Introduction
Gastric cancer (GC) is a worldwide health problem
with >600,000 cases reported annually. The highest rates occur
in Japan, China, Eastern Europe and South America, with 42% of
worldwide cases occurring in China (1,2). The
occurrence of GC is associated with gene mutations, deletions and
other genetic and epigenetic mechanisms, which are the result of
the interaction between genetic and environmental factors.
Epigenetic modification controls gene expression via DNA
methylation, histone modification, chromatin remodeling and
non-coding RNAs. Abnormal epigenetic modifications may lead to
tumorigenesis (3,4), and their role in the process of tumor
formation is likely to be of increasing interest. DNA methylation
is a type of reaction using S-adenosylmethionine (SAM) as a methyl
donor for converting cytosine into S-methyl cytosine using the DNA
methyltransferase enzyme (DNMT) (5). Mizuno et al(6) showed that the expression of DNA
methyltransferase 1 (DNMT1) in tumor cells is 4-12-fold higher than
that in normal cells, confirming that the DNMT1 increase is
involved in tumorigenesis. 5-Aza-2′-deoxycytidine (5-aza-CdR) is a
nucleoside analog methylation inhibitor that forms a covalent
complex with DNMT1 to inhibit its methyltransferase activity,
resulting in less methylation (7).
Caudal type homeobox transcription factor 2 (CDX2),
a member of the caudal-related homeobox gene family, plays a key
role in early mammalian intestinal development and the maintenance
of intestinal epithelia via regulation of intestine-specific gene
transcription (8,9). CDX2 expression in normal tissue
mainly exists in the small intestinal and colonic mucosa, not in
the normal gastric mucosa, but it appears ectopic expression from
intestinal metaplasia to the intestinal-type GC (10). Numerous studies indicate that CDX2
performs a tumor suppressor role in human colorectal carcinogenesis
(11–14). Recently, several studies reported
that the survival rates for CDX2 expression-positive GC were
significantly higher than those for the negative group, suggesting
a possible tumor suppressor role for CDX2 (15,16).
However, the molecular mechanisms leading to the inactivation of
CDX2 remain unclear.
In the present study, the expression and association
of CDX2 and DNMT1 mRNA in GC tissues and normal tissues distal to
the GC were analyzed. To further elucidate the molecular mechanisms
behind the inactivation of the CDX2 gene, we detected the promoter
methylation status by methylation-specific PCR (MSP) and the
expression of CDX2 mRNA and protein by real-time fluorescence
quantitative polymerase chain reaction (RFQ-PCR) and western
blotting in the GC cell lines AGS, MKN-45 and SGC-7901. We then
investigated the ability of 5-aza-CdR to induce CDX2 gene
re-expression and its effects and mechanisms in GC cell
proliferation and apoptosis by treating MKN-45 cells with the
demethylating agent 5-aza-CdR in vitro. Our study reveals
that the loss of CDX2 function in GC may be attributed to promoter
hypermethylation, and that the reactivation of CDX2 by 5-aza-CdR
inhibits cell proliferation and induces caspase-3-independent
apoptosis in GC cells.
Materials and methods
Tissue samples
Sixty pairs of tissue specimens of GC and the
matching distal non-cancerous gastric mucosal tissues (the distance
from normal tissue to tumor was >5 cm) were obtained from 60
patients. All patients underwent surgery without preoperative
radiation or chemotherapy at the Surgery Department of the
Affiliated Hospital of Nantong University (Nantong, China) between
January 2009 and August 2011. The tumor and the distal
non-cancerous gastric mucosal tissues were snap frozen in liquid
nitrogen (N2) and stored at −80°C until use. All the
specimens were diagnosed separately by two pathologists to
determine the pathological classification of GC according to the
7th edition of the AJCC cancer staging manual for stomach cancer
(17). The detailed profiles of
clinical and pathological variables, including age, gender, tumor
size, Lauren classification, differentiation and lymph node
metastasis status of the patients, are listed in Tables I and II. The tissue samples were collected with
the informed consent of all patients and approval of the ethics
committee of the Affiliated Hospital of Nantong University.
 | Table I.Relative expression of CDX2 and DNMT1
mRNA in gastric cancer and distal non-cancerous gastric tissues
(mean ± SD). |
Table I.
Relative expression of CDX2 and DNMT1
mRNA in gastric cancer and distal non-cancerous gastric tissues
(mean ± SD).
| Group | CDX2 | DNMT1 |
|---|
| Non-cancerous
gastric tissue | 1.34±2.12 | 5.23±3.66 |
| Gastric cancer
tissue | 18.43±16.74a | 40.45±24.45a |
 | Table II.Correlation between CDX2 and DNMT1
mRNA expression and clinical pathological features of gastric
cancer (mean ± SD). |
Table II.
Correlation between CDX2 and DNMT1
mRNA expression and clinical pathological features of gastric
cancer (mean ± SD).
| Clinical
features | N | CDX2 | DNMT1 |
|---|
| Age (years) | | | |
| <60 | 25 | 20.34±3.31 | 38.25±1.28 |
| ≥60 | 35 | 16.98±2.21 | 42.12±3.67 |
| Gender | | | |
| Male | 42 | 18.63±2.13 | 38.57±6.12 |
| Female | 18 | 19.45±4.32 | 43.10±3.59 |
| Tumor size
(cm) | | | |
| <5 | 32 | 17.35±8.54 | 41.17±4.38 |
| ≥5 | 28 | 19.53±5.12 | 39.65±4.17 |
| Lauren
classification | | | |
|
Intestinal-type | 28 | 29.31±13.15a | 38.33±2.56 |
| Diffuse-type | 32 | 7.24±1.75 | 43.43±8.33 |
| TNM staging | | | |
| I+II | 26 | 27.50±11.47a | 29.22±7.21a |
| III+IV | 34 | 10.01±2.39 | 52.46±8.86 |
| Tumor
differentiation | | | |
| Good | 29 | 19.37±2.41 | 30.56±6.04a |
| Poor | 31 | 18.15±2.20 | 51.53±14.23 |
| Lymph node
metastasis | | | |
| Absent | 19 | 26.02±8.72a | 26.37±5.35a |
| Present | 41 | 11.21±2.02 | 54.37±12.15 |
Cell culture and 5-aza-CdR treatment
The human GC cell lines MKN-45, AGS and SGC-7901,
purchased from the Cell Resource Center of Shanghai Institutes for
Biological Sciences affiliated to the Chinese Academy of Sciences
(Shanghai, China) were maintained in RPMI-1640 medium (GIBCO, Grand
Island, NY, USA), supplemented with 10% heat-inactivated fetal
bovine serum (Invitrogen, Carlsbad, CA, USA) and 1%
antibiotic-antimycotic (Invitrogen), and grown at 37°C in a
humidified atmosphere containing 5% CO2. The cell lines
were treated with 2.5, 5 or 10 μmol/l 5-aza-CdR (Sigma, St.
