was referred to the Department of Clinical
Pathology, First Affliated Hospital of Xi’an Jiaotong University
for a secondary consultation.
Pathological features
Low-power examination revealed a well-demarcated
expansive nodule at the dermal-epidermal junction and within the
dermis (Fig. 1A). High-power
examination revealed that the tumor was composed of two cell types.
The two neoplastic cell proliferations were discerned intermingling
within the epidermis and dermis (Fig.
1B). The first cell type observed consisted of slight atypical
squamous cells with abundant eosinophilic cytoplasm and large,
often hyper-chromatic and vesicular nuclei. Squamous pearls,
squamous cysts, dyskeratotic cells, apoptotic bodies and mitotic
figures were also observed. The squamous cell component consisted
of thin anastomosing epithelial cords and small whorled nests,
diffusely and irregularly distributed through the entire tumor,
extending to the deep reticular dermis. The formation of papilla by
overlying squamous epithelial proliferation was accompanied by
hyperkeratosis and dyskeratosis. The continuous sections presented
an area in which the epithelial proliferation formed a branch-like
shape extending into the dermis. The basal layer was absent
(Fig. 1C–E). The second cell type
observed was an irregularly shaped nest of predominant atypical
pigmented epithelioid cells (melanocytes). The cells had marginal
ambiguity with little eosinophilic cytoplasm, in addition to plump
to elongated spindle nuclei, with one or two predominant nucleoli.
The mitotic cell count was ∼9 mitoses per 10 high-power fields. A
number of cells had fine granular, brown to gray cytoplasmic
granules of melanin, primarily arranged in small to large nests at
the dermal-epidermal junction and proliferating to the deep
reticular dermis; however, they did not extend to the hair
follicles or eccrine gland. Single cells invading the epidermis
were identified. (Fig. 1F and G).
