Introduction
Diabetes mellitus has progressively become a serious
public health problem worldwide. Reactive oxygen species
(ROS)-induced pancreatic β cell death has an important role in the
pathogenesis of diabetes and also affects insulin secretion. The
ROS that are particularly responsible for oxidative stress include
superoxide ions (O2−), hydroxyl radicals
(•OH), singlet oxygen (1O2),
hydrogen peroxide (H2O2), nitric oxide (NO)
and peroxynitrite (ONOO−). Oxidative stress induces the
dysfunction of pancreatic β cells, decreases insulin secretion
(1) and leads to diabetic
complications, including retinopathy, nephropathy, neuropathy and
vascular damage (2,3). Generally, mammalian cells contain
various antioxidative compounds, including low molecular mass
antioxidants such as glutathione (GSH), uric acid, vitamin C and
vitamin E, as well as various endogenous antioxidant enzymes
against oxidative stress. Superoxide dismutase (SOD), catalase
(CAT) and glutathione peroxidase (GSH-px) are three important
endogenous antioxidant enzymes with roles against ROS-induced
oxidative stress in living organs. Among these antioxidant enzymes,
SOD catalyses the dismutation of the superoxide anion
(O2−) into hydrogen peroxide
(H2O2) which is transformed into
H2O and O2 by CAT. GSH-px is key in removing
lipid hydroperoxides and reducing free hydrogen peroxide to
water.
Certain drugs which are used in clinical diabetes
mellitus treatment are also associated with undesirable
side-effects, such as gastrointestinal disturbances, edema,
myocardial infarction and risk of cardiovascular disease (4,5). For
these reasons, the development of more effective and safer drugs
for treating diabetes has become essential. Currently, >400
traditional plant treatments for diabetes have been recorded
(6). It is possible that
anti-diabetic components from those natural plants may be ancillary
medicines for diabetes.
Quercus salicina is an evergreen plant which
grows in southern parts of the Korean Peninsula and Japan. It has
exhibited anti-inflammatory, antiedemic, diuretic and litholytic
activities and has been used to treat diarrhea, dysentery,
dermatitis and hemorrhagia in Korean folk medicine (7–9).
The current study was designed to investigate the
potential cytoprotective effects of QSWE on alloxan-induced
oxidative stress and also to elucidate the mechanisms underlying
its protective effects in HIT-T15 cells.
Materials and methods
Plant extract preparation
Fresh Quercus salicina leaves were purchased
from a local market in Chongqing, China in August 2012. Quercus
salicina hot water extract (QSWE) was prepared by boiling 100 g
freeze-dried Quercus salicina leaves in 1 l distilled water
for 2 h, followed by ultracentrifuging at 30,000 × g for 30 min,
filtering with a 0.4-μm filter, concentrating by heat
evaporation and freeze-drying. The QSWE was redissolved in dimethyl
sulfoxide (DMSO) at a concentration of 50 mg/ml and stored at 4°C
until further study.
Cell culture
HIT-T15 Syrian hamster insulin-secreting cells were
obtained from the American Type Culture Collection (ATCC,
Rockville, MD, USA). The cells were maintained in RPMI-1640 medium
supplemented with 10% (v/v) fetal bovine serum (FBS) and 1%
penicillin-streptomycin in a humidified CO2 incubator
(Model 3154; Forma Scientific Inc., Marietta, OH, USA) with 5%
CO2 at 37°C.
Cell viability assay
Cell viability was assessed using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays. Cells were seeded on 96-well plates at a density of
5×103 cells/well. After a 24 h incubation, the cells
were treated with alloxan (1 mM) for 1 h, then incubated with QSWE
(2.5–50 μg/ml) for 24 h. Following incubation, 100 μl
MTT reagent (0.5 mg/ml) was added to each well and the cells were
incubated in a humidified incubator at 37°C to allow the MTT to be
metabolized. After 4 h, the medium was removed and the cells were
resuspended in formazan with 100 μl DMSO. The absorbance of
the samples was measured at 540 nm using a microplate reader (model
680; Bio-Rad, Hercules, CA, USA).
Analysis of intracellular ROS
Intracellular ROS levels were measured using the
fluorescent probe dihydrodichlorofluorescein diacetate (H2DCF-DA).
Following treatment, the HIT-T15 cells were washed with calcium-
and magnesium-free phosphate-buffered saline (PBS) and incubated in
H2DCF-DA (20 μM) containing serum- and
phenol-red-free DMEM for 30 min. After incubation, the medium was
removed and the cells were washed twice with PBS. Fluorescence was
measured using a FLUOstar OPTIMA fluorescence plate reader (BMG
Labtec, Ortenberg, Germany; excitation was read at 485 nm and
emission at 535 nm). Relative ROS production (percentage of the
control) was expressed as the ratio of the fluorescence of the
treated samples to the response in the appropriate controls:
(Fluorescence treatment / fluorescence control) × 100.
