Introduction
Bladder cancer is one category of common
genitourinary cancers and recurrence with metastasis is the main
reason for treatment failure (1).
Metastases are the end result of tumor progression and the most
common cause of mortality in cancer patients. The process of
metastasis is multistep and interruption of this process at any
individual step may arrest the metastatic cascade (2). Therefore, it is important to
investigate the biological mechanisms contributing to the
development and metastatic movement of bladder cancer.
Tumor metastasis suppressor genes are a relatively
new class of genes. These genes, which include NM23 (3–7),
KISS-1 (8–11) and RhoGDI2 (12–14),
reduce the metastatic ability of cancer cells at orthotopic sites,.
However, the mechanism of action and role in human cancer remains
unknown for the majority of these genes.
The LASS (longevity assurance homolog) family
members are highly conserved from yeasts to mammals. Homo
sapiens longevity assurance homolog 2 of yeast LAG1 (LASS2),
also known as tumor metastasis suppressor gene 1 (TMSG1, GenBank
accession number AF189062), is a gene isolated from a human liver
cDNA library by the laboratory of Shanghai Medical College, Fudan
University (Shanghai, China), and it is a human homolog of the
yeast (Saccharomyces cerevisiae) longevity assurance gene,
LAG1. LASS2 has been observed to correlate with the degree of
invasion and recurrence in carcinomas of the prostate (15,16),
liver (17) and breast (18). However, there has been no report on
the expression of LASS2 in human bladder cancer cell lines.
In our previous study, we demonstrated that
LASS2-negative bladder cancer was associated with poor clinical
prognosis. The expression of LASS2 mRNA was significantly
correlated with clinical stage (P<0.001), depth of tumor
invasion (P<0.001) and recurrence (P<0.001) (19).
In order to fully understand the biological
importance of LASS2, we examined the mRNA and protein expression of
LASS2 in human bladder cancer cell lines (BIU-87, T24, EJ and
EJ-M3) with diverse proliferation and invasion potential, and
analyzed the potential role of the tumor metastasis supressor gene
LASS2 in these human bladder cancer cell lines.
Materials and methods
Cell lines and cell culture
The EJ, T24 and BIU-87 human bladder cancer cell
lines were preserved by our department (Department of Urology, the
Second Affiliated Hospital of Kunming Medical University, Yunnan
Institute of Urology, China). The highly invasive human bladder
carcinoma EJ-M3 cell line was established in previous studies
(20,21). The BIU-87, T24, EJ and EJ-M3 cell
lines were cultured in DMEM supplemented with 10% fetal bovine
serum and incubated in 5% CO2/95% air at 37°C.
Cell proliferation assay
The growth of the BIU-87, T24, EJ and EJ-m3 cells
was evaluated by a cell proliferation assay. Briefly, cells
(1×104) were plated in seven 24-well culture plates
after the cell count. The cells were harvested on days 1, 2, 3, 4,
5, 6 and 7 for cell counting experiments, and the values were
normalized to untreated controls. This experiment was repeated
three times. We then created growth curves and compared the
proliferation ability among the four cell lines.
Matrigel invasion assay
The assay was carried out according to the method of
Girnita et al(22). The
invasive ability of BIU-87, T24, EJ and EJ-M3 cells was evaluated
using a Matrigel invasion assay. BD BioCoat Matrigel Invasion
Chambers with 8-mm pore size PET membranes (BD Biosciences, San
Jose, CA, USA) for 24-well plates were prepared by hydrating for 2
h at 37°C. A total of 2×105 cells in 0.2 ml were seeded
into each insert. After culturing for 12 h, the invasion chamber
was removed and the medium in the top wells was aspirated and cells
on the upper surface of the membranes were removed with cotton
swabs. The invading cells which remained attached to the lower side
of the membrane were removed by flushing with a pipette before
migrating cells present in the bottom chambers were labeled. The
fluorescence intensities were plotted on a standard histogram and
the number of invading cells was calculated. All experiments were
performed in triplicate. Data were expressed as the number of
invaded cells.
Total RNA isolation and real-time
quantitative PCR (qPCR)
According to the manufacturer’s instructions, total
RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA).
