Introduction
The efficacy of therapies for hepatocellular
carcinoma (HCC) is poor. Curative therapies, including resection,
liver transplantation or percutaneous treatments benefit only 30%
of patients (1). Even so, the
majority of surgically treated patients show recurrence within 5
years of resection and this is linked to the high mortality of
patients with resected HCC (2).
Patients with large and multiple lesions exceeding the Milan
criteria have been widely treated by transcatheter arterial
embolisation (TAE) due to its precisely targeted, minimally
invasive, repeatable and well-tolerated method. Although occlusion
of tumour-feeding arteries may lead to extensive necrosis in
vascularised HCC, hypoxia and ischemia of tumour tissue may produce
large quantities of factors capable of inducing significant
angiogenesis in the residual viable tumour, promoting recurrence
and metastasis and consequently counteracting the efficacy of TAE
(3,4).
Peri-procedural use of anti-angiogenic agents is
recommended in order to overcome the disadvantages of TAE. However,
the efficacy of those agents remains uncertain (5–7). The
upregulation of cyclooxygenase-2 (COX-2), a key enzyme in
arachidonic acid metabolism, is believed to be involved in
hepatocarcinogenesis (8,9) and induce HCC angiogenesis via
vascular endothelial growth factor (VEGF) (10,11),
making COX-2 a rational therapeutic target for selective COX-2
inhibitors, including celecoxib. Somatostatin (SST) is one of the
regulatory peptides for arresting the growth of HCC and the
overexpression of SST receptors has also been identified in HCC
(12). Our previous studies
demonstrated that a combination of a COX-2 inhibitor with an SST
analogue not only had an enhanced anti-proliferative effect and
suppressed the metastasis of HCC in nude mice (13) but also prolonged the survival of
rabbits with liver cancer that received TAE (14).
Various histopathological factors, including tumour
size, tumour number, vascular invasion and tumour encapsulation,
have been reported to be related to the prognosis of HCC. One study
indicated that encapsulation is a favourable factor in large HCCs
(>5 cm) and that encapsulation may act as a barrier to prevent
the spread of tumour cells (15).
However, few antitumour regimes stimulate the encapsulation of HCC.
The current study aimed to evaluate the encapsulation of VX2
hepatic allografts in rabbits induced by octreotide and celecoxib
administration following TAE.
Materials and methods
Animal experiments
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Sichuan University
and were conducted according to local laws set by Sichuan
University. Adult New Zealand White male rabbits weighing 2.3–2.5
kg were purchased from the Experimental Animal Centre of West China
Medical Centre, Sichuan University. VX2 allograft-bearing rabbits
were purchased from the Union Hospital, Huazhong University of
Science and Technology (Wuhan, China).
The establishment of VX2 hepatic allografts in
rabbits, TAE procedure and experimental grouping were the same as
in our previous study (14).
Briefly, 72 rabbits were randomly assigned into four groups. The
VX2 tumours were orthotopically implanted into the livers of the
rabbits. A total of 67 VX2 allograft-recipient rabbits were divided
into 4 groups and treated as follows: i) control (n=18), the
sham-operated animals received normal saline (NS) daily,
intragastrically and subcutaneously; ii) TAE (n=17), the animals
received the TAE procedure and then NS in the same way as the
control group; iii) octreotide + celecoxib (O+C; n=16), the animals
received sham surgery and then subcutaneous administration of
octreotide (Novartis Diagnostics, Emeryville, CA, USA) at a dose of
37 μg/kg/day plus intragastric administration of celecoxib
(Pfizer, New York, NY, USA) at a dose of 22.2 mg/kg/day and iv)
multimodality therapy (TAE+O+C; n=16), the animals received TAE
followed by subcutaneous administration of octreotide at a dose of
37 μg/kg/day plus intragastric administration of celecoxib
at a dose of 22.2 mg/kg/day. Eight animals of each group were
sacrificed after 30 days of treatment. The other animals were
raised until spontaneous mortality or were sacrificed after 80 days
of treatment.
