Introduction
The estimated incidence of squamous cell carcinoma
of the head and neck (SCCHN) in the USA in 2010 was ∼49,260 cases
with 11,480 mortalities (1). The
majority of patients with newly diagnosed SCCHN present with
locally advanced disease. Multiple treatment modalities have been
utilized for locally advanced SCCHN (LA-SCCHN), including various
combinations of chemotherapy, radiation therapy and surgery.
However, the most effective combination has yet to be determined
(2,3). When chemoradiotherapy is employed,
the most commonly used standard regimen is daily radiation
concurrent with three cycles of bolus cisplatin. Only bolus
cisplatin and weekly cetuximab are supported by level I evidence
(4).
Although chemoradiation has the potential to cure
stage IV disease, a significant number of patients will relapse,
particularly those with higher nodal status at presentation
(5). Induction chemotherapy prior
to definitive chemoradiation in patients with LA-SCCHN is one
approach presently used to improve outcomes, although its use is
controversial. Two phase III trials published in 2007, TAX 323 and
TAX 324 (6,7), studied induction chemotherapy with
docetaxel, cisplatin and fluorouracil (TPF) and provided indirect
evidence of the efficacy of induction chemotherapy. The addition of
docetaxel to cisplatin/fluorouracil in both studies increased
response rates (RRs) as well as overall survival (OS) compared with
that achieved with PF alone, however, neither study featured
definitive standard of care chemoradiotherapy in the control arm.
While these studies resulted in the FDA approval of docetaxel as
part of induction chemotherapy in the USA, they were heavily
criticized for comparing two experimental regimens, instead of
comparing either to an accepted standard of care (8).
The high rates of toxicity reported in TAX 323 and
TAX 324 led many oncologists to question the feasibility of TPF. In
TAX 324, 21% of patients did not proceed to protocol-defined
chemoradiotherapy and 7% of patients did not proceed to potentially
curative therapy (7,9). Following the presentation of DeCIDE
(10) and PARADIGM (11) trial data at ASCO 2012, enthusiasm
for TPF diminished further. However, the ultimate utility of
induction chemotherapy is yet to be resolved. Whether induction as
a concept is flawed or whether it has been tested with the wrong
regimen thus far remains unknown.
One of the most promising induction chemotherapy
regimens evaluated to date consists of weekly cetuximab (C225),
carboplatin and paclitaxel. Carboplatin and paclitaxel were used
together in a phase II study consisting mainly of stage IV patients
where a clinical complete RR of 100%, 3-year OS of 70% and a 3-year
progression-free survival (PFS) of 90% were demonstrated following
completion of definitive chemoradiotherapy (12). Although the regimen was less toxic
compared with TPF, RR to induction chemotherapy was preserved; RR
to TPF in TAX 323 and TAX 324 was 68 and 72%, respectively. In the
study by Haraf et al (12),
the RR to weekly carboplatin/paclitaxel was 82%. Contemporaneously,
cetuximab was demonstrated to be efficacious in head and neck
cancer yielding improvements in survival when used concurrently
with radiation (13,14) and palliative chemotherapy (15). This led to interest in grafting
cetuximab onto the carboplatin/paclitaxel induction backbone. This
was first studied in a phase II clinical trial published in 2010 by
Kies et al (16) as well as
in a second phase II study by Wanebo et al (17). The Kies study employed six weekly
doses of cetuximab, carboplatin and paclitaxel followed by
radiation alone, chemoradiation or surgery using an adaptive
risk-based strategy. All 47 patients proceeded to potentially
curative therapy, and PFS and OS rates were 87 and 91%,
respectively, following a median follow-up of 33 months. Response
to induction was 96%. The Wanebo ‘organ preservation’ study
included 74 operable patients with stage III or IV disease, each of
whom were treated with weekly cetuximab, carboplatin and
paclitaxel, followed by serial biopsies. In total, 65% of patients
demonstrated a pathological complete response following induction
and 100% demonstrated a pathological complete response following
subsequent chemoradiation. Most recently, cetuximab, carboplatin
and paclitaxel were evaluated with a different schedule, where
carboplatin was administered on a monthly bolus (18). The RR to the induction portion of
this study was 92%. The abstract did not report any toxicity from
induction therapy alone, however, the total toxicity was high,
which is likely based in part on the choice of subsequent
chemoradiotherapy regimen.