Louis, MO, USA) for 24, 48 or 72 h.
RFQ-PCR
Total RNA from cultured cells and tissue samples was
isolated using TRIzol reagent (Takara, Dalian, Japan) according to
the manufacturer’s instructions. Primer pairs designed by Premier
5.0 (Premier Biosoft, Palo Alto, CA, USA) were synthesized by
Sangon Biotech (Shanghai) Co. Ltd (Shanghai, China) The primers
were as follows: CDX2, forward: 5′-CGC CGC AGA ACT TCG TCA G-3′ and
reverse: 5′-CGT AGC CAT TCC AGT CCT CCC-3′; DNMT1, forward: 5′-CTA
CCA GGG AGA AGG ACA GG-3′ and reverse: 5′-CTC ACA GAC GCC ACA
TCG-3′; β-actin (used as an internal control), forward: 5′-TGA CGT
GGA CAT CCG CAA AG-3′ and reverse: 5′-CTG GAA GGT GGA CAG CGA
GG-3′. Polymerase chain reaction (PCR) system: 10 μl SYBR
Premix Ex Taq™ (2X), 0.4 μl PCR forward primer,
0.4 μl PCR reverse primer, 2.0 μl PCR template (cDNA
solution), 0.4 μl ROX Reference Dye (50X) and 6.8 μl
sterile double steamed water. The mRNA was amplified for 40 cycles
and the cycling parameters were: 95°C for 30 sec, 95°C for 5 sec
and 60°C for 34 sec. Each measurement was performed in triplicate
and the average was calculated. For relative quantification,
2−ΔΔCt was calculated and used as an indication of the
relative expression levels (18).
Western blotting
Western blotting analysis of the CDX2 and internal
control β-actin proteins was performed as described previously
(19). Monoclonal antibody to CDX2
protein (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was diluted
at 1:100.
DNA isolation and MSP
The DNA was extracted using a DNeasy Blood &
Tissue kit (Qiagen, New York, NY, USA). The DNA (2 μg) was
modified using an EpiTect Bisulfite kit (Qiagen). Methylation of
the CDX2 gene CpG islands was analyzed by an MSP procedure, as
previously described (20). The
primers and PCR conditions have been described previously (21). The primers were as follows: CDX2
methylated sense: 5′-CGT CGG TTT GGG GTT TCG TAC-3′; antisense:
5′-GAT ACT CCG CTA ACT CCT CGC G-3′, expected fragment length, 169
bp; CDX2 unmethylated sense: 5′-GAA GTT GTT GGT TTG GGG TTT TGT
AT-3′; antisense: 5′-CCC ACA ATA CTC CAC TAA CTC CTC ACA-3′,
expected fragment length, 180 bp. The PCR products were
electrophoresed in 2.5% agarose gels. The MSP procedures were
performed in triplicate.
Cell proliferation assay
Cell proliferation was determined using a WST-8 Cell
Counting Kit-8 (CCK-8; Dojindo Laboratories, Kunamoto, Japan)
according to the manufacturer’s instructions. Briefly, cells
(5×107 cells/l) suspended in RPMI-1640 medium (100
μl) containing 10% fetal bovine serum were seeded in 96-well
plates and incubated for 24, 48, 72 and 96 h. CCK-8 solution (10
μl) was added to each well and the cultures were incubated
at 37°C for 3 h. Absorbance at 450 nm was measured using an
immunoreader. The results were plotted as means ± standard
deviation of three separate experiments having four determinations
per experiment for each experimental condition.
Annexin V-FITC/propidium iodide (PI)
assay
Cell apoptosis was analyzed by flow cytometry with
an Annexin V-FITC Apoptosis Detection kit (Beyotime, Jiangshu,
China) according to the manufacturer’s instructions. Briefly,
MKN-45 cells were treated with 0, 2.5, 5 or 10 μmol/l
5-aza-CdR for 72 h, then collected and washed twice with cold
phosphate-buffered saline (PBS). Following the addition of 195
μl binding buffer, 5 μl FITC-labeled annexin V was
added and the cells were incubated for 10 min at room temperature.
Each sample was then centrifuged at 1000 x g for 5 min, resuspended
in 190 μl binding buffer and 10 μl PI working
solution was added. The samples were analyzed by flow cytometry
(FCM).
Hoechst 33258 staining
Morphological observation of nuclear change was
assayed with Hoechst 33258 staining (Beyotime) according to the
manufacturer’s instructions. MKN-45 cells
(1×106cells/ml) were seeded in 6-well plates and treated
with 0, 2.5, 5 or 10 μmol/l 5-aza-CdR for 72 h at 37°C. The
cells were collected, washed and fixed in 4% paraformaldehyde for
30 min and then stained with 5 μg/ml Hoechst 33258 for 5 min
at room temperature. The apoptotic cells were visualized using an
inverted fluorescence microscope (Olympus, Tokyo, Japan).
