Introduction
Fractional exhaled nitric oxide (FeNO) has been
proposed as a biomarker of airway inflammation in asthma (1). The use of FeNO measurement in the
diagnosis and monitoring of asthma necessitates the identification
of reference values for FeNO measured with commercially available
equipment (2). However, reference
values vary between countries. Studies have shown that race is one
of the most important variables when considering reference values
(3) and the values from Caucasian
children cannot be applied to Asian individuals (2–4).
Currently, FeNO reference values exist for healthy Asian children
in the southern areas of Hong Kong and Taiwan (3,4).
This study aimed to establish FeNO reference values for the
northern areas of China to supplement the existing body of research
on Asian individuals.
Materials and methods
Study subjects
The participants included 300 healthy students
recruited randomly from two primary schools and one middle school
in Shenyang, China. The sample contained males and females, aged
6–14 years. The students were invited by the International Study of
Asthma and Allergic Disease in Children (ISAAC) to complete a
questionnaire (5). The subjects
had no reported history of wheezing or whistling in the chest at
any time in the past, including during or after exercise.
Additionally, the subjects had no reported history of nasal
(6,7) or asthmatic (8) problems and no history of dry cough at
night, apart from a cough associated with a typical cold or chest
infection in the last month. Moreover, any subject who in the
previous two weeks had any episode of infection, active or passive
exposure to cigarettes or parental or sibling disposition to asthma
or atopy, was excluded. The parents of the subjects provided the
relevant medical history information. The study was approved by the
local Education Bureau and by the schools. Written informed consent
was provided by parents and the subjects gave consent when aged 10
years or older. Forty-two subjects were excluded based on parental
responses to the medical history questions. A total of 258 students
participated in the FeNO meaurement and pulmonary function portions
of the study following the survey.
Measurements of FeNO
Testing occurred for 3 h in the morning. The
temperature of the test room was fixed at 22–24°C. Single-breath
online measurements of FeNO were taken by the Nitric Oxide
Monitoring System (NIOX) according to the American Thoracic Society
(ATS) guidelines (9–11). All subjects were asked not to eat,
drink or participate in heavy exercise during the hour before
testing. Testing began with the subject quiet and standing
comfortably. First, the subject’s nose was clipped and the subject
fully expelled air from the lungs, after which the subject inserted
the mouthpiece of the NIOX system and inhaled NO-free air calmly to
total lung capacity over a period of 2–3 sec. The subject then
exhaled steadily, maintaining a constant air speed. If the subject
breathed correctly, the machine emitted a continuous humming sound.
Subjects exhaled at a flow rate of 50 ml/sec. Children ≥130 cm in
height used a standard card to exhale for 10 sec while children
≤130 cm in height used a special card to exhale for 6 sec. The
system automatically calculated the value during the last 3 sec of
exhalation and displayed the value on the screen. Each subject
repeated the measurements at least 3 times to obtain 3 reliable
FeNO values, defined as three values varying by <10% from each
other or two values varying by <5%. The means of the three
values were then calculated. The subjects had a 2-min rest between
individual FeNO measurements.
Measurements of pulmonary function
Spirometry parameters for all subjects were measured
using a Power-Cube Spirometer (Ganshorn, Niederlauer, Germany)
according to the ATS guidelines (12). The guidelines included the use of
nose clips and the adoption of a standing position after
measurements of FeNO were taken. We received the predicted values
of lung function variables, including forced vital capacity (FVC),
forced expiratory volume (FEV1), FEV1/FVC and peak expiratory flow
(PEF). If the FEV1 for a subject was <80%, the subject was
excluded from further analyses. Values from 219 students were
used.
Measurements of body mass index
(BMI)
The height and weight of the subjects were also
recorded at the time of FeNO measurement.
