Introduction
Propyl gallate (PG), an ester that is also known as
propyl 3,4,5-trihydroxybenzoate and belongs to the polyphenolic
compound family, is synthesized by the condensation of propanol and
gallic acid. PG is used in foods, cosmetics, hair products,
adhesives and lubricants due to its antioxidative properties, which
protect foods containing oils and fats from oxidation by hydrogen
peroxide and free oxygen radicals.
The formation, metabolism, catabolism, and
physiological and pathophysiological roles of prostanoids are
associated with the development of inflammation and carcinogenesis
(1). Cyclooxygenase-2 (COX-2) is
an enzyme that is critical for prostanoid synthesis (2). This process is initiated by the
enzymatic release of arachidonic acid (AA) from cellular stores by
phospholipase A2 (3). AA is then
metabolized to prostaglandins via the COX-2 pathway through a
series of enzymatic steps (4).
COX-2 is inducible and may be associated with one or more
pathophysiological states or reactions (5). Since a number of the metabolic steps
in this pathway generate reactive oxygen species (6), it is important to develop
COX-2-specific agents that are effective anti-inflammatory drugs or
have cancer preventive activity.
The discovery of the molecular associations between
inflammation and cancer was a major breakthrough in chemoprevention
research. Components of the cell signaling network have been
implicated in the promotional stage of carcinogenesis, particularly
those that converge on the redox-sensitive transcription factor
nuclear factor-κB (NF-κB), which is involved in mediating the
inflammatory response (7,8). One of the major target molecules
subjected to NF-κB-driven transactivation is COX-2, which is
involved in the biosynthesis of prostaglandins and inflammation
(9). Inappropriate upregulation of
COX-2 has frequently been observed in various premalignant and
malignant tissues (10). The role
of abnormally high levels of COX-2 in tumorigenesis has further
been corroborated by the increased susceptibility of
COX-2-overexpressing mice (11)
and the relatively increased resistance of COX-2-knockout animals
to spontaneous or experimentally induced carcinogenesis (12). Therefore, targeted inhibition of
COX-2 is regarded as a promising and practical approach to prevent
cancer (13).
NF-κB generally exists as a heterodimer of the p50
and RelA (p65) polypeptides, bound in an inactive state in the
cytoplasm by the inhibitor protein IκB (14). Following cellular stimulation by a
variety of agents, IκB is phosphorylated and degraded by the
proteasome, allowing NF-κB to translocate to the nucleus and
regulate the expression of the target genes, including a number
that control cellular growth properties and apoptotic cell death
(15,16). The transactivation of
NF-κB-regulated genes requires not only the binding of NF-κB to the
promoter regions, but also the phosphorylation of p65/RelA, which
is the active subunit of NF-κB. Topical applications of
12-O-tetradecanoylphorbol-13-acetate (TPA) have been shown to cause
an increase in p65/RelA phosphorylation at serine 536 (17).
PG is an antioxidant which affects and/or inhibits
the inflammatory pathway. Although it appears that the inhibition
of TPA-induced COX-2 by PG occurs through the NF-κB pathway, the
details of the mechanism remain to be elucidated. In the present
study, TPA-induced COX-2 was used in human THP-1 cells as an
inflammation model to evaluate the effect of PG on COX-2 expression
and prostaglandin E2 (PGE2) production. The effect of PG on the
NF-κB pathway was also determined.
Materials and methods
Cell line and reagents
The human THP-1 cell line was obtained from the
Bioresource Collection and Research Center (Hsinchu, Taiwan,
R.O.C.). Roswell Park Memorial Institute-1640 (RPMI-1640) and fetal
bovine serum (FBS) were obtained from Hyclone (South Logan, UT,
USA). Anti-COX-2, anti-NF-κB, anti-IκB, anti-IKKα and anti-β-actin
primary antibodies and a secondary antibody labeled with
horseradish-peroxidase were purchased from Santa Cruz Lab Vision
(Santa Cruz, CA, USA). Anti-phospho-IKKα (Ser180)/IKKβ (Ser181),
anti-p65/RelA and anti-phospho-p65 (Ser536) primary antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
PG, TPA and other chemicals were purchased from Sigma Chemical Co.
(St. Louis, MO, USA) and the PGE2 enzyme immunoassay kit was
purchased from Stressgen (Ann Arbor, MI, USA).
Cell culture and treatment
The basal medium for the THP-1 cell line culture was
composed of RPMI-1640 supplemented with 10% FBS, 100 U/ml
penicillin G and 100 μg/ml streptomycin. The PG stock
solution (100 mM) was dissolved in dimethylsulfoxide (DMSO) and
various concentrations were prepared in the basal medium with a
final DMSO concentration of 0.1%, which was considered to cause
little or no damage in the THP-1 cells.