Analysis of caspase activities
Caspase activities were measured using caspase
activity assay kits C1115, C1151 and C1157 (Beyotime) according to
the manufacturer’s instructions. Briefly, cells were washed with
PBS, resuspended in lysis buffer and left on ice for 15 min. The
lysate was centrifuged at 20,000 x g at 4°C for 15 min. The
activities of caspase-3, −8 and −9 were measured using substrate
peptides acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA),
acetyl-Ile-Glu-Thr-Asp p-nitroanilide (Ac-IETD-pNA) and
acetyl-Leu-Glu-His-Asp p-nitroanilide (Ac-LEHD-pNA), respectively.
The release of p-nitroanilide (pNA) was qualified by determining
the absorbance with a microplate reader (Model 550, Bio-Rad,
Hercules, CA, USA) at 405 nm. Each plate contained multiple wells
of a given experimental condition and multiple control wells.
Statistical analysis
Results were presented as the mean ± standard
deviation. All data were analyzed using statistical software Stata
version 11.0 (Stata, College Station, TX, USA). Statistical
differences between the groups were analyzed using either one-way
ANOVA or Student’s t-test. Linear regression was calculated between
the expression levels of CDX2 and DNMT1 mRNA in tissues. P<0.05
was considered to indicate a statistically significant result.
Results
Expression of CDX2 and DNMT1 mRNA in GC
and distal non-cancerous gastric tissues
The expression levels of CDX2 and DNMT1 mRNA in 60
GC tissue samples and the matching non-cancerous gastric mucosa
tissue samples was detected by RFQ-PCR. The expression levels of
CDX2 and DNMT1 mRNA were significantly higher in the GC tissues
than in the non-cancerous tissues (P<0.05). The expression of
CDX2 mRNA was significantly correlated with Lauren classification,
TNM stage and lymph node metastasis (all P<0.05). DNMT1 mRNA
expression was significantly correlated with TNM stage,
pathological differentiation and lymph node metastasis (all
P<0.05; Tables I and II). Linear correlation analysis showed
that the expression of CDX2 mRNA was inversely correlated with that
of DNMT1 mRNA in GC (r=−0.385, P<0.05).
Association between methylation of the
CDX2 gene promoter and gene expression in GC cells
The expression levels of CDX2 mRNA and protein in
the human GC cell lines AGS, MKN-45 and SGC-7901 were detected by
RFQ-PCR and western blotting. The results suggested that CDX2 mRNA
and protein were strongly expressed in the AGS cell line but
extremely low or absent in MKN-45 and SGC-7901 cells (P<0.05;
Fig. 1A and B).
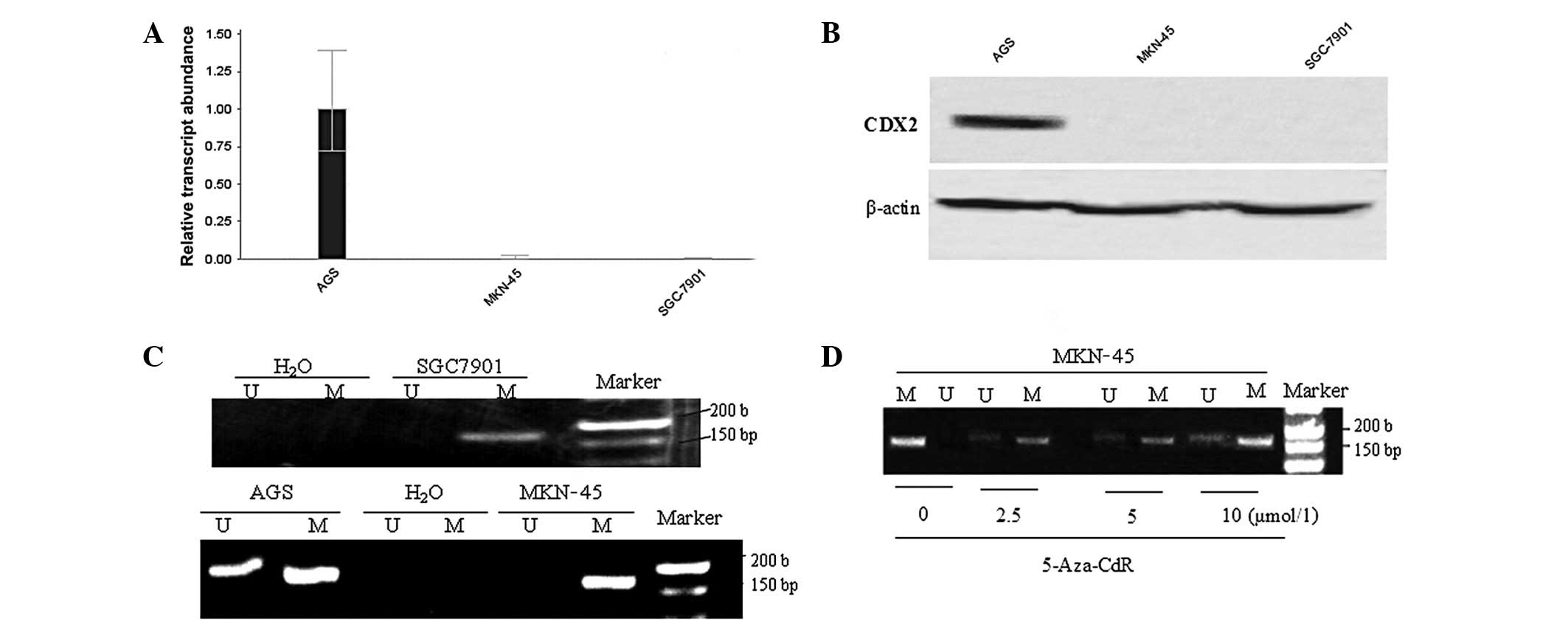 | Figure 1.Association between methylation of
the CDX2 gene 5′CpG island and gene expression in gastric cancer
cells. The expression levels of CDX2 (A) mRNA and (B) protein were
markedly high in AGS cells, but absent in MKN-45 and SGC-7901 cells
(P<0.01). (C) Methylation analysis of the CDX2 gene 5′CpG island
using methylation-specific polymerase chain reaction (MSP) showed
hypermethylation in MKN-45 and SGC-7901 cells, however, AGS showed
a partial methylation status. (D) Representative MSP analyses of
the CDX2 gene in MKN-45 following treatment with different
concentrations (0, 2.5, 5 and 10 μmol/l) for 72 h.