A significant amount of inflammatory and lymphocyte infiltrate was
identified in the stroma.
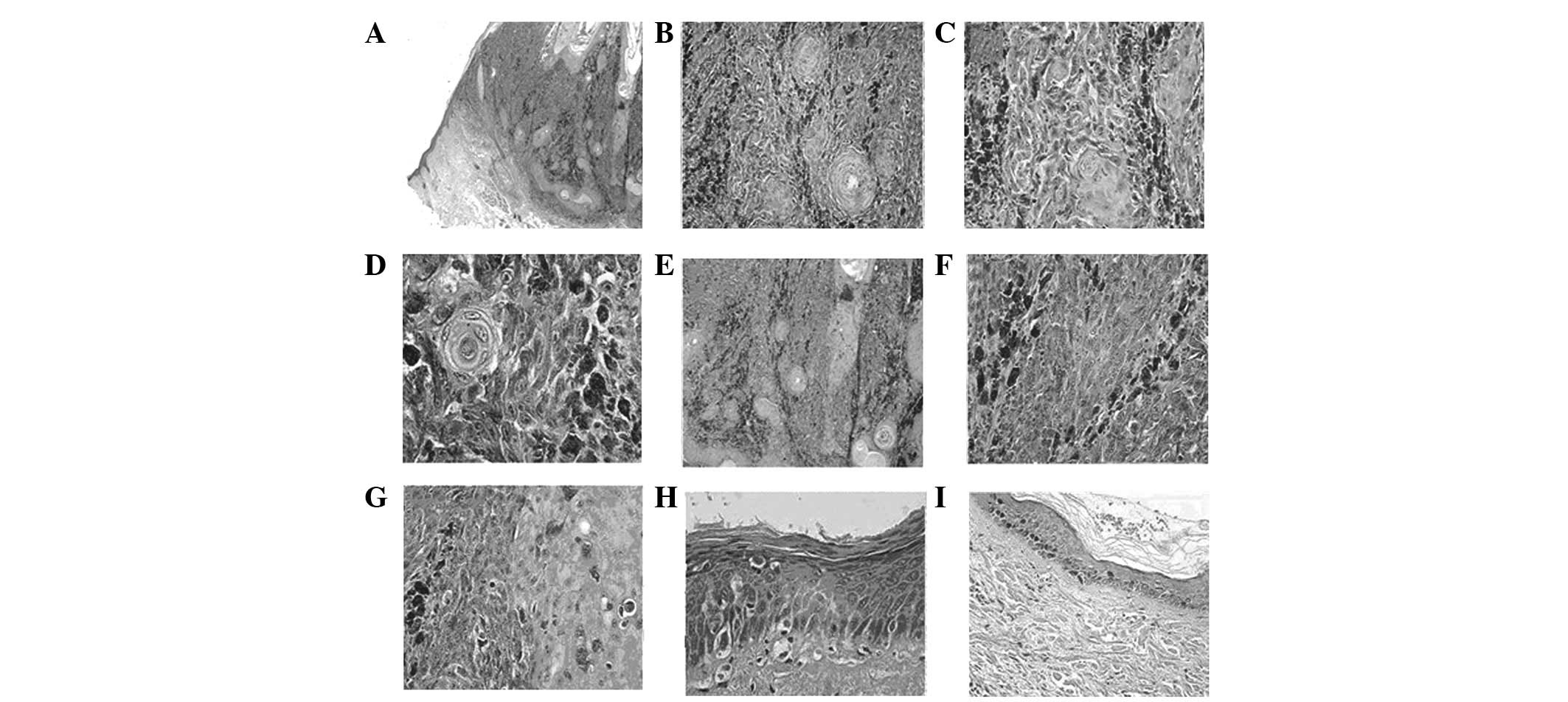 | Figure 1.(A) A well-demarcated expansive nodule
at the dermal-epidermal junction and within the dermis (original
magnification, ×2). (B) Two different neoplastic cell
proliferations were observed intermingling to exhibit a combination
of nested growth patterns (original magnification, ×10). (C) The
squamous component consisted of thin anastomosing epithelial cords
and small whorled nests (original magnification, ×10). (D) Slight
atypical squamous cells contained abundant eosinophilic cytoplasm
and large, often hyperchromatic and vesicular nuclei (original
magnification, ×40). (E) Epithelial proliferation formed a
branch-like shape extending into the dermis and the basal layer was
absent (original magnification, ×20). (F) Irregularly shaped nest
of predominant atypical pigmented epithelioid cells (original
magnification, ×20). (G) Single atypical melanocyte dispersed in
the epidermis and mitotic figures were observed (original
magnification, ×20). (H) Atypical epidermic basal cells lost
polarity and presented larger, hyperchromatic and pleomorphic
nuclei with mitosis (original magnification, ×40). (I) A lentigo
was present with increased melanin in the basal layer (original
magnification, ×10). Hematoxylin and eosin staining. |
Chronic solar keratosis was observed near the tumor
and disruption of collagenous fibers was observed under the
dyskeratotic epidermis, consistent with the chronic solar damage.
Atypical epidermic basal cells had lost polarity and presented
larger, hyperchromatic and pleomorphic nuclei with mitosis
(Fig. 1H).
A solar lentigo was present beside the area of solar
keratosis. Increased melanin and melanocytes in the basal layer, as
well as abnormal basal cells manifesting a benign process and
lentigo maligna existed in the adjacent epidermis (Fig. 1I).