PGE2 assay
A PGE2 EIA kit from Stressgen was used to measure
the PGE2 secreted in conditioned cell culture media of
serum-starved cells for 24 h with and without TPA or PG, according
to the manufacturer’s instructions.
Western blot analysis
Following treatment, cells were washed with
phosphate-buffered saline (PBS), resuspended in a protein
extraction buffer for 10 min, then centrifuged at 12,000 rpm for 10
min at 4°C to obtain the total extracted proteins. The protein
concentrations were evaluated with a Bio-Rad protein assay reagent
(Bio-Rad, Richmond, CA, USA). The expression levels of various
intracellular proteins were then evaluated by western blot
analyses. Briefly, the total extracted protein content was boiled
in a loading buffer and an aliquot corresponding to 50 or 100
μg protein was separated by 12% SDS-polyacrylamide gel.
Following electrophoresis, proteins were electrotransferred onto a
polyvinylidene fluoride transfer membrane. After blotting, the
membranes were incubated with various primary antibodies overnight
at 4°C and then washed with PBST solution (0.05% Tween-20 in PBS).
Following washing, the secondary antibody, labeled with
horseradish-peroxidase, was incubated for 1 h at room temperature
and then washed with the PBST solution. The antigen-antibody
complexes were detected by enhanced chemiluminescence using a
chemiluminescence analyzer (Amersham Pharmacia Biotech, Piscataway,
NJ, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation from at least 3 independent experiments and were analyzed
using Student’s t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
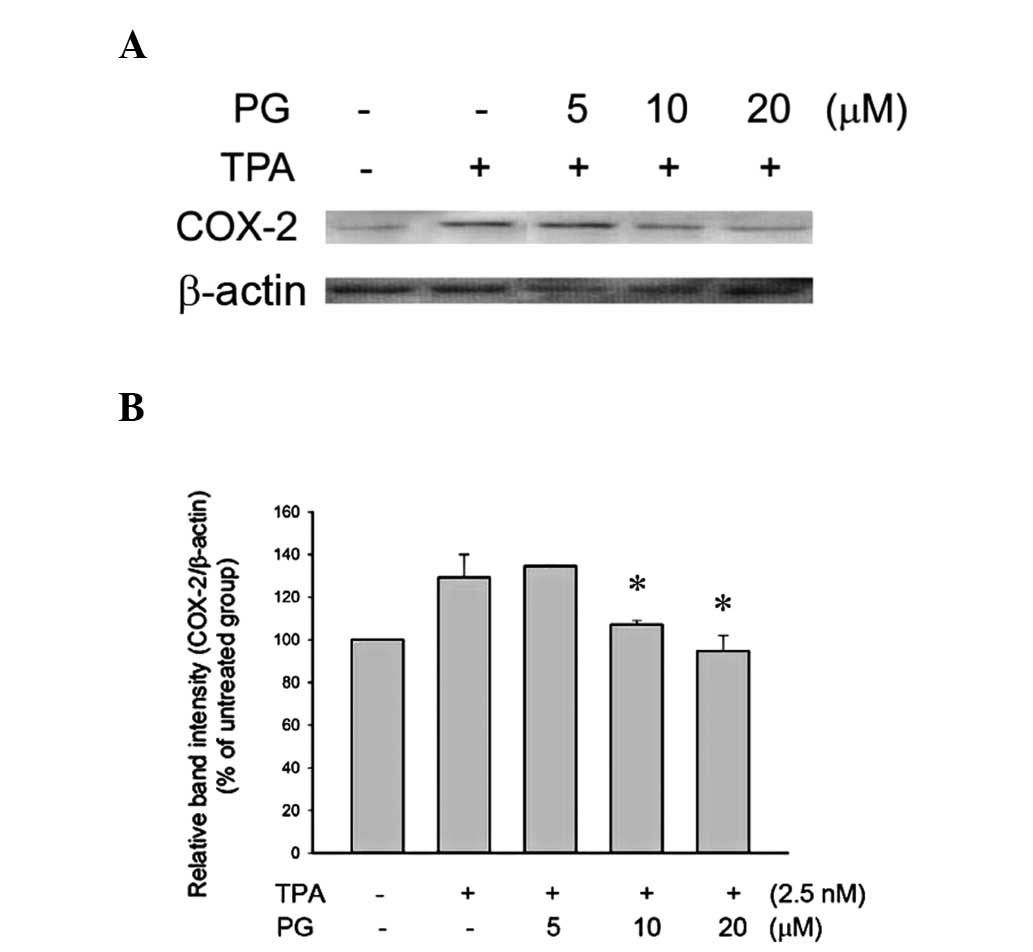
PG inhibited TPA-induced COX-2 in THP-1
cells
TPA is an inflammatory agent that targets
intracellular protein kinase C (PKC) and induces inflammation via
the activation of the NF-κB pathway. In order to evaluate the
anti-inflammatory activity of PG, TPA was used to induce
inflammation in THP-1 cells. Treatment with 2.5 nM TPA for 24 h
increased COX-2 expression levels in the THP-1 cells. Cells that
were pretreated with PG (10 or 20 μM) for 2 h before the TPA
treatment showed significantly inhibited COX-2 expression (Fig. 1).