Once the tumours were removed and weighed,
metastatic foci were carefully searched for in organs. The tumour
inhibition rate (%) = [(tumour weight of control − tumour weight of
treatment group)/tumour weight of control] × 100. Tumour tissue was
fixed in neutral buffered formalin for histological examination or
4% glutaraldehyde for transmission electron microscopy, or stored
in −80°C ultra-low freezer for reverse transcription-polymerase
chain reaction (RT-PCR).
Morphological evaluation of VX2
allografts in rabbits
Paraffin-embedded specimens were sliced into
5-μm sections and stained with haematoxylin and eosin
(H&E) for histological evaluation in a single-blinded fashion.
Clear cells in the VX2 allografts, due to cytoplasmic accumulation
of glycogen and fat droplets that dissolved during the H&E
staining process and left behind a ‘clear’ cytoplasm, were detected
and counted in each tumour allograft (16). Encapsulation of the tumours was
evaluated and the capsule thickness of complete capsules was
measured in pixel pitches using Image-Pro Plus 6.0 software (Media
Cybernetics, Rockville, MD, USA) and then was normalised into
μm. Each value was the mean of five visual fields in which
duplicate measurements were made.
Tumour specimens from each group were also immersed
in 4% glutaraldehyde (pH 7.4) at 4°C for 24 h, postfixed in 1%
osmium tetroxide for 1 h and embedded in Epon 812 following
dehydration. Following double staining with uranyl acetate and lead
citrate, ultrathin sections (60 nm) were examined with a
transmission electron microscope (H-600IV, Hitachi, Tokyo,
Japan).
Immunohistochemistry for VEGF and
CD31
Immunohistochemistry was performed on 5-μm
paraffin-embedded tissue sections on poly-L-lysine coated glass
slides. The sections were deparaffinised and treated with
microwaves for 15 min. For non-specific blocking, 10% goat serum
was added and incubated for 20 min at room temperature. Then the
VEGF antibody (ab288775; Abcam, Cambridge, MA, USA) and the CD31
antibody (08-1425; Zymed Laboratories Inc., San Francisco, CA, USA)
at a 1:250 dilution were added to the individual sections. Positive
reactions were revealed by the streptavidin-biotin-peroxidase
technique. Sections were incubated with 3,3′-diaminobenzidine
(0.05% 3,3′-diaminobenzidine in 0.05 M Tris buffer, pH 7.6 and
0.01% hydrogen peroxide) and counterstained with Mayer’s
haematoxylin. Image-Pro Plus 6.0 software was used to score the
integrated optical density (IOD) from the VEGF expression in the
tumour cells and count the number of CD31 per visual field
(magnification, ×200) in a single-blinded fashion. Each value was
the mean of five visual fields in which duplicate measurements were
made.
RT-PCR for VEGF analysis
Total RNA was extracted from allograft tissue using
the TRIzol reagent (15596-026; Invitrogen Life Technologies,
Carlsbad, CA, USA). Quantification and purity of extracted RNA were
determined by the ratio of absorbance at 260 and 280 nm (A260/A280)
with a spectrophotometer (GeneQuant 1300; Biochrom, Holliston, MA,
USA). Reverse transcription and PCR amplification were conducted
using a thermal cycler (PTC-100; Bio-Rad, Hercules, CA, USA), in
accordance with the instructions of the RT-PCR core kit (K1622;
Fermentas, Hanover, MD, USA). The primer sequences for the sense
and antisense chains were as follows: glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; XM_002714697): 5′-TCT CGT CCT CCT CTG GTG CTC
T-3′ and 5′-AAG TGG GGT GAT GCT GGT GC-3′; and VEGF (NM_001082253):
5′-ATG GCA GAA GAA GGA GAC-3′ and 5′-ATT TGT TGT GCT GTA GGA AG-3′.
The PCR cycle profile was 94°C for 30 sec, 52°C for 60 sec and 72°C
for 60 sec, for 30 cycles. The amplification was terminated by a
final extension step at 72°C for 2 min. A positive control (kidney
RNA) and an internal control (GAPDH) were amplified at the same
time. PCR products were quantified using a gel membrane, which was
scanned into an imaging system (Gel Doc 2000, Bio-Rad). The data
were normalised as a ratio of gray scale (IOD) of objective band
over GAPDH.