Water-treated cells served as blank controls. Lane U, amplified
product with primers recognizing the unmethylated CDX2 sequence,
lane M, amplified product recognizing the methylated CDX2 sequence.
CDX2, caudal type homeobox transcription factor 2; 5-aza-CdR,
5-aza-2′-deoxycytidine. |
MSP analysis revealed that the CDX2 promoter region
was fully hypermethylated in the GC cell lines MKN-45 and SGC-7901,
however, partial methylation status was detected in the GC cell
line AGS (Fig. 1C). In the cell
line MKN-45, treatment with the demethylating agent 5-aza-CdR for
72 h at different concentrations (0, 2.5, 5 and 10 μmol/l)
induced a partial promoter demethylation (Fig. 1D).
Reactivation of CDX2 and rescue of gene
expression by 5-aza-CdR in MKN-45
The human GC cell line MKN-45 was treated with
different concentrations (0, 2.5, 5 and 10 μmol/l) of
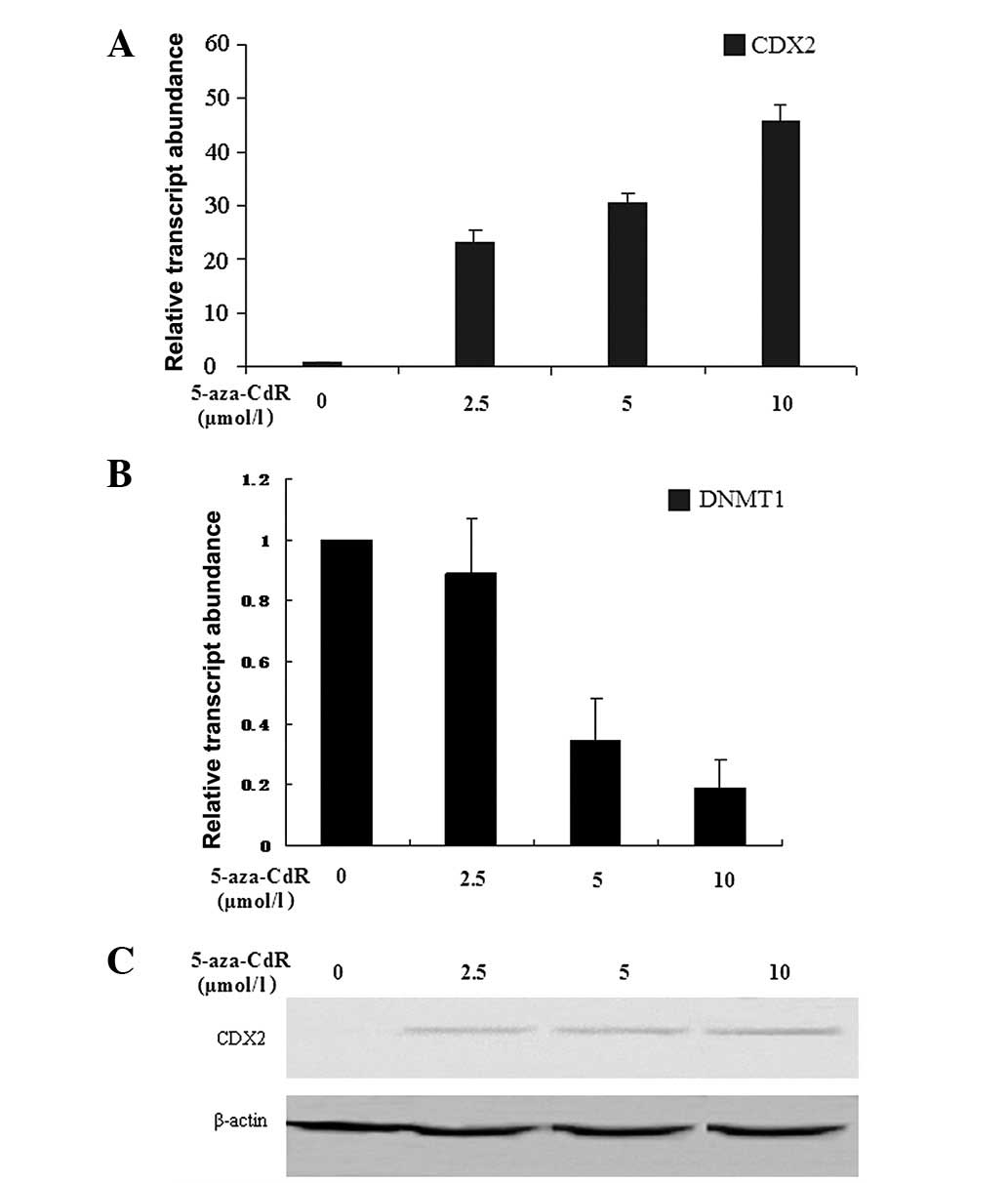
5-aza-CdR for 72 h. The expression levels of CDX2 and DNMT1 mRNA
were detected by RFQ-PCR. The results showed that the expression
level of CDX2 mRNA was increased by 5-aza-CdR in a
concentration-dependent manner (24.65±2.23, 33.59±1.99 and
48.53±1.77, at 2.5, 5 and 10 μmol/l, respectively) compared
with those in the control group (0 μmol/l; P<0.05). A
comparable result was observed for CDX2 protein, the expression
levels of which also increased (1.42±0.01 and 1.86±0.02, at 5 and
10 μmol/l, respectively) in a concentration-dependent manner
compared with those in the other two groups (0 and 2.5
μmol/l; P<0.05). However, the expression level of DNMT1
mRNA showed a marked concentration-dependent decrease (1.00±0.01,
0.89±0.18, 0.34±0.14, 0.19±0.09, at 0, 2.5, 5 and 10 μmol/l,
respectively) in MKN-45 cells following exposure to 5-aza-CdR for
72 h (P<0.05; Fig. 2).