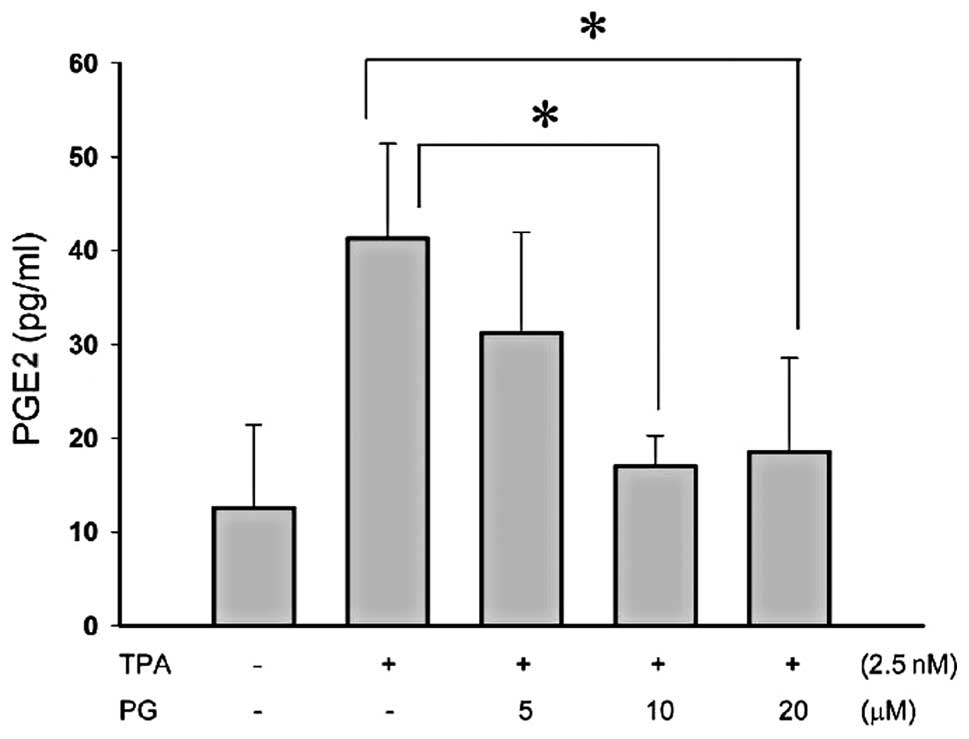
PG inhibited TPA-induced PGE2 in THP-1
cells
PGE2 is an extensively studied prostaglandin owing
to its predominance in inflammation. PGE2 has been of great
interest as a therapeutic target, for example, through the
modulation of its synthesis by COX inhibitors. To evaluate whether
PG suppresses PGE2 production in human monocytes treated with
inflammatory agents, human THP-1 monocyte cell lines were
pretreated with PG (5–20 μM) for 2 h and then exposed to 2.5
nM TPA for 48 h. Using a PGE2 ELISA kit, it was demonstrated that
pretreatment with 10 or 20 μM PG significantly inhibited
PGE2 production (Fig. 2). These
results suggest that PG has anti-inflammatory activity.