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation and tested by one-way analysis of variance
(ANOVA). Qualitative data were tested by the Chi-square test and
correlation analysis was also conducted to verify the correlation
between two parameters. Statistical analysis was performed using
SPSS 13.0 for Windows (IBM, New York, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of O+C treatment on extrahepatic
metastases following TAE
The total intrahepatic foci weight of each
intervention group was significantly lower than that of the control
group on day 30 and during days 30–80 (Table I and Fig. 1, row 1). The inhibition rate of the
TAE+O+C group was the greatest among the three intervention groups
during the whole experiment (Table
I). Extrahepatic metastasis was detected in the control group
on day 30; however, it was greatly reduced in the three
intervention groups (P=0.006; Table
I). During days 30–80, extrahepatic metastasis in the three
intervention groups remained significantly lower than that of the
control group (P=0.007). Over this time period, the TAE+O+C group
demonstrated the least extrahepatic metastasis among the three
intervention groups.
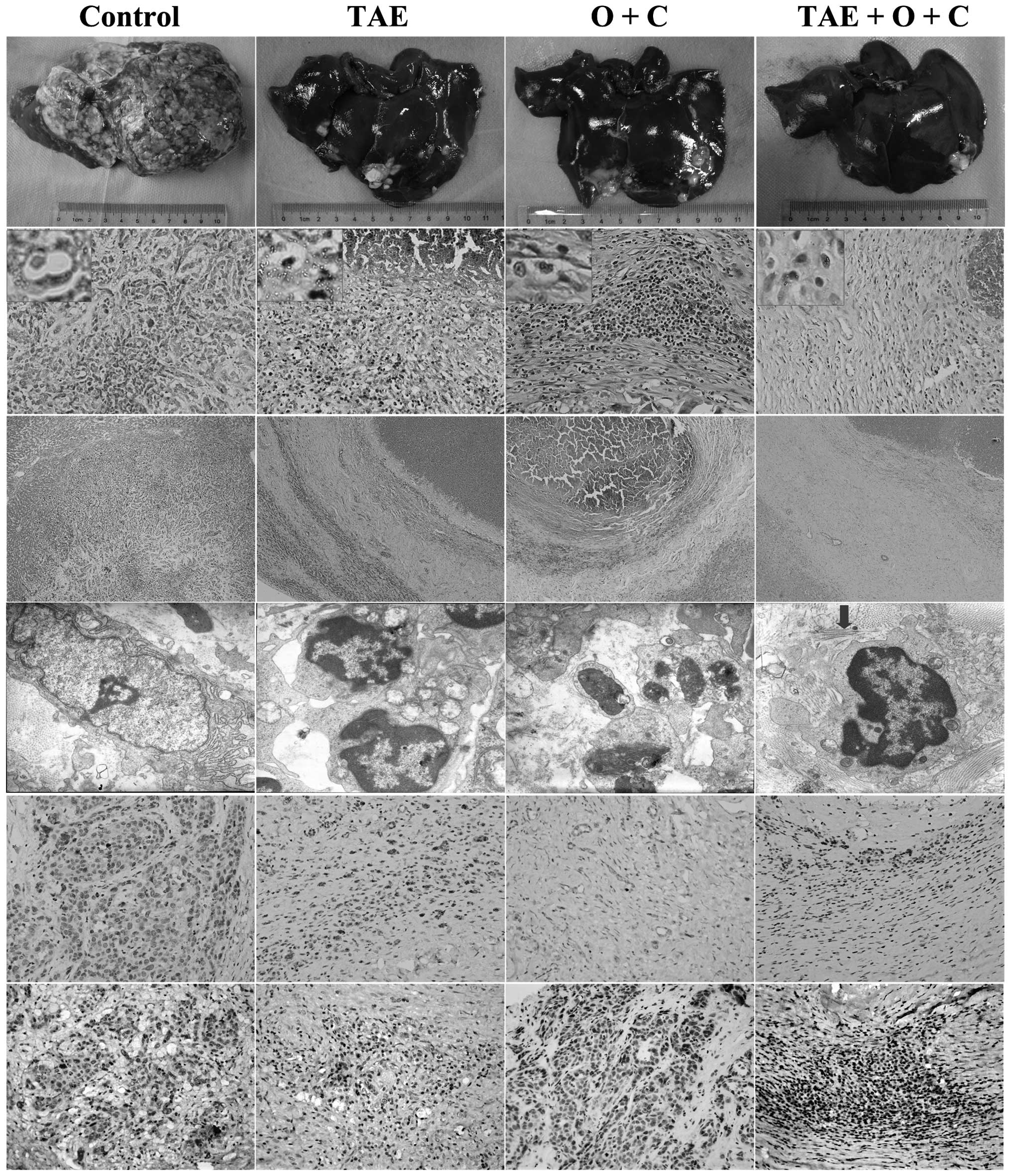 | Figure 1.VX2 allografts of the four groups on
day 30. Row 1, gross morphology of allografts; row 2, clear cells
(haematoxylin and eosin staining; magnification, ×400 and ×100);
row 3, capsules (haematoxylin and eosin staining; magnification,
×100); row 4, ultra-structure of the tumour cells. The arrow
indicates a collagen bundle (transmission electron microscope;
magnification, ×10,000); rows 5 and 6, positive expression of CD31
and vascular endothelial growth factor (VEGF) with brown grains
(immunohistochemical staining; magnification, ×100). TAE,
transcatheter arterial embolisation; O+C, octreotide +
celecoxib. |