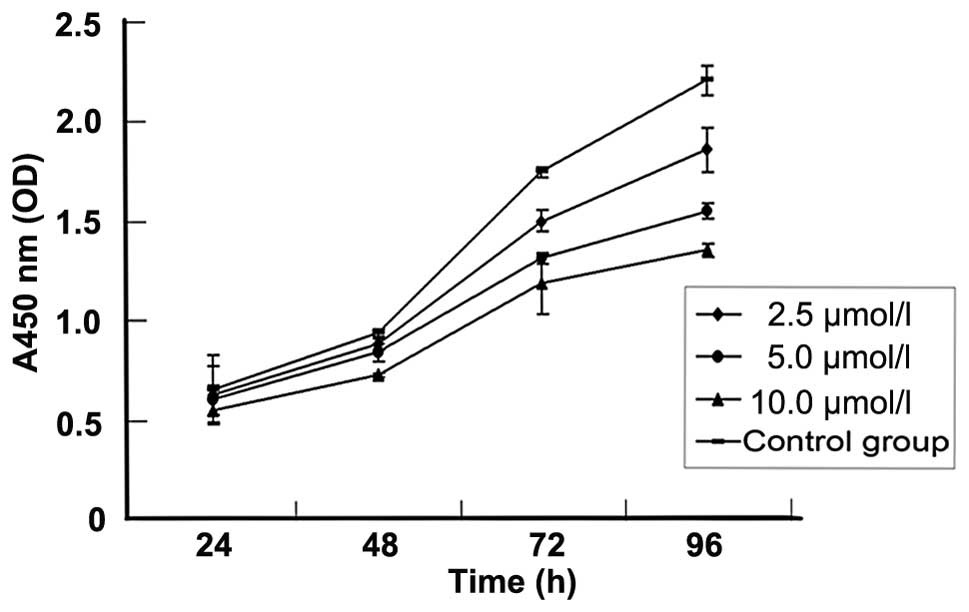
5-Aza-CdR inhibits MKN-45 cell growth by
induction of apoptosis
To investigate the effect of 5-aza-CdR on the growth
of human GC, the MKN-45 cell line was treated with various
concentrations of 5-aza-CdR for 96 h and cell viability was
detected by the CCK-8 assay. A concentration-and time-dependent
growth inhibition of cell proliferation was observed in the MKN-45
cells (Fig. 3, Table III).
 | Table III.The inhibitory rate (%) for different
concentrations of 5-aza-CdR at different time points in MKN-45
cells. |
Table III.
The inhibitory rate (%) for different
concentrations of 5-aza-CdR at different time points in MKN-45
cells.
| 5-Aza-CdR
concentration | 24 h | 48 h | 72 h | 96 h |
|---|
| 0 μmol/l
(control group) | - | - | - | - |
| 2.5
μmol/l | 5.5±1.5a | 6.8±2.0a | 15.1±2.2a | 17.0±2.2a |
| 5.0
μmol/l | 9.9±1.8a | 12.2±2.1a | 27.0±2.3a | 31.8±2.4a |
| 10
μmol/l | 21.2±2.0a | 25.95±3.1a | 34.9±5.2a | 41.3±2.5a |
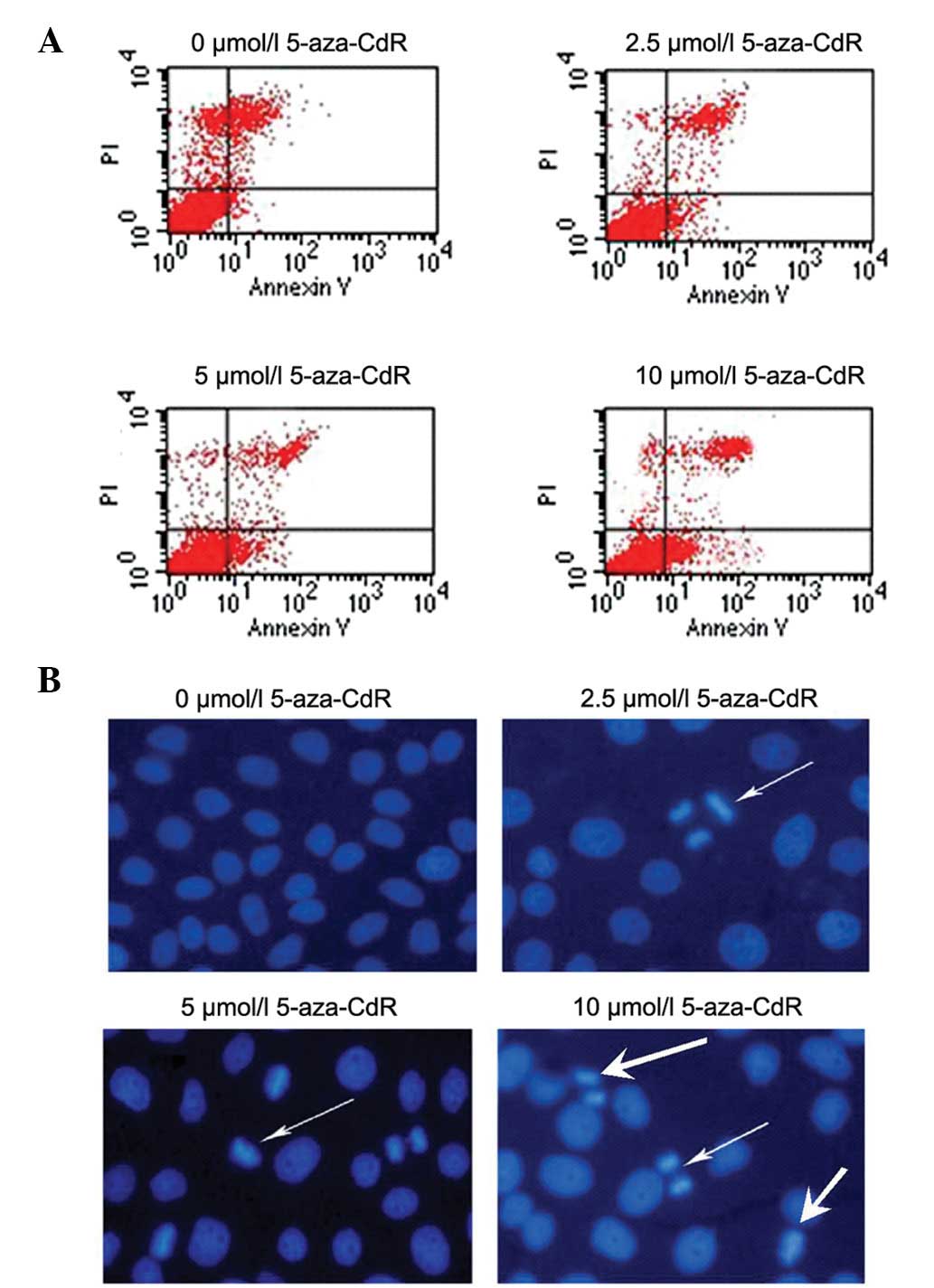
To examine whether 5-aza-CdR is able to efficiently
trigger apoptosis, leading to cytotoxicity against MKN-45 cells,
MKN-45 cells were treated with different concentrations of
5-aza-CdR for 72 h. Apoptosis was detected by Annexin V staining
FCM assay and Hoechst 33258 staining. As shown in Fig. 4, the data revealed that 5-aza-CdR
treatment increased the proportion of apoptotic cells from 0.9±2.3%
in pretreated cells to 4.4±2.2, 7.5±1.5 and 15.5±5.0% after
5-aza-CdR treatment at 2.5, 5 and 10 μmol/l, respectively
(P<0.05). In addition, Hoechst 33258 apoptosis staining showed
morphological changes typical of apoptosis in the nuclear chromatin
using fluorescence microscopy. This result strongly suggests that
apoptosis rather than necrosis was the mechanism of
5-aza-CdR-induced growth inhibition in the MKN-45 cells.
5-Aza-CdR induced apoptosis is mediated
via caspase-dependent pathways
In order to examine the role of caspases in the
apoptosis induced by 5-aza-CdR, we measured the proteolytic
activity of the executioner caspase-3 and the initiator caspase-8
and −9 by measuring Ac-DEVD-pNA, Ac-IETD-pNA and Ac-LEHD-pNA
cleavage of MKN-45 cell lysates collected 72 h after 5-aza-CdR
treatment. As shown in Table IV,
pretreatment with 0, 2.5, 5 and 10 μmol/l 5-aza-CdR caused
marked concentration-dependent increases of caspase-3, −8 and −9
proteolytic activities in MKN-45 cells (P<0.05). These results
indicate that the activation of caspase-3, −8 and −9 was involved
in the 5-aza-CdR-induced cell apoptosis.
 | Table IV.Change in caspase activity in MKN-45
cells following treatment with 5-aza-CdR for 72 h. |