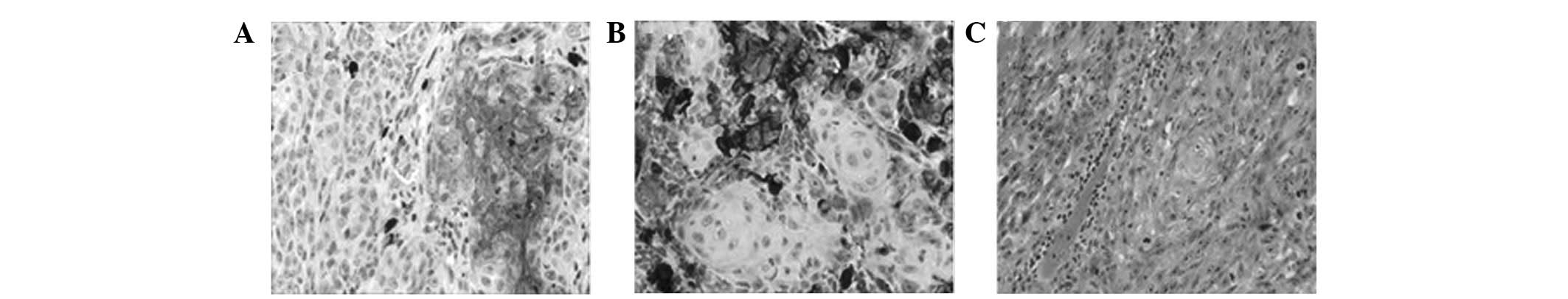
Immunohistochemistry and specific
staining
Immunohistochemistry revealed diffuse cytoplasmic
reactivity for pancytokeratin and high molecular weight cytokeratin
(HMWCK), and local cytomembrane reactivity for epithelial membrane
antigen (EMA) and low molecular weight cytokeratin (LMWCK), in all
areas revealing histopathological features of SCC (Fig. 2A). Antibodies directed to the
nucleus and cytoplasm demonstrated reactivity for S-100 protein,
and Melanoma Marker (HMB45) and Melan-A antibody reactivity
demonstrated a strong positivity for the atypical melanocytic
component (Fig. 2B). No subset of
the tumor cells demonstrated combined staining for epithelial and
melanocytic markers. The melanin staining identified that melanin
had disappeared from the tumor cells, which excluded a differential
diagnosis of other pigmentation diseases (Fig. 2C). Comparison of the mitotic
activity in the two cellular elements revealed that ∼40%
melanocytic cells and ∼5% squamous cells were positive for the
proliferation marker, Ki-67.
Based on the histopathological findings and the
immunohistochemical profile, the diagnosis of an SMT (12 mm in
depth) was made.
Discussion
SMTs are rare and they each have an identical growth
pattern (1). The tumors
characteristically contain an expansile, lobular,
well-circumscribed nodule arising from the epidermis or from the
deep reticular dermis. The Breslow depth range is 10–27 mm, with or
without connection to the epidermis (1,2,4–11).
The lesions may be infiltrative or have a tongue-like extension
into the adjacent normal tissue, including vessels and hair
follicles. The tumor demonstrates an intimate admixture of
melanocytic and squamous cells. Immunohistochemistry indicates two
clear populations: an atypical melanocytic component expressing
S-100 and HMB-45 and atypical squamous cells expressing
cytokeratin. High-index staining of cellular proliferation with
Ki-67 ranged from 20 to 50%, for different components.
By reviewing the 18 previously documented cases
(Table I), we identified that the
patient age range was 32–94 years and only 6 cases occurred in
individuals aged ≤55 years (2,7,8,12).
No predominant gender predilection has been noted for SMT variants.
Two cases of SMT occurred in patients with burn scars from their
childhood, indicating that this stimulating condition increases the
risk of developing malignancies. The most common anatomical
locations are the head (9 cases, including forehead, eyebrow,
canthus, temple, nose and ear), trunk (3 cases, including shoulder,
scapula and back) and the extremities (6 cases, including thigh and
leg). Our case concerned a 63-year-old female with a lesion in the
face, combined with solar keratosis and solar lentigo with SMT.
This is the first reported case with such a specific growth
pattern.
 | Table I.Squamomelanocytic tumors reported in
the literature. |
Table I.