 | Table I.Variation of VX2 allografts and their
metastasis in each group. |
Table I.
Variation of VX2 allografts and their
metastasis in each group.
| Control | TAE | O+C | TAE+O+C | P-value |
|---|
| Total animal
number | 18 | 17 | 16 | 16 | - |
| Data on day 30 | | | | | |
| Total intrahepatic
lesions | | | | | |
| Weight (g) | 63.8±57.8 | 8.2±7.0a | 14.6±21.5a | 5.7±4.9a | 0.002 |
| Inhibition rate
(%) | - | 87.1 | 77.1 | 91.1 | - |
| Allografts | | | | | |
| Clear cells
(%) | 5.1±1.9b | 6.6±3.6 | 5.7±2.5b | 9.4±2.7 | 0.043 |
| VEGF (IOD,
×105) | 3.43±2.01 | 1.05±0.44a | 0.71±0.59a |
0.44±0.30a,c | 0 |
| Capsules | | | | | |
| Partial/complete
(n/n) | 4/4 | 1/7a | 0/8a | 0/8a | 0.017 |
| Thickness
(μm) | 213±59 | 681±290a,b | 757±302a,b | 1143±322a | 0 |
| CD31
(number/field) | 22.5±6.1 | 38.6±4.6a | 12.2±2.6a,c | 11.0±2.2a,c | 0 |
| Extrahepatic
metastasis (%) | 100 | 25a | 37.5a | 25a | 0.006 |
| Data during days
30–80 | | | | | |
| Total intrahepatic
lesions | | | | | |
| Weight (g) | 105.5±70.5 | 8.4±13.6a | 19.6±20.8a | 4.8±4.5a | 0 |
| Inhibition rate
(%) | - | 92.0 | 81.4 | 95.5 | - |
| Allografts | | | | | |
| Clear cells
(%) | 3.2±2.8 | 7.6±4.1 | 8.3±5.9 | 12.3±5.2a | 0.026 |
| Capsules | | | | | |
|
Partial/complete (n/n) | 4/6 | 0/9a | 0/8a | 0/8a | 0.010 |
| Thickness
(μm) | 294±130 | 517±235b |
725±229a,b | 1073±432a | 0 |
| Extrahepatic
metastasis (%) | 100 | 55.6a | 37.5a | 25a | 0.007 |
Effect of O+C treatment on clear cell
number and capsule thickness following TAE
Microscopically, the nuclei of clear cells were
mainly located centrally or slightly eccentrically with dense or
occasionally clumpy chromatin. They were detectable in the VX2
hepatic allografts of the four groups (Fig. 1, row 2). The TAE+O+C group
demonstrated the highest proportion of clear cells during the whole
experiment (P<0.05; Table
I).