Table IV.
Change in caspase activity in MKN-45
cells following treatment with 5-aza-CdR for 72 h.
| OD405
value
|
|---|
| 5-Aza-CdR
concentration | Caspase-3 | Caspase-8 | Caspase-9 |
|---|
| 0 μmol/l
(control group) | 0.083±0.003 | 0.060±0.005 | 0.069±0.002 |
| 2.5
μmol/l | 0.087±0.004a | 0.069±0.001 | 0.078±0.005a |
| 5.0
μmol/l | 0.096±0.005a | 0.075±0.003a | 0.099±0.001a |
| 10
μmol/l | 0.101±0.007a | 0.089±0.004a | 0.102±0.007a |
Dicussion
It is generally accepted that the pathogenesis of GC
is a multistage process that often takes years, with each stage
influenced by environmental, genetic, social and behavioral
factors. The characteristics of GC, as Hanahan et
al(22,23) described, include sustaining
proliferative signaling, evading growth suppressors, resisting cell
death, enabling replicative immortality, inducing angiogenesis,
activating invasion and metastasis, reprogramming energy metabolism
and evading immune destruction. Oncogenes and the inactivation of
tumor suppressor genes are key molecular factors in the tumor
microenvironment in gastric carcinogenesis.
CDX2, as an important nuclear transcription factor,
has an essential role in the proliferation and differentiation of
intestinal epithelial cells in fetal and adult tissues (24). CDX2 is expressed specifically in
colonic and small intestinal mucosa and has been implicated in
disorders involving abnormal intestinal differentiation and
neoplasia (25,26). The use of CDX2 as an
immunohistochemical marker has been described previously in studies
of human gastric and colonic cancer (27–30).
Several studies have reported that patients with low expression
levels of CDX2 in intestinal metaplasia and dysplasia are more
likely to progress into GC, and GC patients who were positive for
CDX2 expression showed a higher survival rate than those who were
CDX2 negative. CDX2 expression levels also gradually during the
progression from gastric dysplasia, to early and advanced GCs. In
addition, a negative correlation was observed between CDX2
expression and the depth of tumor invasion and lymph node
metastasis, suggesting that CDX2 may serve as a powerful predictor
for GC (31–37). A recent study showed that the
overexpression of CDX2 was capable of inhibiting cell growth and
proliferation in vitro and effectively inhibited GC
progression (38). Therefore, CDX2
acts as a tumor suppressor in the upper gastrointestinal tract.
However, the expression of CDX2 gradually declined in the process
of intestinal metaplasia-dysplasia-GC (34) and the molecular mechanisms leading
to the inactivation of CDX2 remain unclear.