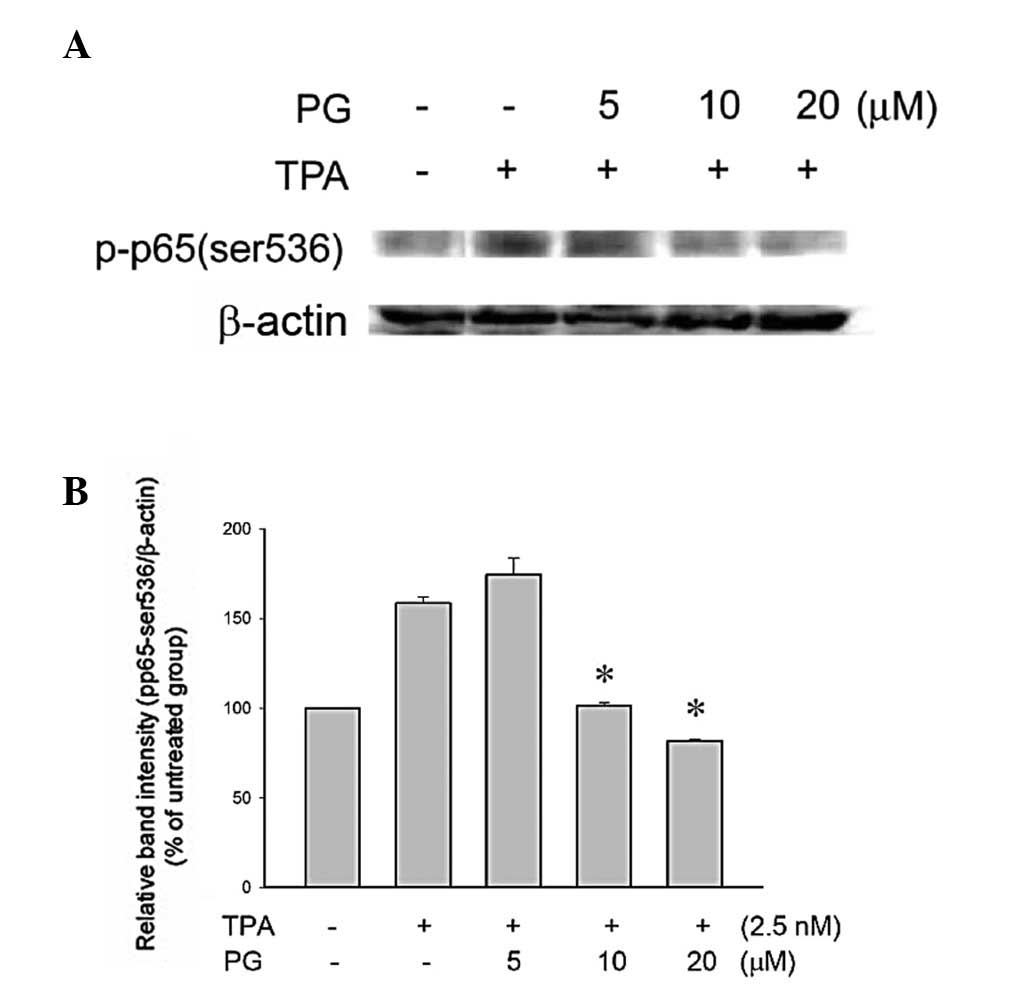
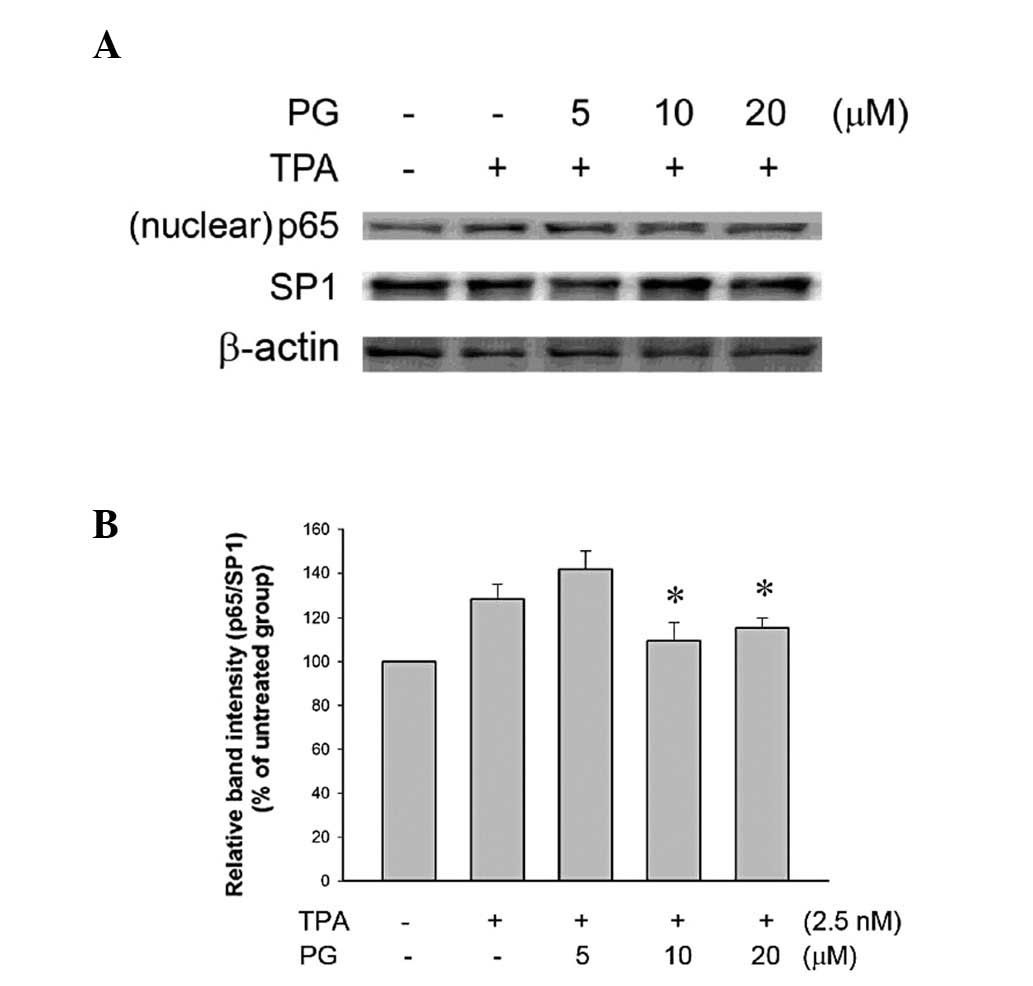
PG inhibited TPA-induced activation of
NF-κB and nuclear translocation in THP-1 cells
NF-κB, a component of the cell signaling network, is
a redox-sensitive transcription factor involved in the mediation of
the inflammatory response. One of the major target molecules
subjected to NF-κB-driven transactivation is COX-2, which is
involved in the biosynthesis of prostaglandins and inflammation
(9). Therefore, whether the
observed anti-inflammatory activity of PG occurred via NF-κB
signaling was investigated. The transactivation of NF-κB-regulated
genes requires the phosphorylation of p65/RelA (p-p65), the active
subunit of NF-κB. The cells were pretreated with PG (5, 10 or 20
μM) for 2 h followed by incubation with TPA (2.5 nM) for 2.5
h. The phosphorylation of p65/RelA (Ser536) was significantly