Lipid peroxidation levels
Lipid peroxidation was evaluated by thiobarbituric
acid (TBA)-reactive substance (TBARS) assays (10). In brief, the treated cells were
washed with cooled PBS, scraped into trichloroacetic acid (TCA;
2.8%, w/v) and sonicated. Total protein was determined with a
bicinchoninic acid (BCA) assay. The suspension was mixed with 1 ml
TBA (0.67%, w/v) and 1 ml TCA (25%, w/v), heated (30 min, 95°C) and
centrifuged (1,500 rpm, 10 min, 4°C). TBA reacts with the oxidative
degradation products of lipids to yield red complexes that absorb
at 535 nm. The amount of TBA-reactive substance was determined
using a UV-2401PC spectrophotometer (Shimadzu, Kyoto, Japan).
Antioxidant enzyme activity
HIT-T15 cells grown in 10-cm cell culture dishes
were first treated with alloxan (1 mM) for 1 h and then incubated
with QSWE (2.5–50 μg/ml) for 24 h for further analysis. The
cells were washed with PBS, detached by scraping and centrifuged,
and the resulting cell pellet was stored at −80°C. Cell pellets
were thawed, resuspended in 300 μl cold lysis buffer (PBS,
1mM EDTA), homogenized and centrifuged (1,200 rpm, 10 min, 4°C).
The resulting supernatants were used for activity measurements. CAT
activity (U/mg protein) was measured according to the method
described by Nelson and Kiesow (11) in which the disappearance of the
substrate H2O2 was measured
spectophotometrically at 240 nm. SOD activity (U/mg protein) was
assayed using a modified autoxidation of pyrogallol method
(12). One unit of SOD activity
was defined as the amount of enzyme that inhibited the rate of
autoxidation of pyrogallol by 50%. GSH-px activity (U/mg protein)
was assayed according to the method of Hafemen et