First-strand cDNA was synthesized in a volume of 20 μl using
1 μg total RNA and TaqMan reverse transcription reagents
(Applied Biosystems, Foster City, CA, USA). The target gene
sequences were obtained from the National Center for Biotechnology
Information GenBank databases. The purity of RNA samples was
determined by the OD260/OD280 (between 1.7
and 2.0) and the OD260/OD230 (>1.7) values
and by analysis of the ribosomal RNA band integrity by conventional
denaturing agarose RNA electrophoresis (23). PCR was performed with an ABI PRISM
7000 (Applied Biosystems) and 2X qPCR MasterMix (Eurogentec,
Seraing, Belgium) according to the manufacturer’s instructions. To
quantify target mRNA levels, LASS1, LASS2 and LASS3 Genes
Expression assays were purchased from Applied Biosystems. The
primer sequences were as follows: LASS1 forward
5′-CACACACATCTTTCGGCCC-3′, and reverse 5′-ACCTGGCAGCATCTCTAGGC-3′;
LASS2 forward 5′-TCTCCTGGTTTGCCAATTACG-3′, reverse
5′-CCGGGCAGGGACCCTCATCA-3′; LASS3 forward 5′- GAGCGCCAGGT TGA A
AGATG-3′, and reverse 5′-GGAATTTCTGCAGCCTGCA-3′. All primers
spanned an intron to ensure discrimination between cDNA and genomic
DNA. The relative amount of specific mRNA was normalized to GAPDH