inhibited by treatment with 10 or 20 μM PG (Fig. 3). In addition, treatment with these
two concentrations of PG (10 and 20 μM) significantly
inhibited the nuclear translocation of p65 (Fig. 4), indicating that the
anti-inflammatory activity of PG occurred via the inhibition of the
NF-κB signaling pathway.
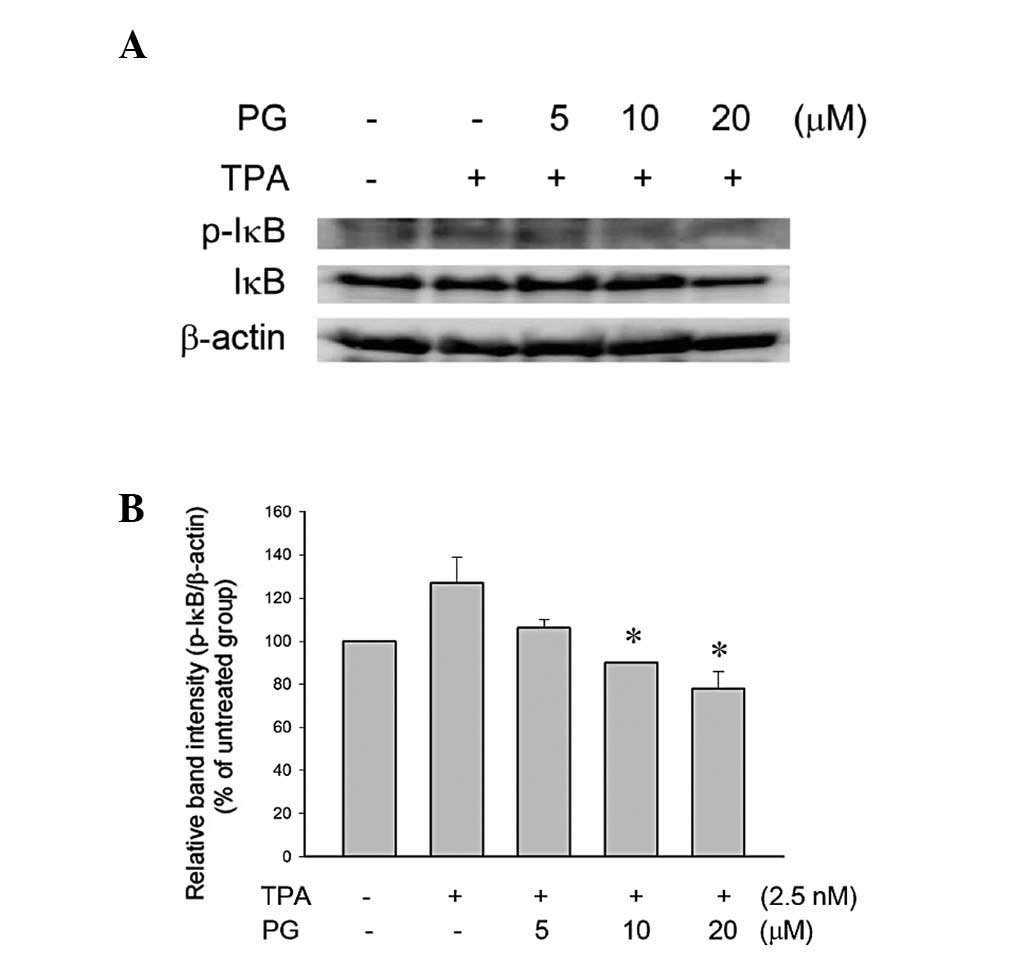
PG inhibited TPA-induced phosphorylation
of IκB in THP-1 cells
NF-κB is bound in an inactive state in the cytoplasm
by the inhibitor protein IκB (14). Following cellular stimulation by a
variety of agents, IκB is phosphorylated and then degraded by the
proteasome, allowing NF-κB to translocate to the nucleus and
regulate the expression of the target genes. The phosphorylation of
IκB was increased by the TPA treatment (Fig. 5), indicating that TPA activates
NF-κB signaling via the phosphorylation of IκB. The phosphorylation
of IκB was significantly inhibited by treatment with 10 or 20
μM PG (Fig. 5).