al(13). Protein contents were
determined using a Bio-Rad protein assay kit according to the
manufacturer’s instructions.
Insulin secretion assay
Insulin secretion was measured with an ELISA assay.
The cells were seeded at 5×105 cells/well in 96-well
plates. The cells were first treated with alloxan (1 mM) for 1 h
and then treated with QSWE (2.5–50 μg/ml) for 24 h. To
measure the amount of insulin secreted, aliquots of samples (10
μl/well) were collected from the experimental medium after
the 24-h QSWE treatment and subjected to an insulin antiserum
immunoassay according to the manufacturer’s instructions (LINCO
Research, St. Charles, MO, USA). The absorbance was read at 450 and
590 nm in a microplate reader (model 680).
Statistical analysis
Data were presented as the mean ± SD. Differences
between the mean values for individual groups were assessed by
one-way ANOVA with Duncan’s multiple range tests. P<0.05 was
considered to indicate a statistically significant difference. The
SAS v9.1 statistical software package (SAS Institute Inc., Cary,
NC, USA) was used for the analyses.
Results
Effects of QSWE on alloxan-induced
oxidative damage in HIT-T15 cells
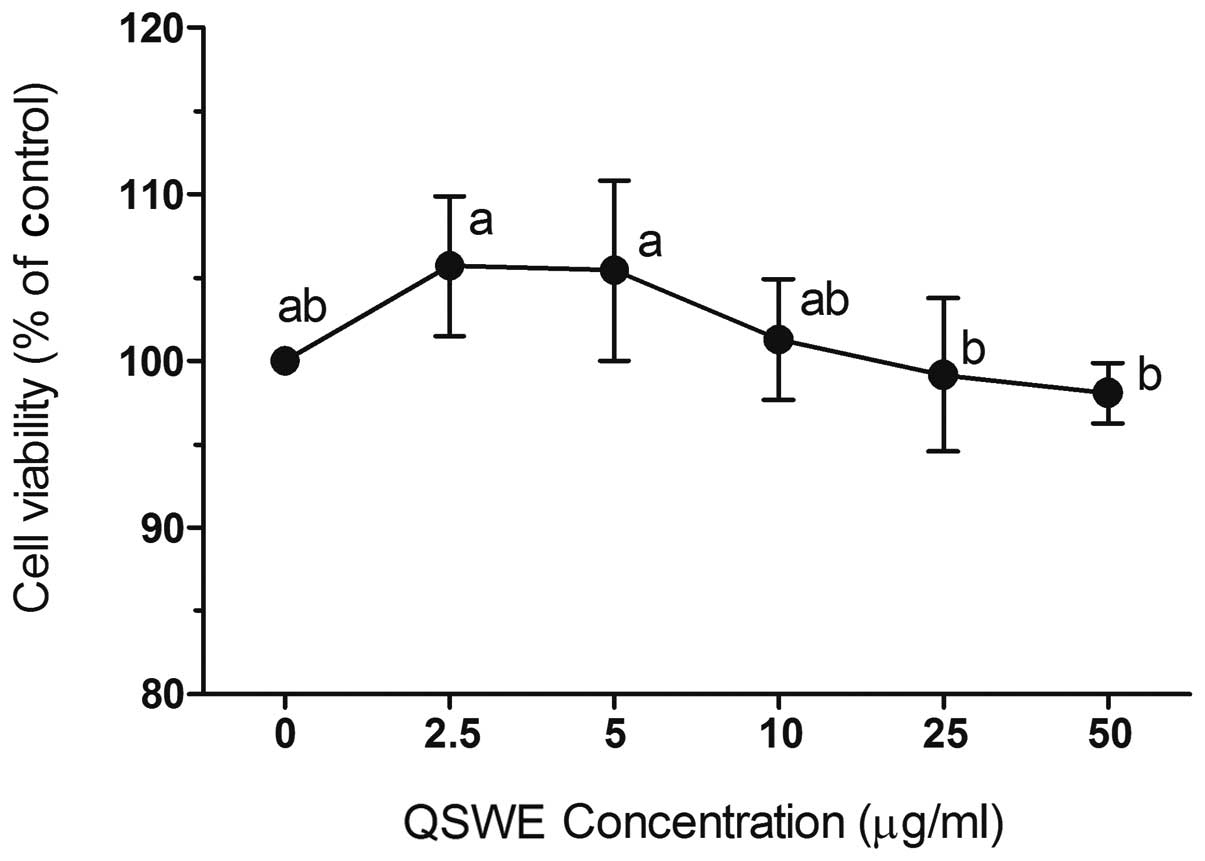
To investigate QSWE-induced cytotoxicity, HIT-T15
cells were first treated with various concentrations of QSWE
(2.5–50 μg/ml) for 24 h and the cell viability was
determined using MTT assays. QSWE did not exhibit any significant
cytotoxicity and the cell viabilities were >90% (Fig. 1). Based on these results,
concentrations between 2.5 and 50 μg/ml were used for
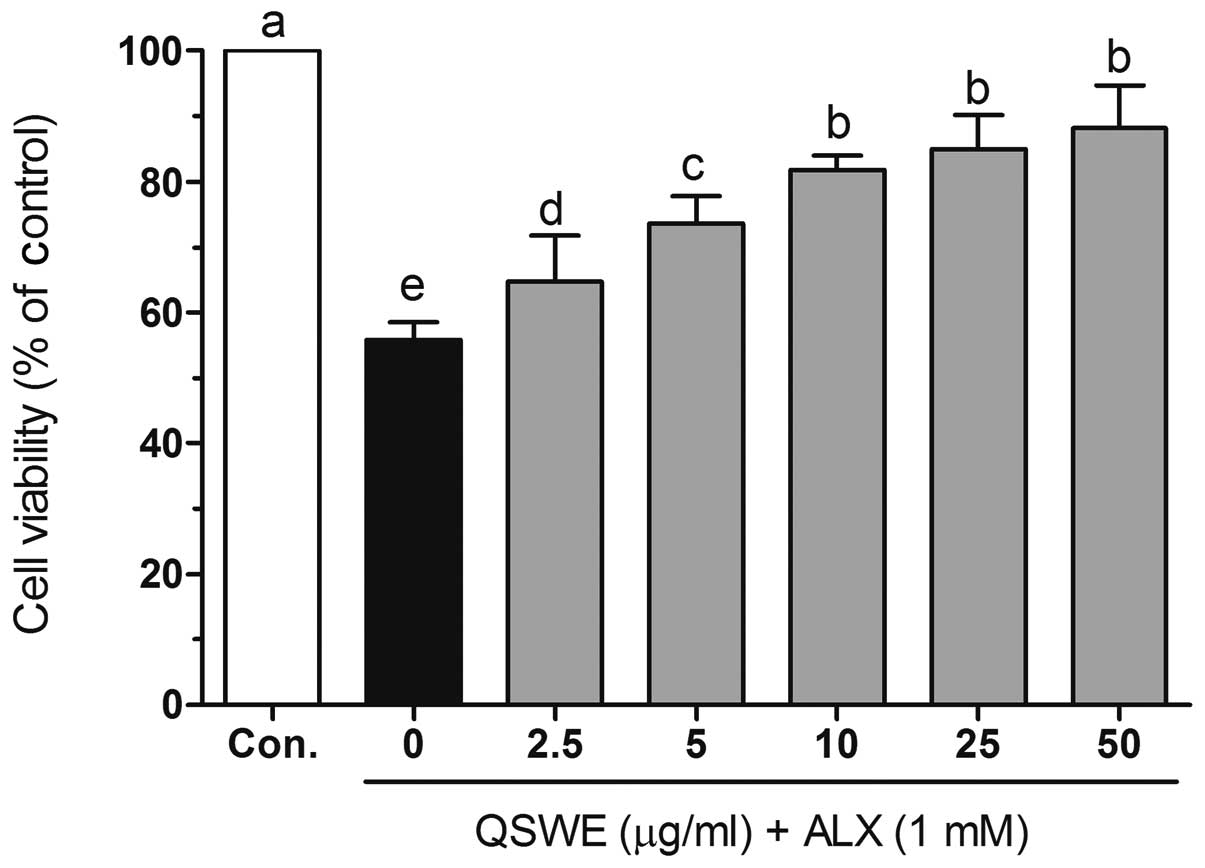
further studies. As shown in Fig.