using the following primer sequences: forward
5′-GGTCTCCTCTGACTTCAACA-3′, and reverse 5′-GAGGGTCTCTCTCTTCCT-3′.
All PCR reactions were run in duplicate and were performed with 40
cycles. A dilution series was carried out for each gene and the 18S
ribosomal subunit was used as an internal control. qPCR analysis
was carried out using the 2−ΔΔCt method
(24).
Western blot analysis
Western blots of whole-cell lysates from a known
number of cells were prepared. The whole-cell lysates were made by
lysing cells in the buffer. Protein (50 mg) was separated by
SDS-PAGE gels and transferred to a membrane. LASS2 was detected in
100 μg of protein from whole cell extracts, according to the
procedure described by Baron et al(25) and Calogero et al(26), and actin was used as a loading
control. The primary and secondary antibodies were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). The protein was
visualized by enhanced chemoluminescence (ECL; Santa Cruz) and
normalized with respect to the actin content of each sample.
Statistical analysis
The results were expressed as mean ± the standard
error of the mean (SEM) of at least three separate experiments.
Statistical significance was assessed using a two-tailed unpaired
Student’s t-test. The correlation between gene expression and
potential causative variables, were evaluated with the Chi-square
test. P<0.05 was considered to indicate a statistically
significant result. Each statistical analysis was performed using
the SPSS 11.0 software for Windows (SPSS Inc., Chicago, IL,
USA).
Results
Growth curves
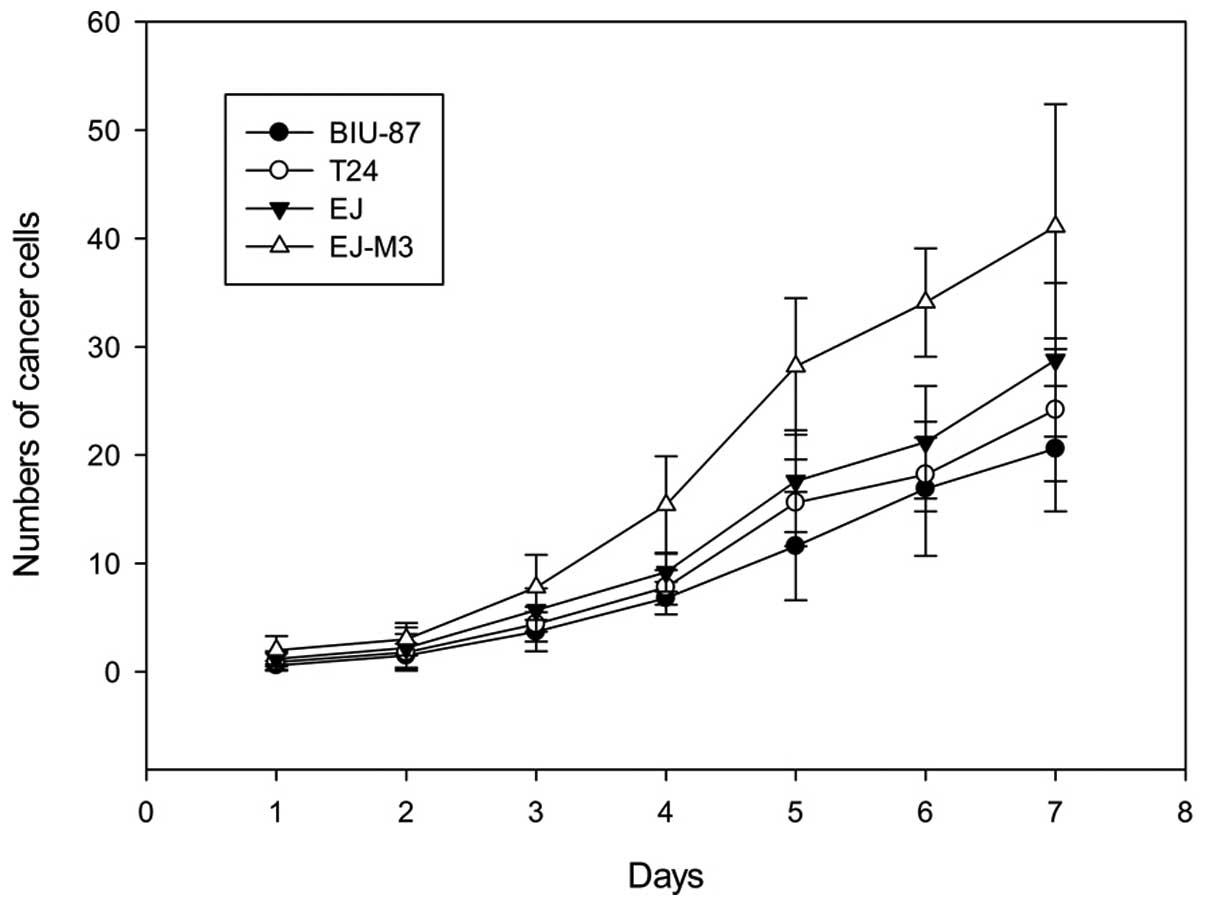
The population-doubling time of BIU-87, EJ, T24 and
EJ-M3 cell lines were 31.7±0.1, 27.6±0.2, 28.5h±0.1 and 20.8±0.2h,
respectively. The cell growth rate was significantly different
between EJ-M3 and the other three cell lines (P<0.05). There was
no significant difference between the growth rates of BIU-87 and
T24 (P>0.05). The growth curves are shown in Fig. 1.
In vitro invasion
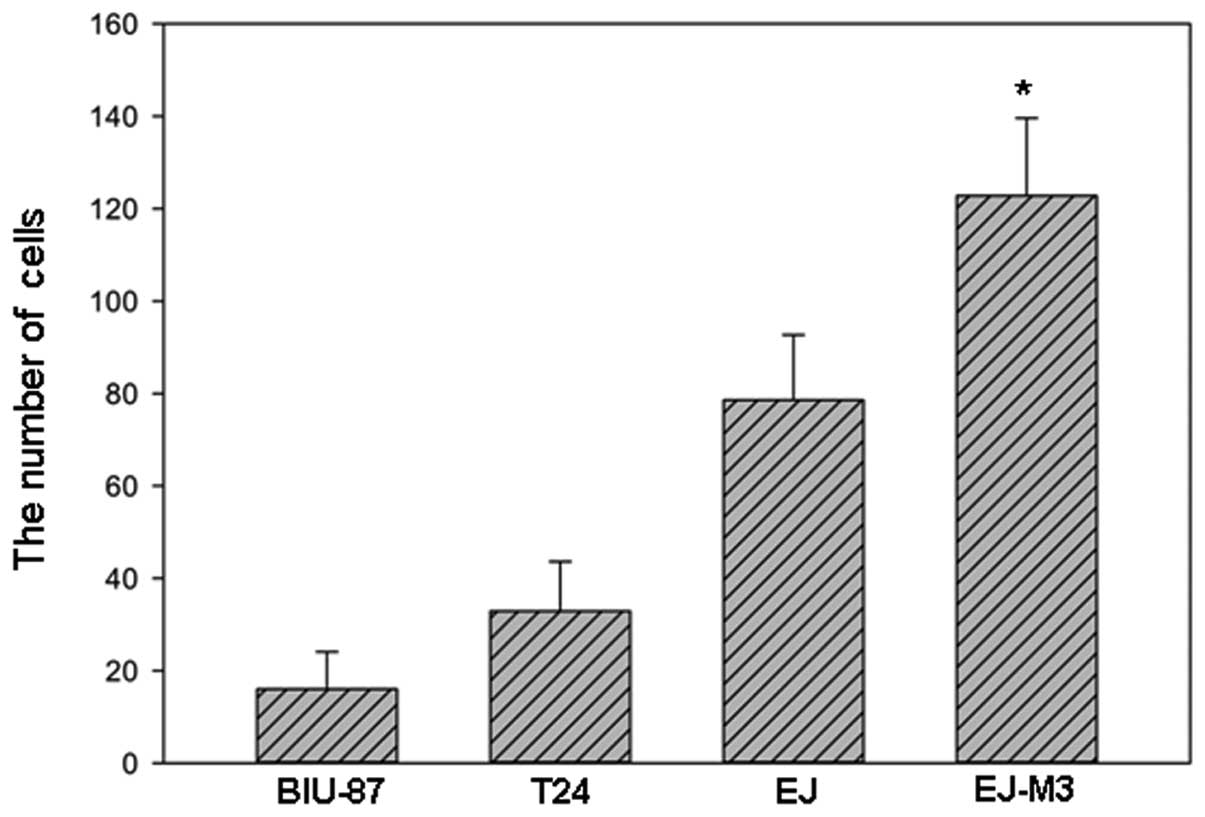
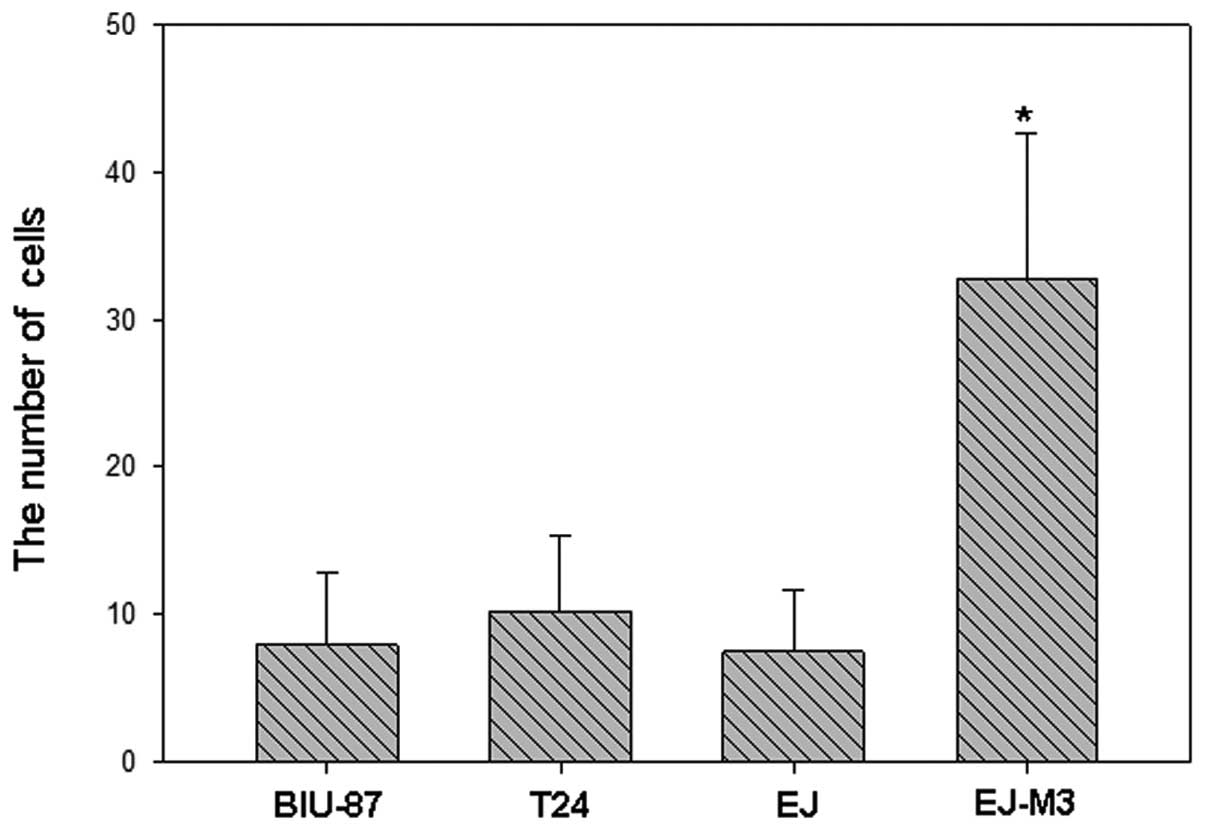
The numbers of EJ-M3, EJ, T24 and BIU-87 cells that
attached to the lower side of the membrane were 122.8±16.8,
78.6±14.1, 32.9±10.7 and 16.0±8.1, respectively. The numbers of
EJ-M3, EJ, T24 and BIU-87 cells that invaded into the 24-well plate