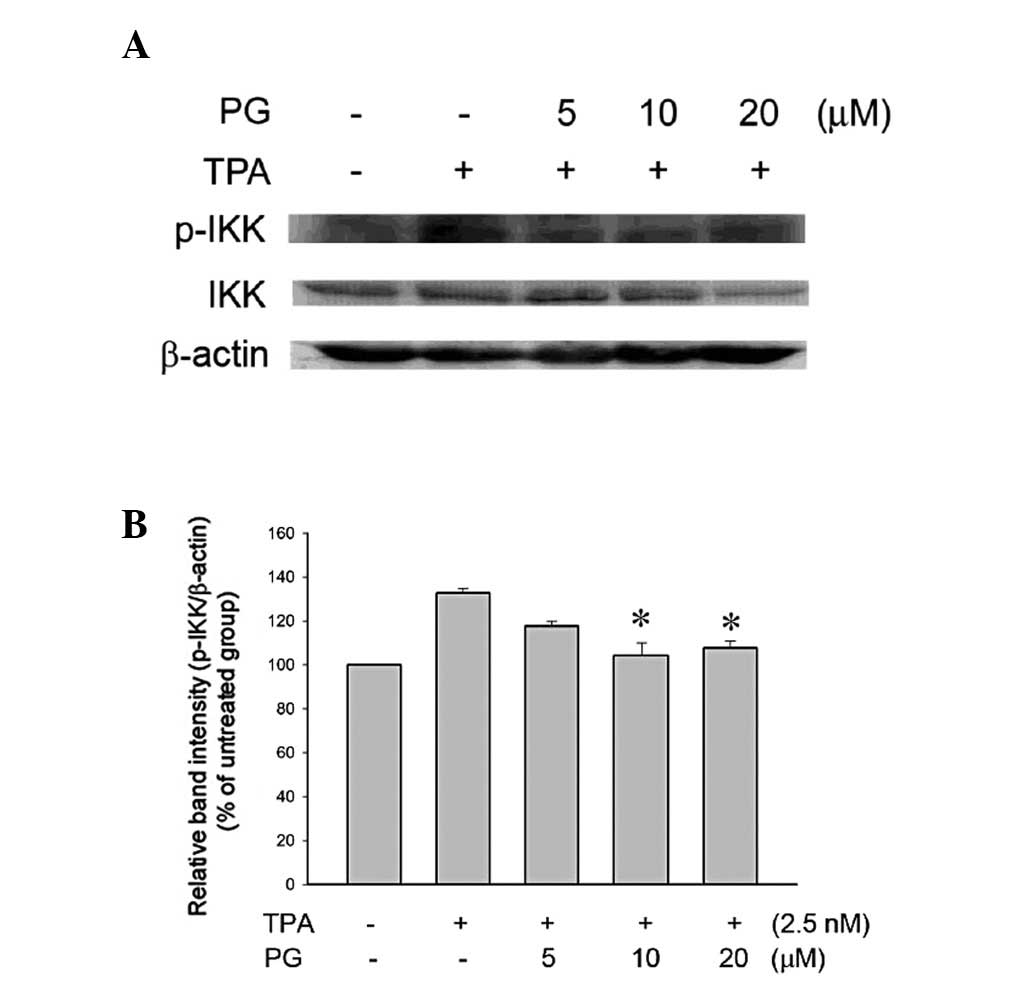
PG inhibited TPA-induced phosphorylation
of IκB kinase (IKK) in THP-1 cells
IKK is able to phosphorylate IκB and cause its
degradation by ubiquitination. It was further evaluated whether PG
was able to inhibit the IKK activity in TPA-treated THP-1 cells. As
shown in Fig. 6, the PG treatment
(10 or 20 μM) significantly inhibited the phosphorylation of
IKK. The expression of IKK was also inhibited by treatment with 20
μM PG. These results indicated that PG inhibits the
upregulated NF-κB signaling pathway, including IKK and IκB.