Complete capsule morphology was displayed in only
half of the allografts in the control group on day 30; however,
they were formed in the allografts in the majority of the TAE, O+C
and TAE+O+C group animals (Table
I). Forty percent of the allografts in the control group
exhibited partial formation of capsules at spontaneous mortality.
There were complete capsules around all the allografts in the three
intervention groups on day 80. The three interventions
significantly increased the thickness of the complete capsules
during the whole experiment (P=0; Table I and Fig. 1, row 3). The thickest capsules,
∼5.4 times the size of that in the control group, were observed in
VX2 allografts treated with the TAE+O+C regime. The capsules were
often adjacent to the necrotic tissues.
An irregular shape of the nuclear membrane and the
nucleolus and extension of the endoplasmic reticulum were observed
in the control group. Swelling mitochondria were displayed in the
tumour cells of the TAE group. A greater number of apoptotic bodies
in the cells of VX2 allografts were detected in the O+C group.
Additionally, collagen bundles surrounding the tumour cells were
clearly increased in the TAE+O+C group (Fig. 1, row 4).
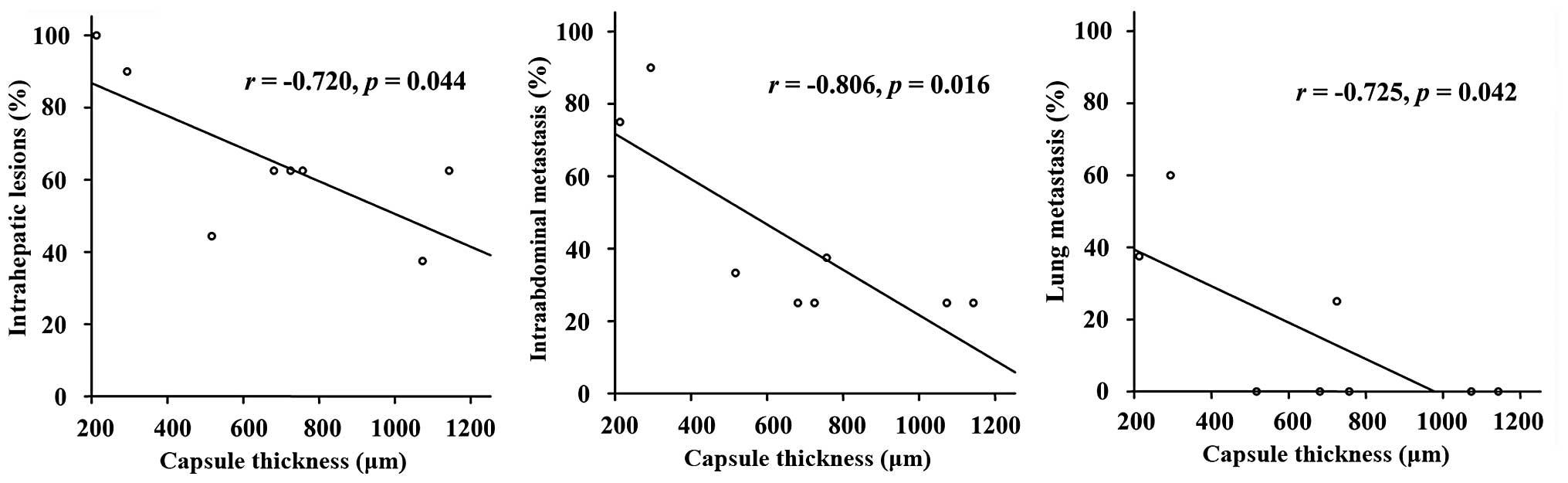
Compared with partial capsules, complete capsules
greatly reduced the intrahepatic lesions and intra-abdominal
metastasis, as well as lung metastasis (P<0.05; Table II). The thickness of the complete
capsules was negatively correlated with the intrahepatic lesions
and intra-abdominal and lung metastasis (P<0.05; Table II and Fig. 2).
 | Table II.Effects of capsules on tumour growth
and metastasis. |
Table II.