In the present study, our results indicate that the
expression levels of CDX2 and DNMT1 mRNA were significantly higher
in GC tissues than in non-cancerous tissues. The expression of CDX2
mRNA was correlated significantly with Lauren classification, TNM
stage and lymph node metastasis. DNMT1 mRNA expression was
significantly correlated with TNM stage, pathological
differentiation and lymph node metastasis, which is similar to
previous findings reported in the literature (39). Linear correlation analysis showed
that the expression of CDX2 mRNA was inversely correlated with that
of DNMT1 mRNA in GC. It was then hypothesized that the
downregulation of CDX2 in GC was likely to be correlated with the
hypermethylation of the CDX2 gene promoter region caused by DNMT1
overexpression.
Recent advances in the field of epigenetics have
shown that human cancer cells harbor global epigenetic
abnormalities in addition to numerous genetic alterations. Among
these epigenetic aberrations, DNA hypermethylation is the one which
has been the most extensively studied (40). With regard to our findings, MSP
analysis revealed that the CDX2 promoter region was fully
hypermethylated in the GC cell lines MKN-45 and SGC-7901, however,
partial methylation status was detected in the GC cell line AGS. We
conclude that promoter hypermethylation of CDX2 is an epigenetic
event in GC that may contribute to epigenetic silencing and result
in cancer progression and poor prognosis in patients.
The epigenetic silencing of cancer-related genes has
proven to be reversible. Therefore, epigenetic alterations are
potential targets of interest for molecular targeted therapy in
human malignancies (41). In the
current study, we have shown that treatment of the GC cell line
MKN-45 with different concentrations of the demethylating agent
5-aza-CdR resulted in partial demethylation of the CDX2 promoter
region thus allowing the restoration of a potentially silenced gene
expression, while DNMT1 showed a marked concentration-dependent
decrease following the exposure of MKN-45 cells to different
concentrations of 5-aza-CdR for 72 h. The results of the present
study clearly show that the transcriptional inactivation of CDX2
was due to hypermethylation. Notably, MKN-45 cells were treated
with 5-aza-CdR at various concentrations and the CCK-8 assay showed
that a concentration- and time-dependent growth inhibition of cell
proliferation occurred in the MKN-45 cells. Apoptosis analysis
revealed that 5-aza-CdR treatment increased the proportion of
apoptotoc cells, and morphological changes typical of apoptosis
were observed in the nuclear chromatin.
Previous studies have reported (42–45)
that the intrinsic mitochondrial or death receptor pathways are
able to trigger cell apoptosis, and the mitochondrial apoptotic
pathway, including caspase-dependent or independent apoptosis.
Caspases are able to cleave essential cellular substrates after
aspartic residues and are critical for the initiation and execution
phases of apoptosis. Caspase-8 is involved in the death-receptor
pathway while caspase-9 mediates the mitochondrial pathway. Once
activated, caspase-8 and caspase-9 activate downstream caspase-3,
triggering cell apoptosis. Therefore, we measured the activities of
caspase-3, caspase-8 and caspase-9 and the results showed that they
were all activated, suggesting that the mitochondrial and
death-receptor pathways were involved in 5-aza-CdR-induced
apoptosis. However, compared with caspase-8, the activity of
caspase-9 was higher in response to 5-aza-CdR, suggesting that the
apoptosis proceeded mainly via a caspase-dependent intrinsic
mitochondrial pathway.
In summary, the results of the current study suggest
the detection of CDX2 and DNMTl mRNA will be beneficial in
predicting the GC histological type and patient progression, and
may also be used as markers in the assessment of the biological
behavior of GC. Furthermore, our results confirmed that, in the GC
cell line MKN-45, the transcriptional inactivation of CDX2 was due
to hypermethylation and the high level of DNMT1. Treatment with a
DNMT1 inhibitor rescued the expression of CDX2, inhibited cell
proliferation and induced caspase-independent apoptosis. Therefore,
our study further emphasizes the importance of the CDX2 gene in
gastric carcinogenesis and progression, and a better understanding
of DNA methylation is likely to provide us with a potential
therapeutic target for GC.
Acknowledgements
This study was supported by the Social
Development Foundation of Nantong City (No.S2009022).
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Leung WK, Wu MS, Kakugawa Y, et al:
Screening for gastric cancer in Asia: current evidence and
practice. Lancet Oncol. 9:279–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Bird A: Perceptions of epigenetics.
Nature. 447:396–398. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Jones PA and Baylin SB: The epigenomics of
cancer. Cell. 128:683–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Turek-Plewa J and Jagodziński PP: The role
of mammalian DNA methyltransferases in the regulation of gene
expression. Cell Mol Biol Lett. 10:631–647. 2005.PubMed/NCBI
|
|
6.
|
Mizuno S, Chijiwa T, Okamura T, et al:
Expression of DNA methyltransferases DNMT1, 3A, and 3B in normal
hematopoiesis and in acute and chronic myelogenous leukemia. Blood.
97:1172–1179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lyko F and Brown R: DNA methyltransferase
inhibitors and the development of epigenetic cancer therapies. J
Natl Cancer Inst. 97:1498–1506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Silberg DG, Swain GP, Suh ER and Traber
PG: Cdx1 and cdx2 expression during intestinal development.
Gastroenterology. 119:961–971. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Almeida R, Silva E, Santos-Silva F, et al:
Expression of intestine-specific transcription factors, CDX1 and
CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol.
199:36–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kang JM, Lee BH, Kim N, et al: CDX1 and
CDX2 expression in intestinal metaplasia, dysplasia and gastric
cancer. J Korean Med Sci. 26:647–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Freund JN, Domon-Dell C, Kedinger M and
Duluc I: The Cdx-1 and Cdx-2 homeobox genes in the intestine.
Biochem Cell Biol. 76:957–969. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Baba Y, Nosho K, Shima K, et al:
Relationship of CDX2 loss with molecular features and prognosis in
colorectal cancer. Clin Cancer Res. 15:4665–4673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mallo GV, Rechreche H, Frigerio JM, et al:
Molecular cloning, sequencing and expression of the mRNA encoding
human Cdx1 and Cdx2 homeobox. Down-regulation of Cdx1 and Cdx2 mRNA
expression during colorectal carcinogenesis. Int J Cancer.