Discussion
In anti-inflammatory studies, three general
categories of inflammatory inducers are considered including, i)
gram-negative bacterial cell wall lipopolysaccharide (LPS); ii)
proinflammatory cytokines such as tumor necrosis factors (TNF-α);
and iii) the tumor promoter agent TPA. With LPS, the cells are able
to simulate pathogenic infections caused by inflammation. TNF-α
directly binds to specific receptors (TNFR1 and TNFR2), which then
activate the NF-κB signaling pathway to induce inflammation. TPA
penetrates intracellularly and activates PKC and thus, the NF-κB
signaling pathway is activated to induce inflammation. Compared
with LPS, TPA is chemically more stable, less variable and requires
a lower concentration to induce inflammation. Furthermore, unlike
TNF-α, TPA does not induce caspase activation. Therefore, in the
present study, TPA was selected as the inflammation inducer.
Numerous antioxidants extracted from food show
excellent anti-inflammatory effects. For example, piperine, a major
component of black (Piper nigrum Linn) and long (Piper
longum Linn) pepper, exhibits antioxidant activity. The
dose-dependent decrease of phorbol 12-myristate 13-acetate
(PMA)-induced COX-2 expression and PGE2 production in murine RAW
264.7 macrophages by piperine was identified to be partially due to
the inhibition of PMA-induced NF-κB nuclear translocation (18). The dietary flavonoid quercetin is
an antioxidant that possesses anti-inflammatory properties.
Quercetin protects cells against TNF-α-induced activation of the
NF-κB signaling pathway (19).
This inhibitory effect of quercetin was mediated, at least in part,
by extracellular regulated kinase, c-jun amino-terminal kinase and
reactive oxygen species and accompanied by reduced COX-2 levels
(19).
It is notable that tannin and phenolic compounds
extracted from tomatoes express excellent inhibition effects in
TPA-induced COX-2 expression in KB cells (20). These results are in agreement with
those of the present study showing that the anti-inflammatory
effect of PG occurs via the NF-κB signaling pathway and COX-2
inhibition in TPA-treated THP-1 cells. PG belongs to the
polyphenolic compound family and is synthesized by the condensation
of propanol and gallic acid. In general, PG has been proposed to
act as an antioxidant that protects foods against oxidation by
hydrogen peroxide and oxygen free radicals. It is used in foods,
cosmetics, hair products, adhesives and lubricants. Our present
study shows that apart from its antioxidant activity, PG also
demonstrates anti-inflammatory activity.
A number of studies have reported that gallic acid
(3,4,5-trihydroxybenzoic acid), a natural polyphenol obtained from
gallnuts and green tea, has antioxidant, anti-inflammatory and
radical-scavenging activities. In human mast cells, gallic acid
decreased the PMA plus calcium ionophore A23187-stimulated gene
expression and production of proinflammatory cytokines such as
TNF-α and inteleukin (IL)-6. The inhibitory effect of gallic acid
on the proinflammatory cytokines was demonstrated to be dependent
on NF-κB and p38 mitogen-activated protein kinase (21). Our previous study demonstrated that
the antioxidant activity of PG is higher than that of gallic acid
in THP-1 cells treated with various reactive oxygen species
(22). Another study has
demonstrated that certain derivatives of gallic acid exhibit good
anti-inflammatory activity, as determined by the
carrageenan-induced paw edema test (23). These studies may provide an
explanation for the present results, wherein PG inhibits
TPA-induced inflammatory reactions in THP-1 cells by blocking
COX-2, PGE2 and IKK activity and NF-κB signaling and these effects
may partially be associated with its excellent antioxidant
activity.
Acknowledgements
This study was supported in part by
grants from the National Science Council (NSC
96-2320-B-415-002-MY3, C.H.C.) and Chang Gung Memorial Hospital,
R.O.C. (CMRPG6A0291, H.C.H.).
References
|
1.
|
Mutoh M, Takahashi M and Wakabayashi K:
Roles of prostanoids in colon carcinogenesis and their potential
targeting for cancer chemoprevention. Curr Pharm Des. 12:2375–2382.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Piston D, Wang S, Feng Y, et al: The role
of cyclooxygenase-2/prostanoid pathway in visceral pain induced
liver stress response in rats. Chin Med J (Engl). 120:1813–1819.
2007.PubMed/NCBI
|
|
3.
|
Weerasinghe GR, Coon SL, Bhattacharjee AK,
et al: Regional protein levels of cytosolic phospholipase A2 and
cyclooxygenase-2 in Rhesus monkey brain as a function of age. Brain
Res Bull. 69:614–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nagasaki S, Suzuki T, Miki Y, et al:
17Beta-hydroxysteroid dehydrogenase type 12 in human breast
carcinoma: a prognostic factor via potential regulation of fatty
acid synthesis. Cancer Res. 69:1392–1399. 2009. View Article : Google Scholar
|
|
5.
|
Branski RC, Zhou H, Sandulache VC, et al:
Cyclooxygenase-2 signaling in vocal fold fibroblasts. Laryngoscope.