Effects of capsules on tumour growth
and metastasis.
| Intrahepatic
lesions (%) | Intra-abdominal
metastasis (%) | Lung metastasis
(%) |
|---|
| Integrity of
capsules | | | |
| Complete | 65.5 | 36.2 | 8.6 |
| Partial | 100 | 88.9 | 66.7 |
| P-value | 0.035 | 0.003 | 0 |
| Correlation with
the thickness of capsules | | | |
| R-value | −0.720 | −0.806 | −0.725 |
| P-value | 0.044 | 0.016 | 0.042 |
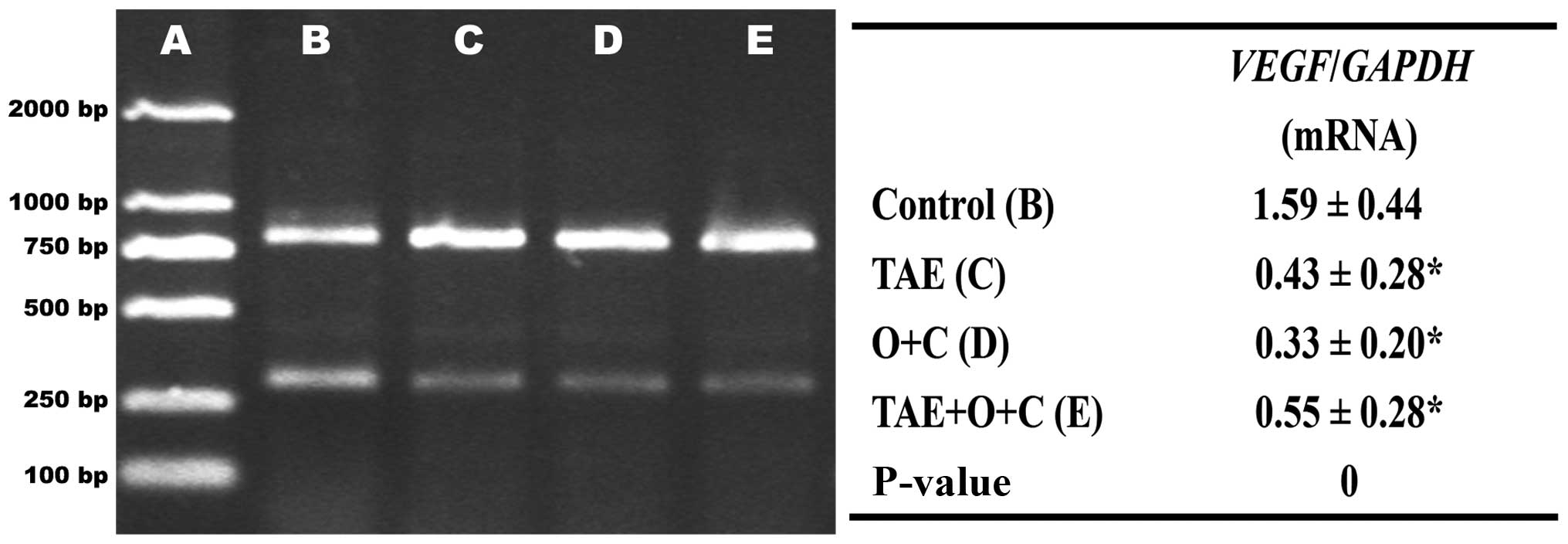
Effect of O+C treatment on VEGF
expression following TAE-induced angiogenesis
The capsules of the VX2 hepatic allografts were rich
in microvessels (Fig. 1, row 2),
which was revealed with the positive staining of CD31 (Table I and Fig. 1, row 5). Following the TAE
procedure, angiogenesis in the capsules significantly increased
(Table I and Fig. 1, row 5). However, the TAE+O+C
regime significantly downregulated the expression of CD31 and VEGF
in the VX2 allografts on day 30, compared with the TAE group
(Table I and Fig. 1, rows 5 and 6). Although the
expression of VEGF mRNA in the three intervention groups were
significantly lower than that in the control group, there was no
significant difference in the expression of VEGF mRNA between the
TAE and TAE+O+C groups (Fig.
3).