were 32.8±9.8, 7.5±4.2, 10.2±5.1 and 8.0±4.9, respectively. Using
the method of analysis of variance (ANOVA), we demonstrated that
there was no statistic difference of invasiveness between EJ, T24
and BIU-87. However, the invasiveness of EJ-M3 was significantly
different from that of EJ, T24 and BIU-87 (P<0.001) and are
shown in Fig.s 2–4.
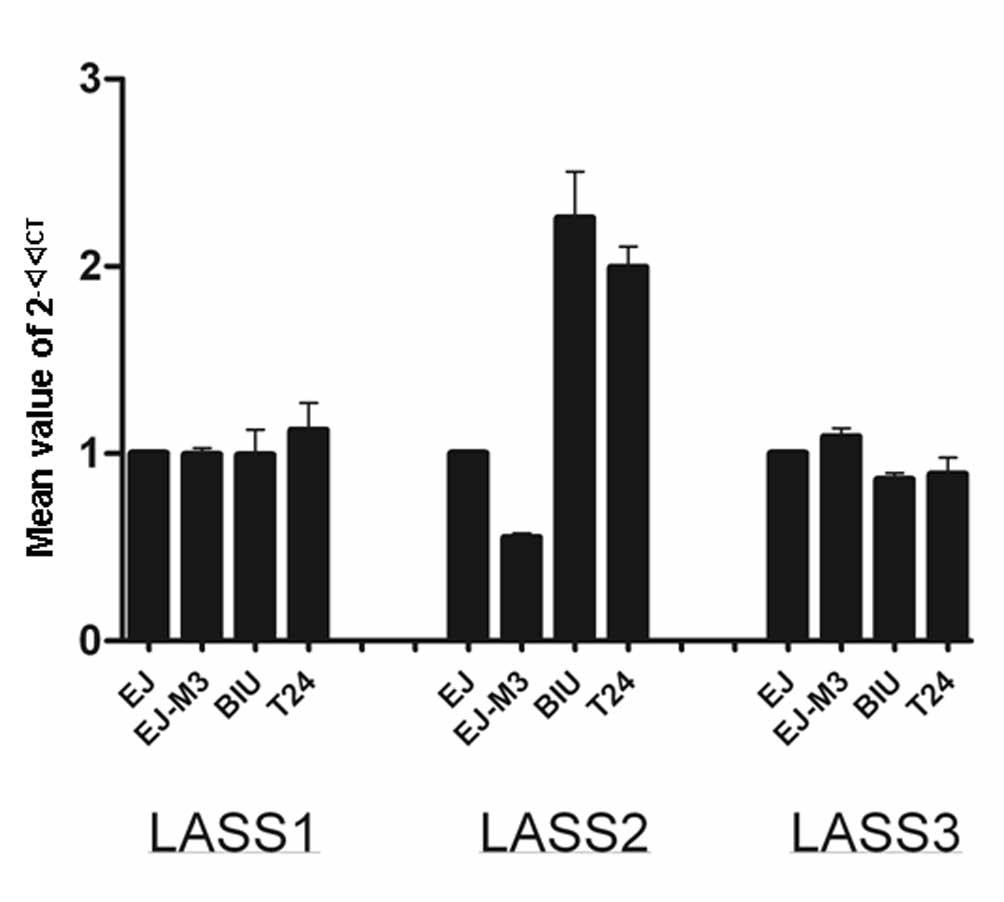
qPCR analysis of LASS1, LASS2 and LASS3
mRNA expression in the EJ-M3, EJ, T24 and BIU-87 cell lines
Prior to the quantitative analysis, optimization
procedures were carried out for qPCR reactions on EJ-M3, EJ, T24
and BIU-87 cell lines, using specific primers for human LASS2,
LASS2 and LASS3 genes, separately. Since LASS1, LASS2 and LASS3
have been reported to be expressed at the mRNA level in normal
human tissues, we used LASS2 expression as a control in qPCR
reactions. To optimize our quantitative technique, a cDNA
synthesized from the cell sample was used in serial dilution and a
PCR efficiency close to 100% was obtained. After optimization of
the qPCR, expression of LASS2, LASS2 and LASS3 genes were analyzed
using qPCR in the bladder carcinoma cell lines EJ-M3, EJ, T24 and
BIU-87. The result is shown in Fig.