Squamomelanocytic tumors reported in
the literature.
| Author | Age (years) | Gender | Site of tumor | Clinical details | Coexisting
condition | Follow-up
(months) | Metastasis |
|---|
| Muhlemann et
al(5) | 59 | M | Scapula | Unknown | Burn scar | Unknown | No |
| Rosen et
al(1) | Unknown | Unknown | Unknown | Unknown | Lentigo maligna | Unknown | No |
| Walker and Walker
(6) | 78 | F | Thigh | Unknown | Burn scar | Unknown | No |
| Akiyama et
al(7) | 55 | M | Right lower leg | Pigmented macule | Burn scar | 108 | Yes |
| Alconchel et
al(8) | 46 | F | Thigh | Unknown | Burn scar | Unknown | Yes |
| Pool et
al(2) | 70 | M | Right medical
canthus | Crusted black
nodule | Unknown | 12 | No |
| 50 | M | Left eyebrow | Brown-black
nodule | Unknown | 24 | No |
| 44 | F | Forehead | Brown-black
nodule | Lentigo maligna | 108 | No |
| 47 | M | Nose | Brown-black
nodule | Unknown | 12 | No |
| Cutlan et
al(18) | 72 | F | Shoulder | Nonpigmented
lesion | Unknown | 3 | No |
| Dorić et
al(19) | 61 | F | Left preauricular
area | Darkly pigmented
nodule | Unknown | Unknown | No |
| Satter et
al(20) | 63 | F | Left leg | Gray macule | Unknown | 31 | No |
| 73 | F | Left forearm | Flesh-colored
papule | Unknown | 27 | No |
| Rongioletti et
al(21) | 94 | M | Back | Purple-brownish
nodule | Hidradenoma | 8 | No |
| Pouryazdanparast
et al(22) | 62 | M | Left ear | Cutaneous lesion | Sunburn | 9 | No |
| Leonard et
al(9) | 68 | M | Left temple | Blue-gray nodule | Solar keratosis | Unknown | No |
| Miteva et
al(10) | 82 | M | Nose | Skin-colored
papule | Unknown | Unknown | No |
| Amerio et
al(12) | 32 | F | Right arm | Brown-black
nodule | Unknown | Unknown | No |
| Present case | 63 | F | Left lateral
canthus | Brown-colored
spot | Solar
keratosis | 14 | No |
The histological differential diagnoses of an SMT
include: i) Collision tumors of MM with keratoacanthoma and SCC:
the clear border and distinct growth pattern of epithelial
neoplasms, including keratoacanthoma and SCC, even if they
occasionally collide with MM, allow easy exclusion. The craterform
appearance of keratoacanthoma presents the benign differentiation
of cells without malignant features. However, SMTs present an
intimate admixture of melanocytic and squamous cells, in contrast
to collision tumors in which there are distinctly separate
components of melanocytic and epithelial cells. ii) Pigmented SCC:
a rare variant of SCC that consists of distinct malignant
epithelial components admixed with benign dendritic melanocytes.
SMTs present the coexistence of two malignant tumors intermingling
in the same histological specimen. The characteristic
immunohistochemical features of SMT are the expression of S-100
protein and positivity to HMB-45 and Melan-A antibodies for the
atypical melanocytic components and cytokeratin positivity for the
atypical squamous cells, while epithelial cells in pigmented SCC
are negative for HMB-45 and S-100 protein (13). iii) Pigmented keratinizing basal
cell carcinoma: this tumor comprises basal cell carcinoma and
benign dendritic melanocytes. The basal cells are arranged in cords
and nodules containing peripherally palisaded cells and have focal
areas of squamous differentiation. Immunohistochemistry reveals
epithelial components negative for HMB-45 and S-100. iv) MM with
pseudoepitheliomatous hyperplasia: reactive epithelial
pseudoepitheliomatous hyperplasia has been described as a reactive
phenomenon in a series of MMs (14). Apart from abnormal serrated
architecture and cytological features characteristic of MM, the
hyperplastic epithelium lacks nuclear atypia, mitoses and prominent
dyskeratosis. Furthermore, the melanocytic and the squamous
components have distinctive cytological atypia and Ki-67
proliferation marker expression, consistent with malignancy.
The exact histogenesis of SMT has yet to be
elucidated. Although a number of hypotheses have been proposed, no
conclusive explanation been confirmed.