Statistical analysis
Data were analyzed with SPSS version 13.5 (SPSS
Inc., Chicago, IL, USA). Due to the skewed distribution of FeNO
values, the FeNO levels in parts per billion (ppb) were presented
as the mean and median with interquartile range (IQR). Logarithmic
transformations were calculated for the FeNO values. The results
were presented using geometric means and upper 95% confidence
limits. Either the Student’s t-test or the Mann-Whitney U test was
used for between-group comparison. Since spirometry values (FVC,
FEV1, FEV1/FVC and PEF) were not normally distributed, the
arithmetic means were not used for further data analysis.
Polynomial linear trend analysis was used to examine the
correlation between FeNO and gender, age, weight, height, BMI, FVC,
FEV1, FEV1/FVC and PEF. Linear regression, multivariate analysis
and Pearson correlation analysis were also employed for this study.
P<0.05 was considered to indicate a statistically significant
difference.
Results
The general characteristics of the participants in
this study are displayed in Table
I. The subjects with a FEV1 <80% were excluded, leaving 219
students available for analyses. Of the 219 Chinese individuals in
the sample, 103 were males. The median FeNO value for the group was
11 ppb (IQR, 8–16 ppb). Table II
shows the comparison of FeNO levels in children from other
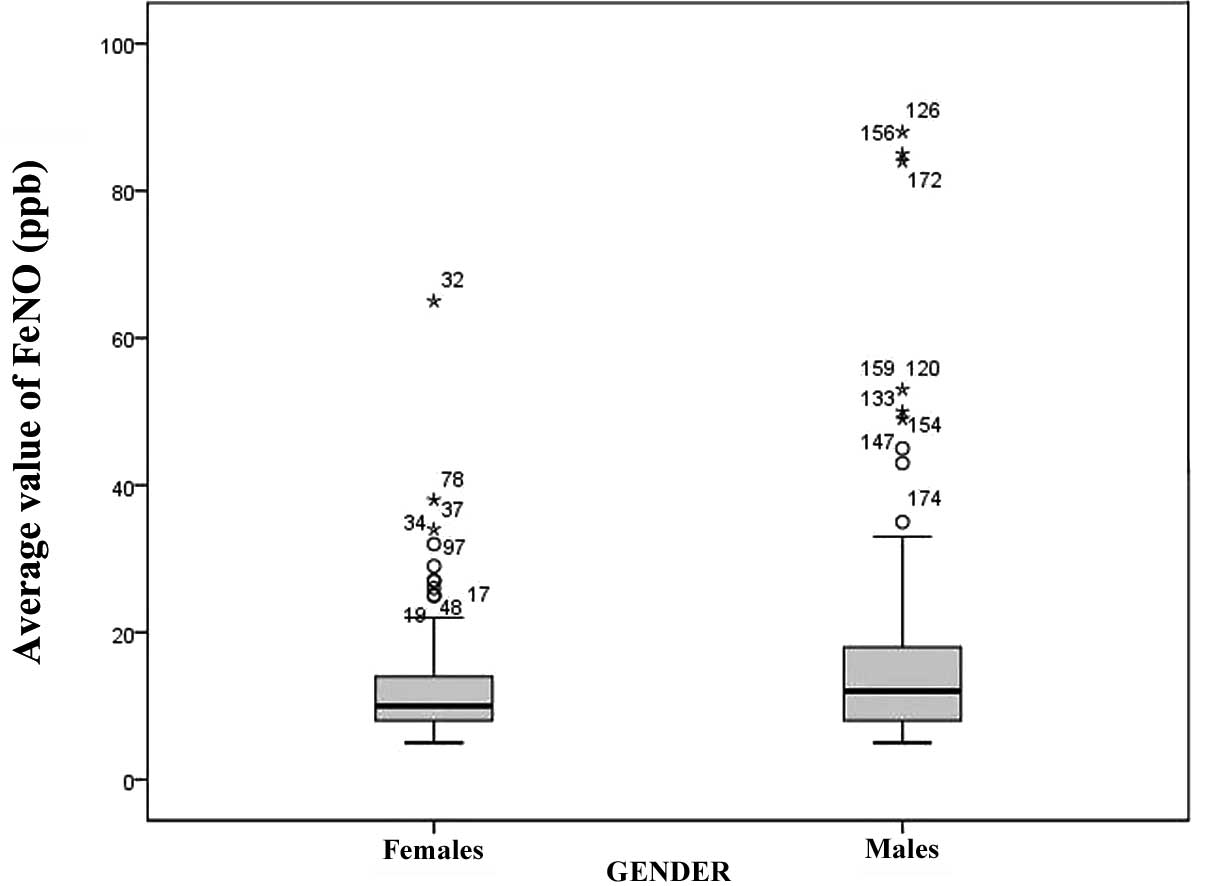
countries and areas. In males, the median FeNO was 13 with an IQR
of 9–18, while in females the median was 10 with an IQR of 8–14.