5.
The differences among the four bladder carcinoma
cell lines were calculated using the ANOVA method. The differences
in LASS2 mRNA expression levels among the four cell lines were
statistically significant (P<0.001). LASS1 and LASS3 expression
levels among the four cell lines were not statistically significant
(P>0.05).
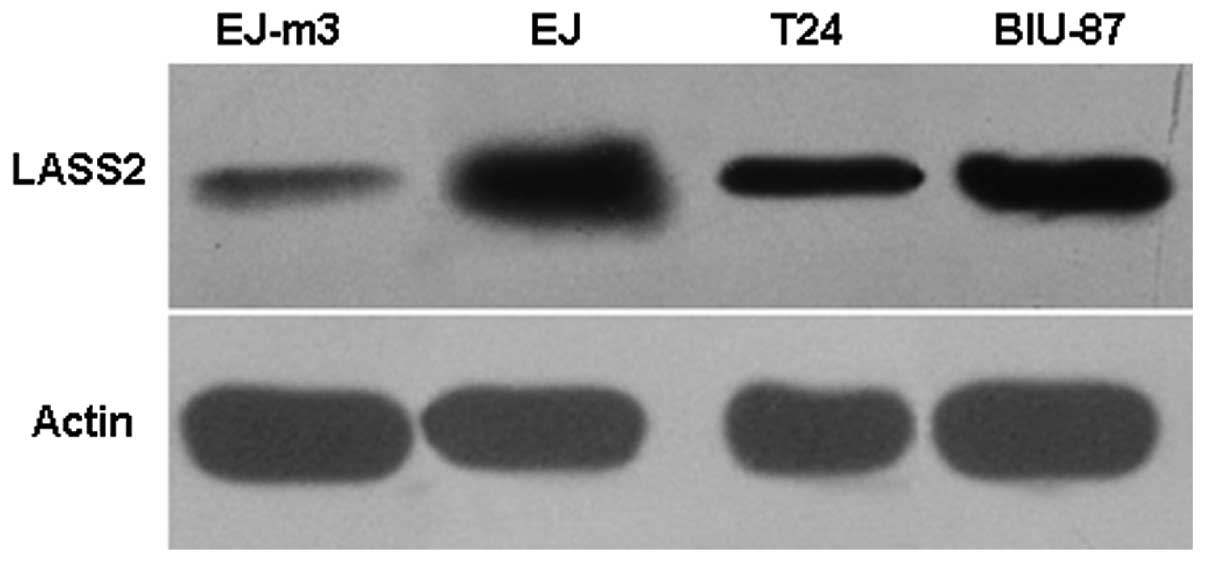
Western blot analysis of LASS2 protein
expression in the EJ-M3, EJ, T24 and BIU-87 cell lines
The expression of LASS2 protein was observed in the
four bladder carcinoma cell lines, EJ-M3, EJ, T24 and BIU-87. The
highest expression level of LASS2 was detected in the human bladder
carcinoma cell line EJ-M3 by western blot analysis. Moderate LASS2
protein expression was observed in EJ and T24 cell lines, and weak
LASS2 expression was observed in the BIU-87 cell line by western
blotting. The result is shown in Fig.
6.
Discussion
The present study identified the presence of LASS2
mRNA and protein in four bladder carcinoma cell lines. The
expression levels of LASS2 among these human bladder cancer cell
lines, which have diverse reproductive activity and invasive
abilities, are distinct at the mRNA and protein level.