Based upon these data, the treating clinicians at
the University of Pennyslvania (PA, USA) viewed the combination of
carboplatin, paclitaxel and cetuximab as a less toxic, potentially
more efficacious alternative to TPF. The regimen was therefore
adopted in 2008 as the exclusive induction strategy for advanced
neck disease in SCCHN, typically N2b or greater.
Patients and methods
Patient identification
To be considered eligible for this retrospective
review, patients had to have been diagnosed with LA-SCCHN between
May 2008 and December 2011, and treated with the induction regimen
of weekly cetuximab, carboplatin and paclitaxel prior to definitive
chemoradiation or surgery. Appropriate patients were identified
through review of all subjects treated during this period at the
University of Pennsylvania with SCCHN. Patients who received this
regimen in the setting of recurrent or metastatic SCCHN were
excluded. None of these identified patients were excluded from the
analysis.
Database
Once patients were identified, we reviewed
electronic medical charts to extract data for baseline
characteristics including TNM status of the tumor, the primary
tumor site, tumor HPV-16 status (if available), date of diagnosis,
gender, performance status, Charlson Comorbidity Index, body weight
throughout therapy and smoking status. Additionally, doses of
induction chemotherapy as well as definitive chemotherapy and
radiation regimens, date of treatment and toxicity of therapy were
obtained. Toxicity was graded retrospectively by CTCAE version 3.
These results were entered into a password-protected tumor
database, which was maintained in Microsoft Access. This
retrospective collection of data was approved by the University of
Pennsylvania Institutional Review Board.
Written informed consent
Written informed consent was not required as data
were obtained retrospectively with the permission of the
institutional review board. Patient data were de-identified upon
chart extraction then maintained in a password-protected database.
All patients receiving chemotherapy and cetuximab signed standard
systemic therapy consent forms prior to receiving treatment.
Data collection
Toxicity data were extracted retrospectively through
chart review of EPIC, the outpatient electronic medical record used
at the University of Pennsylvania. The data were gathered from
review of oncology, nutrition and radiation oncology provider
notes. All laboratory studies acquired subsequent to the initiation
of chemotherapy and cetuximab were reviewed to assess for
hematologic toxicity. All nonhematological toxicity data were
obtained retrospectively only if they were recorded formally in a
complete review of systems or through a standardized, itemized
checklist used by the provider. If the data available were
ambiguous in gradation of symptoms and severity, we defaulted to
the higher grade of toxicity.
Treatment data were extracted from the outpatient
EPIC system and through the inpatient order-entry system, Sunrise,
when chemotherapy was administered on an inpatient basis. The
nature and doses of chemotherapy were documented from scanned order
sheets and electronic medical records. Doses of radiation were
determined in the radiation oncology progress notes and in the
summary completion notes. Reasons for changes in chemotherapy doses
and regimens were documented in the oncology provider notes.
Treatment
Induction chemotherapy with cetuximab, carboplatin
and paclitaxel was most commonly administered over eight weeks,
with an additional loading dose of 400 mg/m2 i.v.
cetuximab administered alone the week prior to starting the
three-drug regimen. During subsequent weeks, patients received
cetuximab 250 mg/m2 in combination with weekly
carboplatin AUC 2 and paclitaxel 90 mg/m2, with standard
premedications of dexamethasone 12 mg i.v., diphenhydramine 50 mg
i.v. and ondansetron 24 mg i.v. Treatment cycles were repeated
weekly for up to 8 weeks and doses were withheld or modified at the
provider’s discretion depending on the types of toxicities
experienced.
Radiotherapy was initiated ∼3 weeks after induction
chemotherapy was completed. Target volumes, duration of therapy and
total radiation dose were determined by the treating radiation
oncologist based on the standard of care. All radiation was planned
for 7 weeks of the total treatment.
Concurrent therapy during radiotherapy was
determined at the discretion of the provider. Depending on
performance status and prior toxicities, the regimens used included
either high-dose cisplatin (100 mg/m2) administered
every 3 weeks for three doses, weekly cetuximab, weekly cisplatin
(30 mg/m2) or weekly carboplatin (AUC 2) and paclitaxel
(30 mg/m2). If toxicities were encountered during
chemoradiotherapy, providers either reduced the dose of the chosen
regimen or changed to a less toxic regimen including weekly
cetuximab (250 mg/m2), weekly carboplatin (AUC 2) or
weekly cisplatin (30 mg/m2). There were no prescribed
criteria. No other agents were administered.