2, alloxan (1 mM) significantly induced cell death in the
HIT-T15 cells. However, following treatment with various
concentrations of QSWE, the cell viability was increased in a
concentration-dependent manner.
Effects of QSWE on alloxan-induced
intracellular ROS levels in HIT-T15 cells
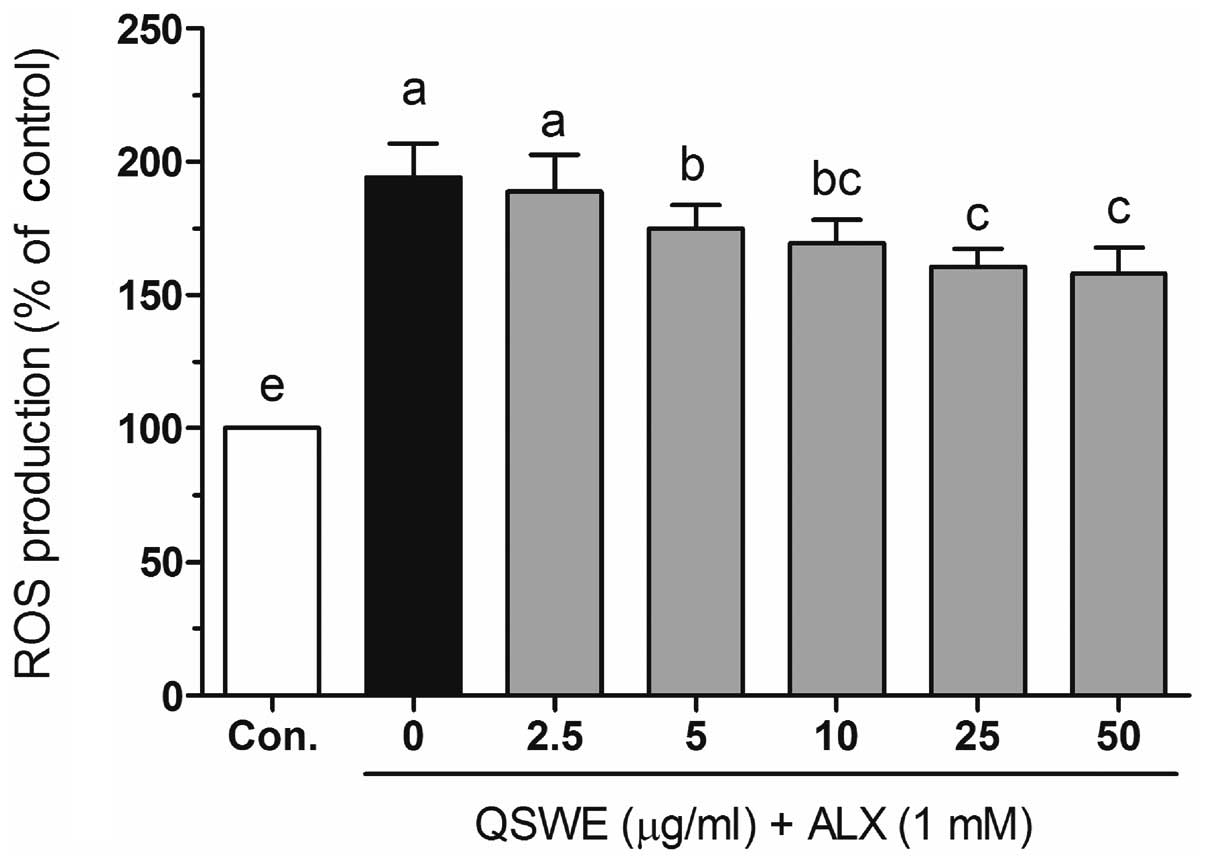
To investigate the protective effects of QSWE in
alloxan-treated HIT-T15 cells, the intracellular ROS levels were
evaluated using a fluorescent probe, H2DCF-DA. As shown
in Fig. 3, alloxan significantly
increased the ROS levels compared with those in the normal cells.
In the presence of alloxan, QSWE significantly reduced ROS
generation in a concentration-dependent manner between 2.5 and 50
μg/ml. The intracellular ROS levels were 188.6±13.7,
174.9±8.9, 169.5±8.8, 160.5±6.8 and 158.1±9.8% at 2.5, 5, 10, 25
and 50 μg/ml QSWE, respectively. Treatment with the same
concentrations of QSWE alone did not significantly increase the
intracellular ROS levels (data not shown). These results suggest
that QSWE is a free radical scavenger.