The FeNO level was significantly higher for males than females
(P<0.05; Fig. 1).
 | Table IGeneral characteristics of subjects
(n=219). |
Table I
General characteristics of subjects
(n=219).
| Parameter | Minimum | Maximum | Mean ± SD |
|---|
| Age (years) | 6 | 15 | 10.16±2.805 |
| Height (cm) | 100 | 185 | 142.85±17.737 |
| Weight (kg) | 19 | 91 | 38.32±14.43 |
| BMI
(kg/m2) | 9.4 | 30.5 | 18.085±3.1934 |
|
FVCpredicted (%) | 69 | 130 | 88.59±9.881 |
|
FEV1predicted (%) | 80 | 140 | 96.17±10.475 |
|
FEV1/FVCpredicted (%) | 71 | 119 | 105.42±6.972 |
| PEF | 36 | 122 | 74.26±18.365 |
 | Table IIComparison of FeNO levels in children
from other countries and areas. |
Table II
Comparison of FeNO levels in children
from other countries and areas.
| Study year | Author (ref) | Country/area
(ethnicity) | N | Age range
(years) | Mean FeNO (ppb) | Median FeNO
(ppb) | FeNO IQR |
|---|
| 2005 | Buchvald et al
(2) | Italy | 405 | 4.0–17.0 | 9.7 | | |
| 2005 | Wong et al
(3) | Hong Kong
(Chinese) | 253 | 11.0–18.0 | 25.3 | 17.0 (M) | 10.7–36.6 |
| | | | | 15.8 | 10.8 (F) | 7.8–17.6 |