Discussion
Clear cell HCC has a particular histological type,
and accounts for between 0.4 and 37% of all HCC cases (16–19).
Patients with primary clear cell carcinoma of the liver often have
a higher rate of HCV infection and capsule formation associated
with suppressed vascular invasion. The prognosis is better than
that of patients with common type HCC, which is related to the
ratio of clear cells. There are few studies that demonstrate the
effect of enhancing the proportion of clear cells in HCC. TAE
followed by long-term administration of octreotide and celecoxib
synergistically induces the secondary clear cells in HCC and
therefore greatly prolongs the survival of rabbits with VX2 hepatic
allografts (14).
Encapsulation, defined as the formation of a clear
fibrous layer with collagen content, acts as a barrier to prevent
the spread of tumour cells. Capsule formation has been observed in
∼90% of primary clear cell carcinomas of the liver (20,21).
The TAE procedure significantly enhanced the completeness of the
capsules when compared with controls. Furthermore, TAE+O+C
combination therapy significantly enhanced capsule thickness
resulting in increased number of clear cells in the VX2 hepatic
allografts. Hepatic stellate cells (HSCs) are the major cellular
component of the HCC capsule and the formation of a HCC capsule may
start from the activation of HSCs (15). Encapsulation was usually related to
ischemic necrosis of surrounding tissue that may increase
production of extracellular matrix in VX2 hepatic allografts. The
collagen bundles adjacent to the tumour cells may also induce
apoptosis of the tumour cells.
One study considered that an early HCC tumour is an
ill-defined nodule without fibrous capsule formation, the fibrous
capsule appears as the tumour size increases and the survival of
patients with encapsulated HCCs is poorer than that of patients
with HCC without encapsulation (22). In contrast to this observation, the
current study revealed that encapsulation of VX2 hepatic allografts
was negatively related to tumour growth and metastasis. Moreover,
there are a number of controversies on vascular invasion and
capsule formation (15,23). The present study identified that
the TAE+O+C regimen significantly inhibits TAE-induced angiogenesis
and VEGF expression in the capsules, as well as increases the
completeness and the thickness of the capsules. The main fibrogenic
stimuli for stromal cells requires further elucidation.
Although encapsulation of HCC is considered as an
important bio-behavior, which would be beneficial to the host, no
regime has previously been reported to encapsulate HCC as
demonstrated in this study. The potential inhibition of VX2 hepatic
allograft growth and metastasis with the TAE+O+C regime relates to
the increased proportion of clear cells, the encapsulation and
anti-angiogenesis effects.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (no. 30770984). The
authors would like to thank Professor Jian-Ying Wang, Department of
Surgery and Pathology, University of Maryland School of Medicine,
Baltimore, MD, USA, for the language modification of this paper.
The authors also thank Associate Professor Rui Liu, technicians
Xian Li and Ou Qiang, Division of Peptides Related with Human
Diseases, State Key Laboratory of Biotherapy, West China Hospital,
Sichuan University, Chengdu, China, for their technical
assistance.
References
|
1.
|
Bruix J and Llovet JM: Major achievements
in hepatocellular carcinoma. Lancet. 373:614–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Shah SA, Greig PD, Gallinger S, et al:
Factors associated with early recurrence after resection for
hepatocellular carcinoma and outcomes. J Am Coll Surg. 202:275–283.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Lee EH, Kao WWY and Schwarz RI: Cell
density regulates prolyl 4-hydroxylase activity independent of mRNA
levels. Matrix Biol. 19:779–782. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gupta S, Kobayashi S, Phongkitkarun S,
Broemeling LD and Kan Z: Effect of transcatheter hepatic arterial
embolisation on angiogenesis in an animal model. Invest Radiol.
41:516–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Doffoel M, Bonnetain F, Bouche O, et al:
Multicentre randomised phase III trial comparing tamoxifen alone or
with transarterial lipiodol chemoembolisation for unresectable
hepatocellular carcinoma in cirrhotic patients (Fédération
Francophone de Cancérologie Digestive 9402). Eur J Cancer.