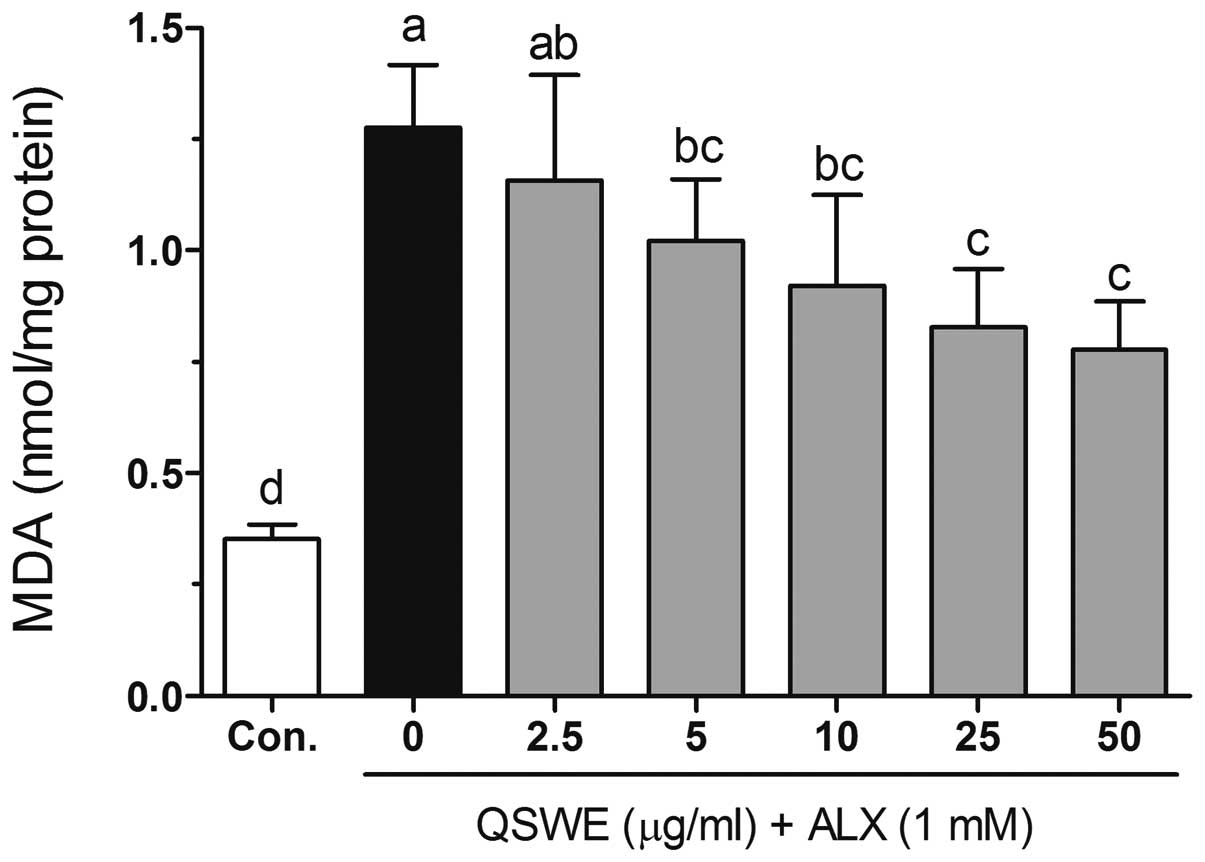
Effects of QSWE on lipid peroxidation in
alloxan-treated HIT-T15 cells
Free radicals and ROS-induced oxidative damage were
markedly associated with the lipid peroxidation of cell membranes
and increased the levels of malondialdehyde (MDA), which is a
biomarker of cell membrane lipid peroxidation. As shown in Fig. 4, alloxan significantly increased
the level of MDA (to 1.27±0.14 nmol/mg protein) compared with that
in the normal cells (0.35±0.03 nmol/mg protein). QSWE significantly
reduced the MDA levels in a concentration-dependent manner between
2.5 and 50 μg/ml. The MDA levels were 1.16±0.23, 1.02±0.14,
0.92±0.20, 0.83±0.13 and 0.78±0.11 nmol/mg protein at 2.5, 5, 10,
25 and 50 μg/ml QSWE, respectively.
Effects of QSWE on the activity of
antioxidant enzymes in alloxan-treated HIT-T15 cells
Table I shows the
intracellular antioxidant enzyme activities of QSWE in the
alloxan-treated HIT-T15 cells. The activity of SOD was reduced by
alloxan (to 7.25±0.68 U/mg protein) and this reduction was
attenuated by various concentrations of QSWE; the SOD activity was
7.76±1.07, 8.85±1.26, 10.37±0.57, 10.65±1.65 and 11.60±1.18 U/mg
protein at 2.5, 5, 10, 25 and 50 μg/ml QSWE, respectively.
Following treatment with alloxan, the cellular CAT activity was
reduced (1.25±0.15 U/mg protein) compared with that in the normal
cells (2.11±0.24 U/mg protein). However, the reduction in CAT
activity was significantly attenuated (P<0.05) by treatment with
QSWE. In addition, QSWE also attenuated the alloxan-induced
reduction in GSH-px activity in the HIT-T15 cells. The GSH-px
activity of the alloxan-treated cells significantly increased
following treatment with QSWE; the increased levels ranged from
3.29±0.15 to 4.85±0.20 U/mg protein.
 | Table I.Effect of QSWE on the activity of CAT,
SOD and GSH-px in HIT-T15 cells exposed to alloxan. |
Table I.