Response
Partial or complete response to the combined
induction chemotherapy and subsequent chemoradiotherapy or surgery
was evaluated retrospectively through physical exam notation in
provider notes, nasopharyngolaryngoscopy (NPL) where available and
imaging (PET, CT or MRI). Partial and complete RRs were defined by
the Response Evaluation Criteria in Solid Tumors (RECIST)
criteria.
Statistical analysis
Data from Microsoft Access were exported into
Microsoft Excel where all analysis was completed. The primary
endpoint of this retrospective analysis was feasibility, as
measured by the number of induction cycles tolerated. Other
pre-specified secondary endpoints included percentage of patients
progressing to definitive chemoradiotherapy, toxicity, response and
overall and median survival. Overall and median survival curves
were calculated with the standard Kaplan-Meier method.
Results
Patients
Thirty patients were identified in this
retrospective analysis between May 2008 and December 2011. These
constituted all treatment-naïve patients receiving induction
therapy for LA-SCCHN at the University of Pennsylvania during that
period. Patient and tumor characteristics are summarized in
Table I. The median age was 58.3
years (range, 37.4–83.6), median performance status was 0 and
median Charlson Comorbidity index (combined condition and
age-related score) was 4. Thirty percent were never smokers; of the
smokers, 33% smoked ≤10 pack-years and 37% smoked >10
pack-years. Base of the tongue or tonsil were the primary tumor
sites in 77% of the patients. Table
II displays the tumor and nodal distribution matrix. No patient
had metastatic disease at the start of induction chemotherapy. All
but one patient had stage IVa or IVb SCCHN.
 | Table IPatient and tumor characteristics
(n=30). |
Table I
Patient and tumor characteristics
(n=30).
| Characteristic | Value |
|---|
| Age (years) | |
| Median | 58.3 |
| Range | 37.4–83.6 |
| Gender, n (%) | |
| Male | 30 (100) |
| Female | 0 (0) |
| Performance status, n
(%) | |
| 0 | 25 (83.3) |
| 1 | 5 (16.7) |
| Charlson score, n
(%) | |
| 2 | 7 (23.3) |
| 3 | 7 (23.3) |
| 4 | 8 (26.7) |
| 5 | 7 (23.3) |
| 6 | 0 (0) |
| 7 | 1 (3.3) |
| Smoking status, n
(%) | |
| Never smokers | 9 (30.0) |
| 1–5 pk/year | 8 (26.7) |
| 6–20 pk/year | 7 (23.3) |
| 20–40 pk/year | 3 (10) |
| >40 pk/year | 3 (10) |
| Site of primary
tumor, n (%) | |
| Base of tongue | 15 (50) |
| Tonsil | 8 (26.7) |
| Hypopharynx | 1 (3.3) |
| Larynx | 2 (6.7) |
| Oral cavity | 4 (13.3) |
| HPV status, n
(%) | |
| p16 status
unknown | 17 (56.7) |
| p16 positive | 10 (33.3) |
| p16 negative | 3 (10) |
 | Table IITumor (T) and nodal (N) distribution
(n=30). |
Table II
Tumor (T) and nodal (N) distribution
(n=30).
| N | No. of patients by T
classification
| Total no. of
patients |
|---|
| Tx | T1 | T2 | T3 | T4 |
|---|
| N0 | | | 1 | | | 1 |
| N1 | | | | | | 0 |
| N2a | | | 1 | | 1 | 2 |
| N2b | 2 | | 5 | 2 | 3 | 12 |
| N2c | | | 2 | 1 | 8 | 11 |
| N3 | | 1 | 1 | | 2 | 4 |
| Total | 2 | 1 | 10 | 3 | 14 | 30 |
HPV data
HPV data were available for 13 patients. p16
staining as a surrogate for HPV was reported as an addendum to
pathology reports from patient specimens. Ten patients tested
positive. The primary sites of these ten cases were all either base
of tongue or tonsil. The primary sites of the three with negative
p16 staining were base of tongue, hypopharynx and oral cavity.