44:528–538. 2008.
|
|
6.
|
Kudo M, Imanaka K, Chida N, et al: Phase
III study of sorafenib after transarterial chemoembolisation in
Japanese and Korean patients with unresectable hepatocellular
carcinoma. Eur J Cancer. 47:2117–2127. 2011. View Article : Google Scholar
|
|
7.
|
Britten CD, Gomes AS, Wainberg ZA, et al:
Transarterial chemoembolization plus or minus intravenous
bevacizumab in the treatment of hepatocellular cancer: a pilot
study. BMC Cancer. 12:162012. View Article : Google Scholar
|
|
8.
|
Breinig M, Schirmacher P and Kern MA:
Cyclooxygenase-2 (COX-2)-a therapeutic target in liver cancer? Curr
Pharm Design. 13:3305–3315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Wu T: Cyclooxygenase-2 in hepatocellular
carcinoma. Cancer Treat Rev. 32:28–44. 2006. View Article : Google Scholar
|
|
10.
|
Zhao QT, Yue SQ, Cui Z, et al: Potential
involvement of the cyclooxygenase-2 pathway in hepatocellular
carcinoma-associated angiogenesis. Life Sci. 80:484–492. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Cheng AS, Chan HL, To KF, et al:
Cyclooxygenase-2 pathway correlates with vascular endothelial
growth factor expression and tumor angiogenesis in hepatitis B
virus-associated hepatocellular carcinoma. Int J Oncol. 24:853–860.
2004.
|
|
12.
|
Reubi JC, Zimmermann A, Jonas S, et al:
Regulatory peptide receptors in human hepatocellular carcinomas.
Gut. 45:766–774. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Xie Y, Chen S, Wang C and Tang C: SOM230
combined with celecoxib prolongs survival in nude mice with HepG-2
xenografts. Cancer Biol Ther. 12:86–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Tong H, Li X, Zhang CL, et al:
Transcatheter arterial embolization followed by octreotide and
celecoxib synergistically prolongs survival of rabbits with hepatic
VX2 allografts. J Dig Dis. Oct 5–2012.(Epub ahead of print).
|
|
15.
|
Wu TH, Yu MC, Chen TC, et al:
Encapsulation is a significant prognostic factor for better outcome
in large hepatocellular carcinoma. J Surg Oncol. 105:85–90. 2012.
View Article : Google Scholar
|
|
16.
|
Emile JF, Lemoine A, Azoulay D, Debuire B,
Bismuth H and Reynes M: Histological, genomic and clinical
heterogeneity of clear cell hepatocellular carcinoma.
Histopathology. 38:225–231. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kashala LO, Conne B, Kalengayi MM, et al:
Histopathologic features of hepatocellular carcinoma in Zaire.
Cancer. 65:130–134. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lai CL, Wu PC, Lam KC and Todd D:
Histologic prognostic indicators in hepatocellular carcinoma.
Cancer. 44:1677–1683. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Adamek HE, Spiethoff A, Kaumann V, et al:
Primary clear cell carcinoma of noncirrhotic liver:
immunohistochemical discrimination of hepatocellular and
cholangiocellular origin. Dig Dis Sci. 43:33–38. 1998. View Article : Google Scholar
|
|
20.
|
Murakata LA, Ishak KG and Nzeako UC: Clear
cell carcinoma of the liver: a comparative immunohistochemical
study with renal clear cell carcinoma. Mod Pathol. 13:874–881.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Liu Z, Ma W, Li H and Li Q:
Clinicopathological and prognostic features of primary clear cell
carcinoma of the liver. Hepatol Res. 38:291–299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Iguchi T, Aishima S, Sanefuji K, et al:
Both fibrous capsule formation and extracapsular penetration are
powerful predictors of poor survival in human hepatocellular
carcinoma: a histological assessment of 365 patients in Japan. Ann
Surg Oncol. 16:2539–2546. 2009. View Article : Google Scholar
|
|
23.
|
Tanaka T, Yamanaka N, Oriyama T, Furukawa
K and Okamoto E: Factors regulating tumor pressure in
hepatocellular carcinoma and implications for tumor spread.
Hepatology. 26:283–287. 1997. View Article : Google Scholar : PubMed/NCBI
|