Effect of QSWE on the activity of CAT,
SOD and GSH-px in HIT-T15 cells exposed to alloxan.
| Group | QSWE concentration
(μg/ml) | CAT (U/mg
protein) | SOD (U/mg protein)
GSH- | px (U/mg
protein) |
|---|
| Normal | - | 2.11±0.24a | 14.78±0.40a | 5.34±0.35a |
| ALX (1 mM) +
QSWE | 0.0 | 1.25±0.15c | 7.25±0.68d | 3.19±0.24e |
| 2.5 | 1.68±0.24b | 7.76±1.07d | 3.29±0.15e |
| 5.0 | 1.70±0.14b | 8.85±1.26cd | 3.74±0.32d |
| 10.0 | 1.89±0.23ab | 10.37±0.57bc | 4.19±0.16c |
| 25.0 | 1.95±0.07ab | 10.65±1.65b | 4.43±0.18c |
| 50.0 | 2.04±0.14a | 11.60±1.18b | 4.85±0.20b |
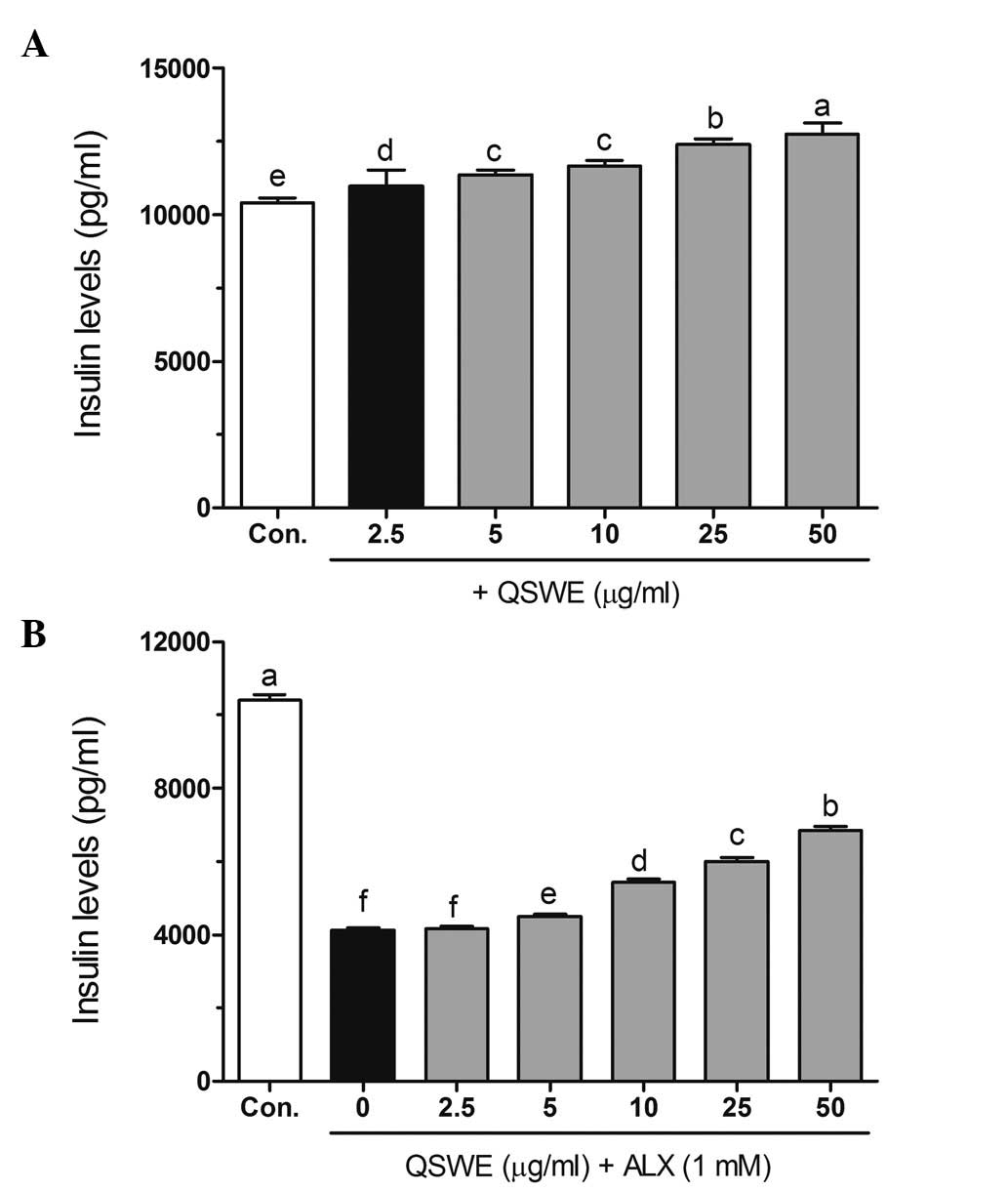
Effects of QSWE on insulin secretion in
alloxan-treated HIT-T15 cells
As shown in Fig.