74:35–44. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Vider BZ, Zimber A, Hirsch D, et al: Human
colorectal carcinogenesis is associated with deregulation of
homeobox gene expression. Biochem Biophys Res Commun. 232:742–748.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Park do Y, Srivastava A, Kim GH, et al:
CDX2 expression in the intestinal-type gastric epithelial
neoplasia: frequency and significance. Mod Pathol. 23:54–61.
2010.PubMed/NCBI
|
|
16.
|
Saad RS, Ghorab Z, Khalifa MA and Xu M:
CDX2 as a marker for intestinal differentiation: Its utility and
limitations. World J Gastrointest Surg. 3:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Washington K: 7th edition of the AJCC
cancer staging manual: stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Mao ZB, Zhang JF, Xu Z, et al: Ectopic
expression of guanylyl cyclase C in gastric cancer as a potential
biomarker and therapeutic target. J Dig Dis. 10:272–285. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Herman JG, Graff JR, Myöhänen S, et al:
Methylation-specific PCR: a novel PCR assay for methylation status
of CpG islands. Proc Natl Acad Sci USA. 93:9821–9826. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yuasa Y, Nagasaki H, Akiyama Y, et al: DNA
methylation status is inversely correlated with green tea intake
and physical activity in gastric cancer patients. Int J Cancer.
124:2677–2682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
23.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Walters JR, Howard A, Rumble HE, et al:
Differences in expression of homeobox transcription factors in
proximal and distal human small intestine. Gastroenterology.
113:472–477. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Tamai Y, Nakajima R, Ishikawa T, et al:
Colonic hamartoma development by anomalous duplication in Cdx2
knockout mice. Cancer Res. 59:2965–2970. 1999.PubMed/NCBI
|
|
26.
|
Beck F, Chawengsaksophak K, Waring P, et
al: Reprogramming of intestinal differentiation and intercalary
regeneration in Cdx2 mutant mice. Proc Natl Acad Sci USA.
96:7318–7323. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Barbareschi M, Murer B, Colby TV, et al:
CDX-2 homeobox gene expression is a reliable marker of colorectal
adenocarcinoma metastases to the lungs. Am J Surg Pathol.
27:141–149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Werling RW, Yaziji H, Bacchi CE, et al:
CDX2, a highly sensitive and specific marker of adenocarcinomas of
intestinal origin: an immunohistochemical survey of 476 primary and
metastatic carcinomas. Am J Surg Pathol. 27:303–310. 2003.
View Article : Google Scholar
|
|
29.
|
Moskaluk CA, Zhang H, Powell SM, et al:
Cdx2 protein expression in normal and malignant human tissues: an
immunohistochemical survey using tissue microarrays. Mod Pathol.
16:913–919. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kaimaktchiev V, Terracciano L, Tornillo L,
et al: The homeobox intestinal differentiation factor CDX2 is
selectively expressed in gastrointestinal adenocarcinomas. Mod
Pathol. 17:1392–1399. 2004. View Article : Google Scholar
|
|
31.
|
Seno H, Oshima M, Taniguchi MA, et al:
CDX2 expression in the stomach with intestinal metaplasia and
intestinal-type cancer: Prognostic implications. Int J Oncol.
21:769–774. 2002.PubMed/NCBI
|
|
32.
|
Mizoshita T, Tsukamoto T, Nakanishi H, et
al: Expression of Cdx2 and the phenotype of advanced gastric
cancers: relationship with prognosis. J Cancer Res Clin Oncol.
129:727–734. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Fan Z, Li J, Dong B and Huang X:
Expression of Cdx2 and hepatocyte antigen in gastric carcinoma:
correlation with histologic type and implications for prognosis.
Clin Cancer Res. 11:6162–6170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Liu Q, Teh M, Ito K, et al: CDX2
expression is progressively decreased in human gastric intestinal
metaplasia, dysplasia and cancer. Mod Pathol. 20:1286–1297. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Song JH, Kim CJ, Cho YG, et al: Genetic
alterations of the Cdx2 gene in gastric cancer. APMIS. 116:74–80.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Okayama H, Kumamoto K, Saitou K, et al:
CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for
prediction of lymph node metastasis in primary gastric cancer.
Oncol Rep. 22:745–755. 2009.PubMed/NCBI
|
|
37.
|
Qin R, Wang NN, Chu J, et al: Expression
and significance of homeodomain protein Cdx2 in gastric carcinoma
and precancerous lesions. World J Gastroenterol. 18:3296–3302.
2012.PubMed/NCBI
|
|
38.
|
Xie Y, Li L, Wang X, et al: Overexpression
of Cdx2 inhibits progression of gastric cancer in vitro. Int J
Oncol. 36:509–516. 2010.PubMed/NCBI
|
|
39.
|
Ding WJ, Fang JY, Chen XY and Peng YS: The
expression and clinical significance of DNA methyltransferase
proteins in human gastric cancer. Dig Dis Sci. 53:2083–2089. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar
|
|
41.
|
Gilbert J, Gore SD, Herman JG and Garducci
MA: The clinical application of targeting cancer through histone
acetylation and hypomethylation. Clin Cancer Res. 10:4589–4596.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Susin SA, Daugas E, Ravagnan L, et al: Two
distinct pathways leading to nuclear apoptosis. J Exp Med.
192:571–580. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Wang X, Zhu S, Drozda M, et al:
Minocycline inhibits caspase-independent and -dependent
mitochondrial cell death pathways in models of Huntington’s
disease. Proc Natl Acad Sci USA. 100:10483–10487. 2003.PubMed/NCBI
|
|
44.
|
Antonsson B: Mitochondria and the Bcl-2
family proteins in apoptosis signaling pathways. Mol Cell Biochem.
256–257:141–155. 2004.PubMed/NCBI
|
|
45.
|
Stefanis L: Caspase-dependent and
-independent neuronal death: two distinct pathways to neuronal
injury. Neuroscientist. 11:50–62. 2005. View Article : Google Scholar : PubMed/NCBI
|