| | Hong Kong
(Caucasian) | 33 | 11.0–18.0 | 14.9 | 11.6 (M) | 8.2–19.3 |
| | | | | | 9.1 (F) | 7.5–11.9 |
| 2008 | Kovesi et al
(23) | Canada
(Caucasion) | | 9.1–12.9 | 12.7 | | 11.8–13.7 |
| | Canada (Asian) | | | 22.8 | | 17.9–27.7 |
| 2010 | Perzanowski et
al (1) | America | | 7 | | 12 | 10.6–13.7 |
| 2011 | Yao et al
(4) | Taiwan | | | 13.7 | | |
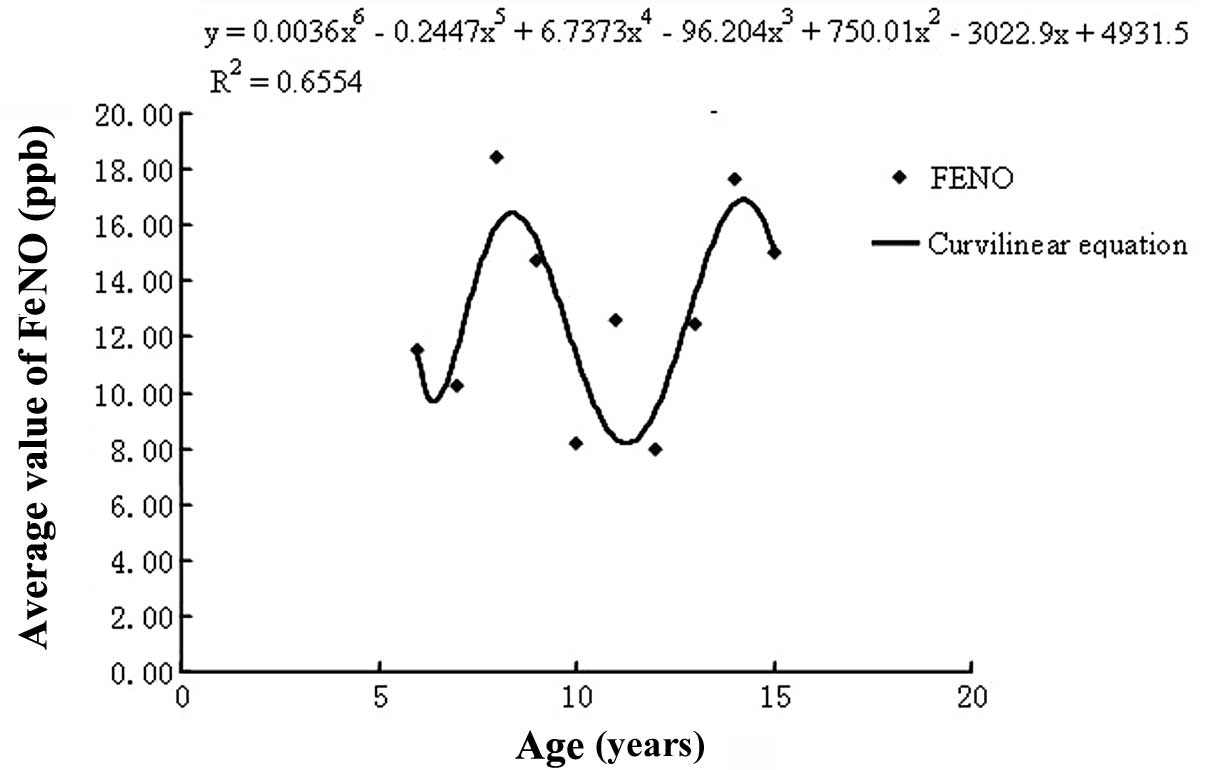
Furthermore, the correlation analyses revealed age
to be correlated with FeNO levels (R2= 0.66). Other
potentially significant factors, including height (R2=
0.14), weight (R2= 0.17), BMI (R2= 0.099),
FVCpredicted (R2=0.037),
FEV1predicted (R2=0.023),
FEV1/FVCpredicted (R2=0.02) and PEF
(R2= 0.019) did not demonstrate a statistical
correlation. We therefore identified reference FeNO values for
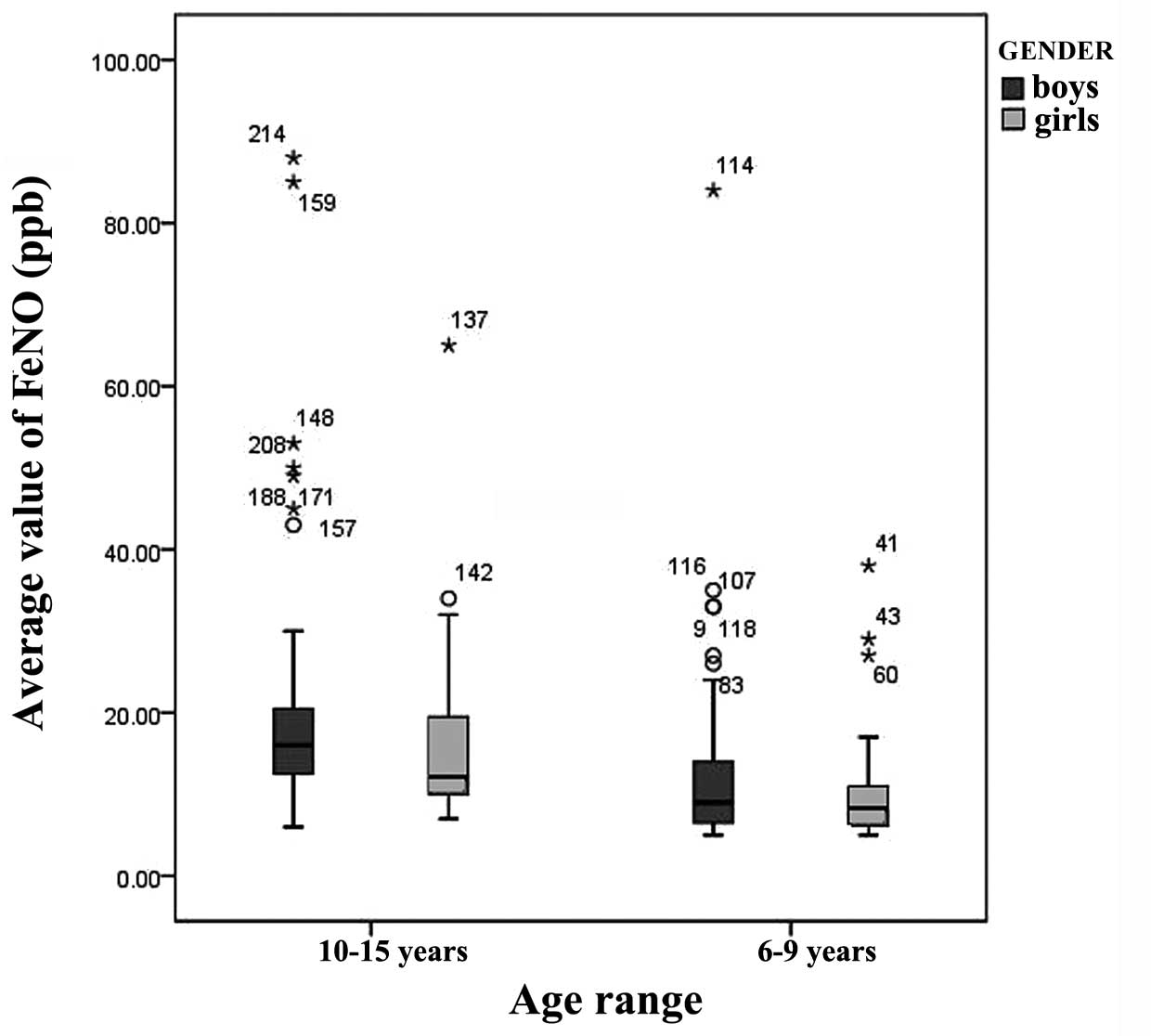
northern Chinese children according to gender and age (Fig. 2). For individuals aged 6–9 years,
the median FeNO level for males was 9 ppb (range, 6.5–14 ppb; 95%
CI, 27.71 ppb) while for females it was 8 ppb (range, 6–11 ppb; 95%
CI, 18.01 ppb). For individuals aged 10–15 years, the median FeNO
level for males was 16 ppb (range, 12.75–20.25 ppb; 95% CI, 46.98
ppb) while for females it was 12 ppb (range, 10–19 ppb; 95% CI,
30.33 ppb; Fig. 3).
Discussion
In the respiratory tract, NO is produced by a wide
variety of cells, including airway epithelial cells, airway and
circulatory endothelial cells and trafficking inflammatory cells,
in large and peripheral airways and alveoli (13). NO is a gaseous signaling molecule
that is generated by three isoenzymes of NO synthase (NOs) that are
differentially regulated and expressed in the airways and that
appear to play different pathophysiologic roles (14). The measurement of FeNO as a marker
of airway inflammation is useful given the positive correlation
with the degrees of airway hyper-responsiveness (AHR) (15) and eosinophilia in induced sputum,
blood, bronchoalveolar lavage and bronchial mucosa. Asthma is
accurately diagnosed in daily practice on the basis of subjective
symptoms and FeNO levels, particularly in atopic patients (16,17).