5A, QSWE effectively increased insulin secretion in normal
HIT-T15 cells. However, alloxan significantly decreased the insulin
level (4,119.58±66.70 pg/ml) compared with that in the normal cells
(10,411.66±159.14 pg/ml). Following treatment with QSWE, the
insulin levels in the alloxan-treated cells were 4,160.78±67.36,
4,490.35±72.70, 5,437.85±88.04, 6,014.59±97.38 and 6,846.02±116.42
pg/ml at at 2.5, 5, 10, 25 and 50 μg/ml QSWE, respectively
(Fig. 5B). These results suggest
that QSWE treatment is effective for increasing pancreatic β cell
survival and maintaining normal biological function in ROS-induced
diabetes.
Discussion
ROS-induced oxidative damage in pancreatic β cells
is considered to have an important role in the pathological process
of diabetes. Certain studies have reported that reducing ROS levels
and treatment with antioxidants (such as NAC, vitamin C and vitamin
E) are able to improve β cell structure and function in
vitro(14,15). However, whether QSWE protects
pancreatic β cells from alloxan-induced oxidative damage has not
been investigated. The present study demonstrated that QSWE was
able to protect HIT-T15 cells from ROS-induced cell damage. The
cytoprotective effects are mainly mediated by upregulated
intracellular antioxidant enzyme activity.
In the present study, it was revealed that QSWE
prevented alloxan-induced cell death, as assessed by MTT assays.
The results showed that QSWE alone was not significantly cytotoxic
to cells at the tested concentrations. Treatment with QSWE
exhibited significant protective effects which may be due to the
free radical scavenging activity of QSWE.
To evaluate the role of the free radicals in the
protective activity of QSWE, the effect on alloxan-induced ROS
generation was analyzed using H2DCF-DA assays. Treatment
with alloxan alone significantly increased the intracellular ROS
generation. Following treatment with QSWE, ROS generation was
observed to decline in a concentration-dependent manner. This
decrease in alloxan-induced ROS may account for the observed
cytoprotective effect.
Lipid peroxidation is the most extensively
investigated process induced by free radicals. ROS participate in
the toxic actions that lead to the apoptosis of insulin-producing
cells. In the present study, increased lipid peroxidation levels
were observed in alloxan-treated HIT-T15 cells. However, treatment
with QSWE resulted in a decrease in lipid peroxidation, indicating
that oxidative stress-related damage was lower in the QSWE-treated
cells. The capacity of QSWE to reduce lipid peroxidation may be due
to its function as a preventive antioxidant for scavenging
initiating radicals.
Overproduced free radicals are scavenged by
endogenous antioxidant enzymes, including SOD, CAT and GSH-px. In
cells, SOD catalyzes the conversion of superoxide
(O2−) to hydrogen peroxide
(H2O2) and H2O2 is
further reduced H2O by the activity of CAT or GSH-px. Pancreatic β
cells have been reported to contain low levels of endogenous
antioxidant enzymes, in particular GSH-px and CAT (16). In the present study, alloxan
significantly decreased the activity of GSH-px and CAT in HIT-T15
cells. However, QSWE treatment increased the activity of these
antioxidant enzymes in the alloxan-treated HIT-T15 cells,
indicating that QSWE was able to reduce alloxan-induced oxidative
stress. Certain studies have reported that the overexpression of
Cu/Zn-SOD showed a protective effect against nitric oxide-induced
cytotoxicity in human islets and INS-1 insulin-secreting cells
(17) and alloxan- and
streptozotocin-induced diabetes (18,19).
CAT also showed a protective effect against
H2O2− and streptozotocin-induced
oxidative stress in vivo(20). In addition, the combined
over-expression of CAT and GSH-px also revealed a protective effect
against ROS-induced oxidative stress by increasing the activity of
Cu/Zn SOD or MnSOD (21–23).
In conclusion, in the present study, QSWE
demonstrated protective activity against alloxan-induced cell death
in HIT-T15 hamster insulin-secreting cells. QSWE was able to
effectively scavenge alloxan-induced intracellular ROS and prevent
pancreatic β cell death by increasing the activity of the
intracellular antioxidant enzymes SOD, CAT and GSH-Px. Furthermore,
QSWE also promoted insulin secretion in the alloxan-treated HIT-T15
cells.
References
|
1.
|
Evans JL, Goldfine ID, Maddux BA and
Grodsky GM: Are oxidative stress-activated signaling pathways
mediators of insulin resistance and β-cell dysfunction? Diabetes.
52:1–8. 2003.PubMed/NCBI
|
|
2.
|
Rahimi R, Nikfar S, Larijani B and
Abdollahi M: A review on the role of antioxidants in the management
of diabetes and its complications. Biomed Pharmacother. 59:365–373.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Robertson RP and Harmon JS: Diabetes,
glucose toxicity, and oxidative stress: a case of double jeopardy
for the pancreatic islet β cell. Free Radic Biol Med. 41:177–184.