120:1826–1831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Oláh O, Németh I, Tóth-Szüki V, et al:
Regional differences in the neuronal expression of cyclooxygenase-2
(COX-2) in the newborn pig brain. Acta Histochem Cytochem.
45:187–192. 2012.PubMed/NCBI
|
|
7.
|
Slattery ML, Lundgreen A, Bondurant KL and
Wolff RK: Interferon-signaling pathway: associations with colon and
rectal cancer risk and subsequent survival. Carcinogenesis.
32:1660–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Setia S and Sanyal SN: Nuclear factor
kappa B: a pro-inflammatory, transcription factor-mediated
signalling pathway in lung carcinogenesis and its inhibition by
nonsteroidal anti-inflammatory drugs. J Environ Pathol Toxicol
Oncol. 31:27–37. 2012. View Article : Google Scholar
|
|
9.
|
Liu D, Kim DH, Park JM, et al: Piceatannol
inhibits phorbol ester-induced NF-kappa B activation and COX-2
expression in cultured human mammary epithelial cells. Nutr Cancer.
61:855–863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Fernández-Martínez AB, Carmena MJ, Arenas
MI, et al: Overexpression of vasoactive intestinal peptide
receptors and cyclooxygenase-2 in human prostate cancer. Analysis
of potential prognostic relevance. Histol Histopathol.
27:1093–1101. 2012.
|
|
11.
|
Muller-Decker K, Neufang G, Berger I, et
al: Transgenic cyclooxygenase-2 overexpression sensitizes mouse
skin for carcinogenesis. Proc Natl Acad Sci USA. 99:12483–12488.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tiano HF, Loftin CD, Akunda J, et al:
Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters
epidermal differentiation and reduces mouse skin tumorigenesis.
Cancer Res. 62:3395–3401. 2002.PubMed/NCBI
|
|
13.
|
Cerella C, Sobolewski C, Chateauvieux S,
et al: COX-2 inhibitors block chemotherapeutic agent-induced
apoptosis prior to commitment in hematopoietic cancer cells.
Biochem Pharmacol. 82:1277–1290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gutierrez H, O’Keeffe GW, Gavaldà N, et
al: Nuclear factor kappa B signaling either stimulates or inhibits
neurite growth depending on the phosphorylation status of p65/RelA.
J Neurosci. 28:8246–8256. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Li X, Wu G, Wu M, et al: In vitro study of
inhibitory millimeter wave treatment effects on the TNF-α-induced
NF-κB signal transduction pathway. Int J Mol Med. 27:71–78.
2011.PubMed/NCBI
|
|
16.
|
Kole L, Giri B, Manna SK, et al:
Biochanin-A, an isoflavon, showed anti-proliferative and
anti-inflammatory activities through the inhibition of iNOS
expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB
nuclear translocation. Eur J Pharmacol. 653:8–15. 2011.PubMed/NCBI
|
|
17.
|
Kim SO, Kundu JK, Shin YK, et al:
[6]-Gingerol inhibits COX-2 expression by blocking the activation
of p38 MAP kinase and NF-kappaB in phorbol ester-stimulated mouse
skin. Oncogene. 24:2558–2567. 2005.
|
|
18.
|
Kim HG, Han EH, Jang WS, et al: Piperine
inhibits PMA-induced cyclooxygenase-2 expression through
downregulating NF-κB, C/EBP and AP-1 signaling pathways in murine
macrophages. Food Chem Toxicol. 50:2342–3428. 2012.PubMed/NCBI
|
|
19.
|
Granado-Serrano AB, Martín MÁ, Bravo L, et
al: Quercetin attenuates TNF-induced inflammation in hepatic cells
by inhibiting the NF-κB pathway. Nutr Cancer. 64:588–598.
2012.PubMed/NCBI
|
|
20.
|
Shen YC, Chen SL, Zhuang SR and Wang CK:
Contribution of tomato phenolics to suppression of COX-2 expression
in KB cells. J Food Sci. 73:C1–C10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kim SH, Jun CD, Suk K, et al: Gallic acid
inhibits histamine release and pro-inflammatory cytokine production
in mast cells. Toxicol Sci. 91:123–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Chen CH, Liu TZ, Chen CH, et al: The
efficacy of protective effects of tannic acid, gallic acid, ellagic
acid, and propyl gallate against hydrogen peroxide-induced
oxidative stress and DNA damage in IMR-90 cells. Mol Nutr Food Res.
51:962–968. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Arunkumar S, Ilango K, Manikandan RS and
Ramalakshmi N: Synthesis and anti-inflammatory activity of some
novel pyrazole derivatives of gallic acid. J Chem. 6(Suppl 1):
S123–S128. 2009.
|