Previous studies have suggested that FeNO is a valid
and effective marker in the monitoring of treatment for pediatric
asthma (18,19). However, it would be imprudent to
recommend the systematic treatment of patients with high FeNO
values and asthma with an allergy component who are asymptomatic or
under good control (20). FeNO
values have not been evaluated prospectively as an aid in asthma
diagnosis; however, these measurements have been shown to
contribute to optimal treatment and responsiveness to steroids
(21) and may be used to
distinguish asthma from other wheezing diseases (22). The values for FeNO in healthy
children require investigation to develop the cut-off points for
the judgement of normal vs. abnormal levels.
Since our study included healthy children from
public school institutions, it was not feasible to measure atopy
objectively by a skin-prick test or total specific immunoglobulin
(Ig)-E. Therefore, we are not able to rule out the possibility that
atopic subjects may have higher FeNO levels. However, a strict
questionnaire was used to select subjects and we excluded those
whose FEV1 was <80%; therefore, we consider our results to be
reliable. In this study of a northern city in China, the median
level of FeNO was 11 ppb (IQR, 8–16 ppb) and the FeNO was
significantly associated with gender and age. Consequently, the
FeNO levels were divided into four catetories, according to gender
and age: males aged ≤9 years; males aged >9 years; females aged
≤9 years and females aged >9 years. Weight, height, BMI, FVC,
FEV1, FVC/FEV1 and PEF were not correlated with FeNO levels.
A number of studies concerning FeNO in healthy
children have been conducted and the conclusions of the current
study are similar. However, these studies differ in the country of
origin, sample ethnicity and time of study, as well as the number
and ages of the individuals studied. While these data were not
statistically analyzed, we compared them with ours to better
understand the condition of FeNO in these children. In 2005 in
Italy, Buchvald et al (2)
reported a mean FeNO level of 9.7 ppb. In 2005 in Canada, Kovesi
et al (23) reported a mean
FeNO level for Caucasian Canadian children of 12.7 ppb (range,
11.8–13.7 ppb) and 22.8 ppb (range, 17.9–27.7 ppb) for Asian
Canadian children aged 9.1–12.9 years. In 2010 in the United
States, Perzanowski et al (1) suggested that the level of FeNO at age
7 is 12 ppb (range, 10.6–13.7 ppb). Two studies of FeNO in Hong
Kong and Taiwan were conducted in 2005 and 2011 (4), respectively. Wong et al
(3) reported that the level of
FeNO for male children was 17 ppb (range, 10.7–36.6 ppb) and for
female children was 10.8 (range, 7.8–17.6). Our results are
consistent with the findings from the studies carried out in Hong
Kong and Taiwan. This suggests that there are similarities in FeNO
in these countries. Our findings are also in agreement with those
from Caucasian Canadians but less so with those from Asian
Canadians (24). We observed that
the FeNO levels of Asian Americans are greater than those of
Chinese individuals. Although observations have been made in
individuals of the same ethnicity, different values for FeNO have
been observed in different environments. Therefore, factors besides
ethnicity may affect the value of FeNO in healthy children.
In FeNO studies of healthy children, a number of
variables have been considered, including gender, age, weight, BMI,
atopy and environmental tobacco smoke (ETS) (1,25,26).
However, in adults, these factors are not correlated with FeNO
(27).
Buchvald et al (2) observed a mean level of FeNO of 9.7
ppb and there was no difference in FeNO between males and females.
FeNO was significantly and positively correlated with age in males
and females, with an increase in FeNO of 5% per year (2). Although data remain equivocal,
sensitization, rhinitis and conjunctivitis may be independent
determinants of FeNO in subjects of all ages without asthma.