2006.PubMed/NCBI
|
|
4.
|
Bell DS: Do sulfonylurea drugs increase
the risk of cardiac events? CMAJ. 174:185–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Home PD, Pocock SJ, Beck-Nielsen H, Gomis
R, Hanefeld M, Jones NP, Komajda M and McMurray JJ; RECORD Study
Group: Rosiglitazone evaluated for cardiovascular outcomes - an
interim analysis. N Engl J Med. 357:28–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bailey CJ and Day C: Traditional plant
medicines as treatments for diabetes. Diabetes Care. 12:553–564.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Redwane A, Lazrek H, Bouallam S, Markouk
M, Amarouch H and Jana M: Larvicidal activity of extracts from
Quercus lusitania var. infectoria galls (Oliv). J
Ethnopharmacol. 79:261–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Goun EA, Petrichenko VM, Solodnikov SU,
Suhinina TV, Kline MA, Cunningham G, Nguyen C and Miles H:
Anticancer and antithrombin activity of Russian plants. J
Ethnopharmacol. 81:337–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kim J, Kim H, Kim S, Lee K, Ham I and
Whang WK: Antioxidative compounds from Quercus salicina
Blume stem. Arch Pharm Res. 31:274–278. 2008. View Article : Google Scholar
|
|
10.
|
Fraga CG, Leibovitz BE and Tappel AL:
Lipid peroxidation measured as thiobarbituric acid-reactive
substances in tissue slices: characterization and comparison with
homogenates and microsomes. Free Radic Biol Med. 4:155–161. 1988.
View Article : Google Scholar
|
|
11.
|
Nelson D and Kiesow L: Enthalpy of
decomposition of hydrogen peroxide by catalase at 25 degrees C
(with molar extinction coefficients of H2O2
solutions in the UV). Anal Biochem. 49:474–478. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Marklund S and Marklund G: Involvement of
the superoxide anion radical in the autoxidation of pyrogallol and
a convenient assay for superoxide dismutase. Eur J Biochem.
47:469–474. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Hafeman DG, Sunde RA and Hoekstra WG:
Effect of dietary selenium on erythrocyte and liver glutathione
peroxidase in the rat. J Nutr. 104:580–587. 1974.PubMed/NCBI
|
|
14.
|
Robertson RP, Harmon J, Tran PO, Tanaka Y
and Takahashi H: Glucose toxicity in β-cells: type 2 diabetes, good
radicals gone bad, and the glutathione connection. Diabetes.
52:581–587. 2003.
|
|
15.
|
Cheng Q, Law PK, de Gasparo M and Leung
PS: Combination of the dipeptidyl peptidase IV inhibitor LAF237
[(S)-1-[(3-hydroxy-1-adamantyl)ammo] acetyl-2-cyanopyrrolidine]
with the angiotensin II type 1 receptor antagonist valsartan
[N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl)-[1, 1′-biphenyl]-4-yl]
methyl]-L-valine] enhances pancreatic islet morphology and function
in a mouse model of type 2 diabetes. J Pharmacol Exp Ther.
327:683–691. 2008.
|
|
16.
|
Zhang H, Öllinger K and Brunk U:
Insulinoma cells in culture show pronounced sensitivity to
alloxan-induced oxidative stress. Diabetologia. 38:635–641. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Moriscot C, Pattou F, Kerr-Conte J,
Richard MJ, Lemarchand P and Benhamou PY: Contribution of
adenoviral-mediated superoxide dismutase gene transfer to the
reduction in nitric oxide-induced cytotoxicity on human islets and
INS-1 insulin-secreting cells. Diabetologia. 43:625–631. 2000.
View Article : Google Scholar
|
|
18.
|
Kubisch HM, Wang J, Bray TM and Phillips
JP: Targeted over-expression of Cu/Zn superoxide dismutase protects
pancreatic β-cells against oxidative stress. Diabetes.
46:1563–1566. 1997.PubMed/NCBI
|
|
19.
|
Kubisch HM, Wang J, Luche R, Carlson E,
Bray TM, Epstein CJ and Phillips JP: Transgenic copper/zinc
superoxide dismutase modulates susceptibility to type I diabetes.
Proc Natl Acad Sci USA. 91:9956–9959. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Xu B, Moritz JT and Epstein PN:
Overexpression of catalase provides partial protection to
transgenic mouse beta cells. Free Radic Biol Med. 27:830–837. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lortz S and Tiedge M: Sequential
inactivation of reactive oxygen species by combined overexpression
of SOD isoforms and catalase in insulin-producing cells. Free Radic
Biol Med. 34:683–688. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lepore DA, Shinkel TA, Fisicaro N, Mysore
TB, Johnson LE, d’Apice AJ and Cowan PJ: Enhanced expression of
glutathione peroxidase protects islet β cells from
hypoxia-reoxygenation. Xenotransplantation. 11:53–59.
2004.PubMed/NCBI
|
|
23.
|
Mysore TB, Shinkel TA, Collins J, Salvaris
EJ, Fisicaro N, Murray-Segal LJ, Johnson LE, Lepore DA, Walters SN,
Stokes R, Chandra AP, O’Connell PJ, d’Apice AJ and Cowan PJ:
Overexpression of glutathione peroxidase with two isoforms of
superoxide dismutase protects mouse islets from oxidative injury
and improves islet graft function. Diabetes. 54:2109–2116. 2005.
View Article : Google Scholar : PubMed/NCBI
|