It is well known that an imbalance of cellular
growth regulation results from certain genetic changes in the
oncogenic process, which lead to uncontrolled tumor growth.
However, unrestrained proliferation does not, by itself, result in
invasion and metastasis. Certain additional genetic changes are
required for tumor cells to be able to invade and metastasize
(27). A tumor metastasis
suppressor gene that is involved in tumor cell metastasis has been
confirmed (28).
LASS2 has previously been demonstrated to function
as a tumor metastasis suppressor gene. Previous studies have shown
that LASS2 gene plays a key role in carcinomas of the prostate
(15,16), liver (17) and breast (18).Chen et al(17) observed that transfection of LASS2
by lipofectamine inhibited the invasion and metastasis of a highly
metastatic liver cancer cell line, HCCLM3. Su et al(15,16)
demonstrated that overexpression of TMSG-1 (LASS2) inhibits the
proliferation, anchorage-independent growth and invasion of a
highly metastatic prostate cell line, PE-3M-1E8. We observed that
LASS2-negative bladder cancer was associated with poor clinical
prognosis and the expression of LASS2 was significantly correlated
with clinical stage, depth of tumor invasion and recurrence in our
preliminary studies (19).
This is the first study investigating the expression
of LASS2 in human bladder carcinoma cell lines. In this study, we
examined the protein and mRNA expression of LASS2 in four bladder
carcinoma cell lines using the methods of qPCR and western
blotting.
We also demonstrated that LASS2 expression
correlated with the biological characteristics of these human
bladder carcinoma cell lines. The results suggest that LASS2
expression is downregulated in cell lines with a high degree of
malignancy and that the more malignant tumor cells expressed lower
amounts of LASS2 at the protein and mRNA levels. This study
supports the role of LASS2 as a metastasis suppressor gene and its
potential utility as a clinical prognostic marker in human bladder
carcinoma.
The results of qPCR are coincidental to the results
of western blotting. LASS2 may be a useful indicator of tumor
invasion and progression. However, due to the increasing
appreciation of the importance of LASS2 in human bladder carcinoma,
as well as other cancers, future studies focusing on how LASS2 is
regulated and the mechanisms of its anti-metastatic functions, with
the goal of critically examining the possibilities of exploiting
LASS2 as a therapeutic target, are required.
In conclusion, using qPCR and western blotting, we
have shown that LASS2 expression may be correlated with the
development and progression of human bladder cancer and may be a
prognostic indicator for this cancer.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. 81260374) and
Doctor Innovation Fund of Kunming Medical University (No. 2012D03).
The authors would like to thank Professor Hongyi Xu and Professor
Yongtang Zheng for their assistance in preparing this
manuscript.
References
|
1.
|
Morgan TM, Keegan KA and Clark PE: Bladder
cancer. Curr Opin Oncol. 23:275–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Valastyan S and Weinberg RA: Tumor
metastasis: molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Saffar H, Sanii S, Heshmat R, et al:
Expression of galectin-3, nm-23, and cyclooxygenase-2 could
potentially discriminate between benign and malignant
pheochromocytoma. Am J Clin Pathol. 135:454–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Kim SH, Lee SY, Park HR, et al: Nuclear
localization of Nm23-H1 in head and neck squamous cell carcinoma is
associated with radiation resistance. Cancer. 117:1864–1873. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Polanski R, Maguire M, Nield PC, et al:
MDM2 interacts with NME2 (non-metastatic cells 2, protein), and
suppresses the ability of NME2 to negatively regulate cell
motility. Carcinogenesis. 32:1133–1142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jiang WX, Song BG and Wang PJ: Expression
of nm23, KAI1 and spiral computed tomography findings in primary
gallbladder carcinoma. Chin Med J (Engl). 122:2666–2668.
2009.PubMed/NCBI
|
|
7.
|
Boissan M and Lacombe ML: NM23, an example
of a metastasis suppressor gene. Bull Cancer. 99:431–440. 2012.(In
French).
|
|
8.
|
Cao GL, Chu MX, Fang L, et al: Analysis on
DNA sequence of KiSS-1 gene and its association with litter size in
goats. Mol Biol Rep. 37:3921–3929. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hiney JK, Srivastava VK and Les Dees W:
Insulin-like growth factor-1 stimulation of hypothalamic KiSS-1
gene expression is mediated by Akt: effect of alcohol.