However, the authors did not study the effect of ethnic background
on FeNO (2). Multiple factors
affect FeNO in healthy children, including race, age and height.
One study used a formula for FeNO: Ln(FeNO) = 1.993 + age x 0.046
(4).
In the current study, gender and age were identified
to be significant factors that affected FeNO in healthy children.
Lung function is an important parameter in the diagnosis and
monitoring of asthma (8). However,
spirometry had no significant correlation with FeNO in healthy
children or adults. Lung function indices, including
FEV1-reversibility or provocation tests, are only indirectly
associated with airway inflammation. FEV1 is ≥80% in a number of
children whose airway clearance technique (ACT) is abnormal and
whose FeNO is higher (28).
Another study observed a correlation between FeNO and FEV1 while
forced expiratory flow (FEF) did not independently contribute to
FeNO variance (28). Official ATS
clinical practice guidelines (21)
recommend the use of FeNO in the monitoring of airway inflammation
in patients with asthma and states that FeNO is a predictor of
steroid responsiveness during the absence of induced sputum. An
increasing number of countries and areas have participated in
studies of FeNO and in the current study we examined more data to
identify the factors that affect FeNO.
Acknowledgements
This study was supported by the
Construction Program of Medical Science Foundation in the Liaoning
Province (grant no: 2009Z012). The authors would like to thank
American Journal Experts for excellent grammar revisions of this
paper.
References
|
1
|
Perzanowski MS, Divjan A, Mellins RB, et
al: Exhaled NO among inner-city children in New York City. J
Asthma. 47:1015–1021. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buchvald F, Baraldi E, Carraro S, et al:
Measurements of exhaled nitric oxide in healthy subjects age 4 to
17 years. J Allergy Clin Immunol. 115:1130–1136. 2005.PubMed/NCBI
|
|
3
|
Wong GW, Liu EK, Leung TF, et al: High
levels and gender difference of exhaled nitric oxide in Chinese
schoolchildren. Clin Exp Allergy. 35:889–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao TC, Lee WI, Ou LS, Chen LC, Yeh KW and
Huang JL; PATCH Study Group: Reference values of exhaled nitric
oxide in healthy Asian children aged 5 to 18 years. Eur Respir J.
39:378–384. 2012.PubMed/NCBI
|
|
5
|
Asher MI and Weiland SK: The International
Study of Asthma and Allergies in Childhood (ISAAC). ISAAC Steering
Committee. Clin Exp Allergy. 28(Suppl 5): 52–66; discussion 90–1,
1998.
|
|
6
|
Ishizuka T, Matsuzaki S, Aoki H, et al:
Prevalence of asthma symptoms based on the European Community
Respiratory Health Survey questionnaire and FENO in university
students: gender differences in symptoms and FENO. Allergy Asthma
Clin Immunol. 7:152011. View Article : Google Scholar
|
|
7
|
Xu F, Zou Z, Yan S, et al: Fractional
exhaled nitric oxide in relation to asthma, allergic rhinitis, and
atopic dermatitis in Chinese children. J Asthma. 48:1001–1006.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Global Strategy for Asthma Management and
Prevention: 2011.Global Initiative for Asthma. https://www.ginasthma.org. Accessed
December 1, 2011.
|
|
9
|
American Thoracic Society and European
Respiratory Society: ATS/ERS recommendations for standardized
procedures for the online and offline measurement of exhaled lower
respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir
Crit Care Med. 171:912–930. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
No authors listed:. Recommendations for
standardized procedures for the on-line and off-line measurement of
exhaled lower respiratory nitric oxide and nasal nitric oxide in
adults and children-1999. This official statement of the American
Thoracic Society was adopted by the ATS Board of Directors, July
1999. Am J Respir Crit Care Med. 160:2104–2117. 1999.
|
|
11
|
Baraldi E and de Jongste JC; European
Respiratory Society/ American Thoracic Society (ERS/ATS) Task
Force: Measurement of exhaled nitric oxide in children. 2001. Eur
Respir J. 20:223–237. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller MR, Hankinson J, Brusasco V, et al:
Standardisation of spirometry. Eur Respir J. 26:319–338. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barnes PJ, Dweik RA, Gelb AF, et al:
Exhaled nitric oxide in pulmonary diseases: a comprehensive review.