Rongioletti et al favored the hypothesis that
a tumor in one cell type is colonized by a population of a second
cell type (11). In the case
reported by the authors, the pathological features suggest that the
epithelial tumor represents an atypical solid-cystic hidradenoma
colonized by the S-100/HMB-45 melanocytic malignant component.
Another hypothesis is that these tumors represent biphenotypic
malignant populations from a common precursor. Rosen et
al(1) reported a case showing
staining for S-100 protein and keratin in the same tumor cells. We
did not identify biphenotype (combined S-100/cytokeratin positivity
in the same cells) in our case.
Pool et al(2) interpreted that true malignant
proliferation of two distinct phenotypes occurs as a result of
close paracrine interactions. The authors considered that SMT is an
unusual melanoma variant, demonstrating divergent epithelial
differentiation.
Four categories of tumor histogenesis are
considered: i) collision tumor, ii) colonization tumor, iii)
combined tumor and iv) biphenotypic tumor.
We favor the hypothesis that SMT is associated with
solar keratosis, and occurs as the final stage in this process.
Epidemiological, clinicalpathological and molecular evidence
indicates that solar keratosis represents an early stage in a
biological continuum that ranges from carcinoma in situ to
invasive SCC and primarily develops on sun-exposed skin. In a
previous study, the prevalence of solar keratosis in the face and
forehead was reported to be 32% in females and 13% in males
(15). This indicates that solar
keratosis may vary according to gender. Our study presents an
elderly female with an oculocutaneous lesion. The continuous
sections revealed an area of epithelial proliferation into the
dermis. This lesion may initiate SCC origin from the epidermis,
which may be a stage in the process of malignant solar
keratosis.
Ultraviolet radiation stimulation of the skin and
surgery in nevus-prone individuals are two causes of melanoma
development. However, the presence of solar keratosis has been
reported as a risk factor for melanoma, particularly for primary
sun-exposed sites (15). An
epidemiological study of 159 individuals, each with at least one
area of solar keratosis, revealed that 39% of cases with melanoma
were in older patients. Niamh et al(16) reported a case with a 2-year history
of solar keratosis in the primary lesion. Two cases demonstrating
that long-term sun exposure causes SMT have been reported (4,5). A
case arising from lentigo maligna was reported by Pool et
al(2). In our case, following
a 20-year history of colored spots considered to be solar
lentigines, SMT was diagnosed. Solar keratosis was also observed in
the periphery of the focus and lentigo maligna was in existence in
the adjacent epidermis. This indicates that SCC and MM are closely
related to solar keratosis. In our case, we confirmed that solar
keratosis induced SCC. Although the process of MM is not clear, we
identified the histogenesis of SMT to be the final stage of solar
keratosis.
The biological behavior of this unique combined
tumor remains obscure. SCC and MM are capable of metastasis and
mortality. MM are responsible for ∼7,300 mortalities each year,
while between 1,300 and 2,300 individuals succumb each year as a
result of nonmelanoma skin cancer, primarily metastatic SCC
(17). Of the surviving cases in
the present study, all have undergone clinical treatment and
complete excision of the tumors with adequate margins, with a mean
follow-up time of 31 months. Two studies have reported regional
metastasis and there have been two reports of mortality as a result
of metastatic melanoma (4,5). A longer follow-up and additional
studies are required to validate the findings presented here and to
elucidate the prognosis and most suitable therapeutic approach for
combined cutaneous tumors.
References
|
1.
|
Rosen LB, Williams WD, Benson J and Rywlin
AM: A malignant neoplasm with features of both squamous cell
carcinoma and malignant melanoma. Am J Dermatopathol. (6 Suppl):
213–219. 1984.PubMed/NCBI
|
|
2.
|
Pool SE, Manieei F, Clark WH Jr and
Harrist TJ: Dermal squamo-melanocytic tumor: a unique biphenotypic
neoplasm of uncertain biological potential. Hum Pathol. 30:525–529.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Boyd AS and Rapini PR: Cutaneous collision
tumors. An analysis of 69 cases and review of the literature. Am J
Dermatopathol. 16:253–257. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Novick M, Gard DA, Hardy SB and Spira M:
Burn scar carcinoma: a review and analysis of 46 cases. J Trauma.