Neuroscience. 166:625–632. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lee KH and Kim JR: Kiss-1 suppresses MMP-9
expression by activating p38 MAP kinase in human stomach cancer.
Oncol Res. 18:107–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Shoji I, Hirose T, Mori N, et al:
Expression of kisspeptins and kisspeptin receptor in the kidney of
chronic renal failure rats. Peptides. 31:1920–1925. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Moon HG, Jeong SH, Ju YT, et al:
Up-regulation of RhoGDI2 in human breast cancer and its prognostic
implications. Cancer Res Treat. 42:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zheng Z, Li J, He X, et al: Involvement of
RhoGDI2 in the resistance of colon cancer cells to 5-fluorouracil.
Hepatogastroenterology. 57:1106–1112. 2010.PubMed/NCBI
|
|
14.
|
Niu H, Li H, Xu C and He P: Expression
profile of RhoGDI2 in lung cancers and role of RhoGDI2 in lung
cancer metastasis. Oncol Rep. 24:465–471. 2010.PubMed/NCBI
|
|
15.
|
Su J, You JF, Wang JL, et al:
Overexpression of tumor metastasis suppressor gene 1 suppresses
proliferation and invasion, but enhances apoptosis of human breast
cancer cells MDA-MB-231 cells. Zhonghua Bing Li Xue Za Zhi.
36:672–676. 2007.(In Chinese).
|
|
16.
|
Su J, You JF, Wang JL, et al:
Overexpression of human tumor metastasis-related gene TMSG-1
suppresses cell proliferation and invasion of a highly metastatic
prostate cancer cell line PC-3M-1E8 in vitro. Zhonghua Zhong Liu Za
Zhi. 30:404–407. 2008.(In Chinese).
|
|
17.
|
Chen SH, Bubb MR, Yarmola EG, et al:
Vacuolar H+-ATPase binding to microfilaments: regulation
in response to phosphatidylinositol 3-kinase activity and detailed
characterization of the actin-binding site in subunit B. J Biol
Chem. 279:7988–7998. 2004.PubMed/NCBI
|
|
18.
|
Schiffmann S, Sandner J, Birod K, et al:
Ceramide synthases and ceramide levels are increased in breast
cancer tissue. Carcinogenesis. 30:745–752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Wang H, Wang J, Zuo Y, et al: Expression
and prognostic significance of a new tumor metastasis suppressor
gene LASS2 in human bladder carcinoma. Med Oncol. 29:1921–1927.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Wang H, Yang D, Wang K and Wang J:
Expression and potential role of chemokine receptor CXCR4 in human
bladder carcinoma cell lines with different metastatic ability. Mol
Med Rep. 4:525–528. 2011.PubMed/NCBI
|
|
21.
|
Yang D, Wang H, Wang J, et al:
Establishment of a fluorescent implantation metastasis model of
bladder cancer and real-time microscopic detection in nude mice.
Asian Pac J Cancer Prev. 12:393–396. 2011.PubMed/NCBI
|
|
22.
|
Girnita A, All-Ericsson C, Economou MA, et
al: The insulin-like growth factor-I receptor inhibitor
picropodophyllin causes tumor regression and attenuates mechanisms
involved in invasion of uveal melanoma cells. Clin Cancer Res.
12:1383–1391. 2006. View Article : Google Scholar
|
|
23.
|
Masek T, Vopalensky V, Suchomelova P and
Pospisek M: Denaturing RNA electrophoresis in TAE agarose gels.
Anal Biochem. 336:46–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Baron V, Adamson ED, Calogero A, et al:
The transcription factor Egr1 is a direct regulator of multiple
tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin.
Cancer Gene Ther. 13:115–124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Calogero A, Arcella A, De Gregorio G, et
al: The early growth response gene EGR-1 behaves as a suppressor
gene that is down-regulated independent of ARF/Mdm2 but not p53
alterations in fresh human gliomas. Clin Cancer Res. 7:2788–2796.
2001.PubMed/NCBI
|
|
27.
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: an imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ichikawa T, Ichikawa Y and Isaacs JT:
Genetic factors and meta-static potential of prostatic cancer.
Cancer Surv. 11:35–42. 1991.
|