Chest. 138:682–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ricciardolo FL, Sterk PJ, Gaston B and
Folkerts G: Nitric oxide in health and disease of the respiratory
system. Physiol Rev. 84:731–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Motomura C, Odajima H, Tezuka J, et al:
Effect of age on relationship between exhaled nitric oxide and
airway hyper-responsiveness in asthmatic children. Chest.
136:519–525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fukuhara A, Saito J, Sato S, et al:
Validation study of asthma screening criteria based on subjective
symptoms and fractional exhaled nitric oxide. Ann Allergy Asthma
Immunol. 107:480–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cordeiro D, Rudolphus A, Snoey E and
Braunstahl GJ: Utility of nitric oxide for the diagnosis of asthma
in an allergy clinic population. Allergy Asthma Proc. 32:119–126.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Jongste JC, Carraro S, Hop WC; CHARISM
Study Group; Baraldi E: Daily telemonitoring of exhaled nitric
oxide and symptoms in the treatment of childhood asthma. Am J
Respir Crit Care Med. 179:93–97. 2009.PubMed/NCBI
|
|
19
|
Verini M, Consilvio NP, Di Pillo S, et al:
FeNO as a marker of airways inflammation: the possible implications
in childhood asthma management. J Allergy (Cairo) 2010.
pii:691425:2010.PubMed/NCBI
|
|
20
|
Ferrer M, Jarque A, Tosca R and Michavila
A: Is it necessary to treat all asthmatic children with raised
levels of exhaled nitric oxide?: treating the patient or the data.
Allergol Immunopathol (Madr). 39:280–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dweik RA, Boggs PB, Erzurum SC, et al: An
official ATS clinical practice guideline: interpretation of exhaled
nitric oxide levels (FENO) for clinical applications. Am J Respir
Crit Care Med. 184:602–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dor-Wojnarowska A, Liebhart J, Grabowski
M, Czapla L, Grabowski K and Panaszek B: Exhaled nitric oxide in
patients with esophagitis. Pneumonol Alergol Pol. 79:272–277.
2011.(In Polish).
|
|
23
|
Kovesi T, Kulka R and Dales R: Exhaled
nitric oxide concentration is affected by age, height, and race in
healthy 9- to 12-year-old children. Chest. 133:169–175.
2008.PubMed/NCBI
|
|
24
|
Linn WS, Rappaport EB, Berhane KT, Bastain
TM, Avol EL and Gilliland FD: Exhaled nitric oxide in a
population-based study of southern California schoolchildren.
Respir Res. 10:282009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mahut B, Peiffer C, Thibaudon M, et al:
What does a single exhaled nitric oxide measurement tell us in
asthmatic children? J Asthma. 46:810–814. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Laoudi Y, Nikasinovic L, Sahraoui F,
Grimfeld A, Momas I and Just J: Passive smoking is a major
determinant of exhaled nitric oxide levels in allergic asthmatic
children. Allergy. 65:491–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsunaga K, Hirano T, Kawayama T, et al:
Reference ranges for exhaled nitric oxide fraction in healthy
Japanese adult population. Allergol Int. 59:363–367. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Papakosta D, Latsios D, Manika K, Porpodis
K, Kontakioti E and Gioulekas D: Asthma control test is correlated
to FEV1 and nitric oxide in Greek asthmatic patients: influence of
treatment. J Asthma. 48:901–906. 2011. View Article : Google Scholar : PubMed/NCBI
|