17:809–817. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Muhlemann MR, Griffiths RW and Briggs JC:
Malignant melanoma and squamous cell carcinoma arising in a burn
scar. Br J Plast Surg. 35:474–477. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Walker AN and Walker GK: Scar-associated
malignant melanoma and squamous carcinoma in situ. South Med J.
82:1419–1421. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Akiyama M, Inamoto N and Nakamura K:
Malignant melanoma and squamous cell carcinoma forming one tumor in
a burn scar. Dermatology. 194:157–161. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Alconchel M, Olivares C and Alvarez R:
Squamous cell carcinoma, malignant melanoma and malignant fibrous
histiocytoma arising in burn scars. Br J Dermatol. 137:793–798.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Leonard N, Wilson N and Calonje JE:
Squamomelanocytic tumor: an unusual and distinctive entity of
uncertain biological potential. Am J Dermatopathol. 31:495–498.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Miteva M, Herschthal D, Ricotti C, Keri H
and Romanelli P: A rare case of a cutaneous squamomelanocytic
tumor: revisiting the histogenesis of combined neoplasms. Am J
Dermatopathol. 31:599–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Rongioletti F, Baldari M, Carli C and
Fiocca R: Squamomelanocytic tumor: a new case of a unique
biphenotypic neoplasm of uncertain biological potential. J Cutan
Pathol. 36:477–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Amerio P, Carbone A, Auriemma M, et al:
Metastasizing dermal squamomelanocytic tumour: more evidences. J
Eur Acad Dermatol Venereol. 25:734–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Satter EK: Pigmented squamous cell
carcinoma. Am J Dermatopathol. 29:486–489. 2007. View Article : Google Scholar
|
|
14.
|
Kamino H, Tam ST and Alvarez L: Malignant
melanoma with pseudocarcinomatous hyperplasia - an entity that can
simulate squamous cell carcinoma. A light-microscopic and
immunohistochemical study of four cases. Am J Dermatopathol.
12:446–451. 1990. View Article : Google Scholar
|
|
15.
|
Salasche SJ: Epidemiology of actinic
keratoses and squamous cell carcinoma. J Am Acad Dermatol. 42(1 Pt
2): 4–7. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Niamh L, Naveen S and Hazel B: Diagnosis
of vulval inflammatory dermatoses: a pathological study with
clinical correlation. Int J Gynecol Pathol. 28:554–558. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chang YM, Barrett JH, Bishop DT, et al:
Sun exposure and melanoma risk at different latitudes: a pooled
analysis of 5700 cases and 7216 controls. Int J Epidemiol.
38:814–830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Cutlan RT, Wesche WA and Chesney TM: A
cutaneous neoplasm with histopathological and immunohistochemical
features of both malignant melanoma and squamous cell carcinoma. J
Cutan Pathol. 27:5512000.
|
|
19.
|
Dorić M, Radović S, Kuskunović S, et al:
Dermal squamomelanocytic tumor: neoplasm of uncertain biological
potential. Bosn J Basic Med Sci. 8:152–155. 2008.PubMed/NCBI
|
|
20.
|
Satter EK, Metcalf J, Lountzis N and
Elston DM: Tumors composed of malignant epithelial and melanocytic
populations: a case series and review of the literature. J Cutan
Pathol. 36:211–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Rongioletti F, Baldari M, Carli C and
Fiocca R: Squamomelanocytic tumor: a new case of a unique
biphenotypic neoplasm of uncertain biological potential. J Cutan
Pathol. 36:477–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Pouryazdanparast P, Yu L, Johnson T and
Fullen T: An unusual squamo-melanocytic tumor of uncertain biologic
behavior: a variant of melanoma? Am J Dermatopathol. 31:457–461.
2009. View Article : Google Scholar : PubMed/NCBI
|