Treatment
All patients received induction chemotherapy with
weekly paclitaxel, carboplatin and cetuximab, followed by combined
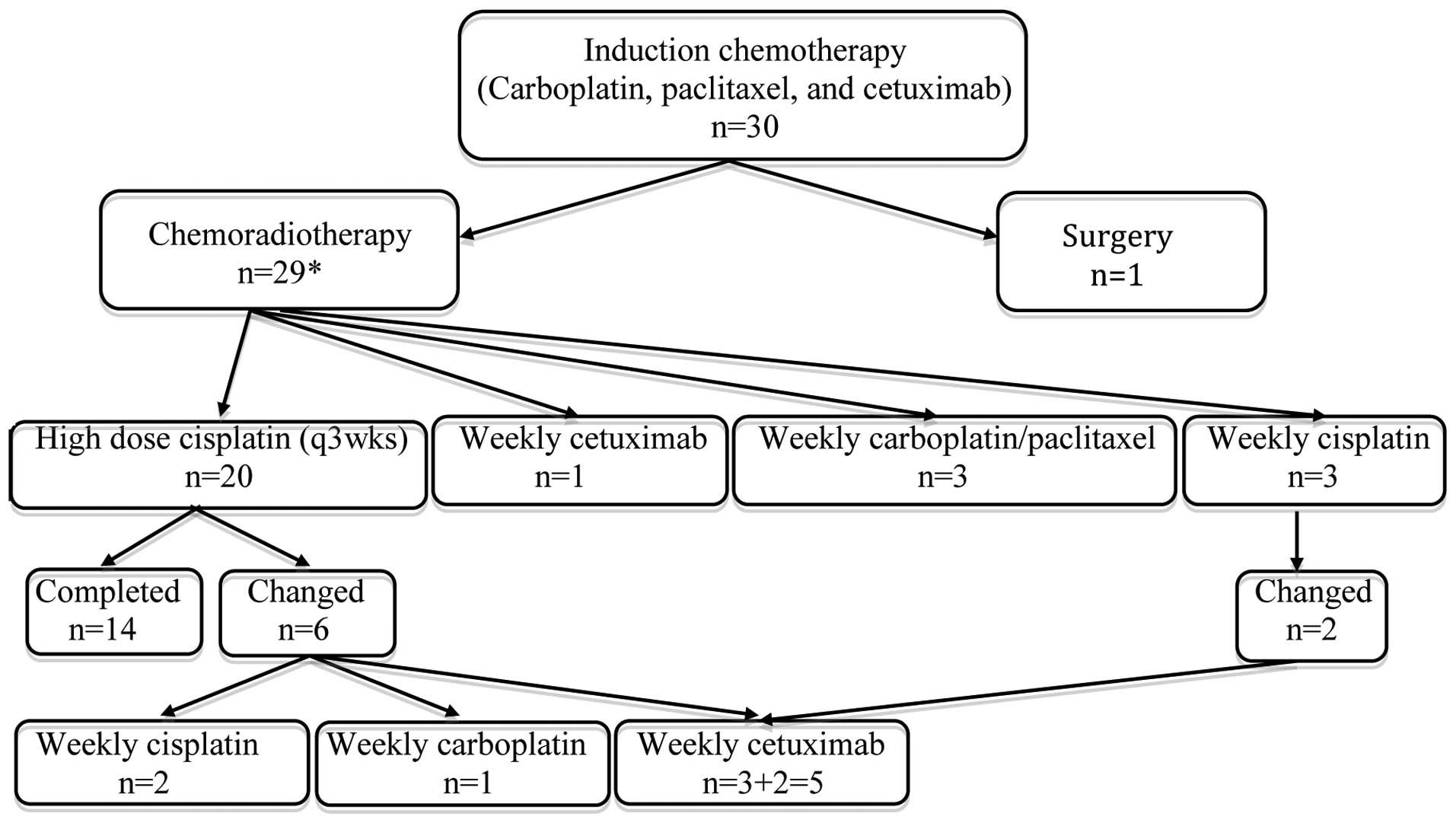
chemoradiotherapy (n=29) or surgery (n=1). Treatment disposition is
further delineated in Fig. 1. In
addition, four patients underwent neck dissections due to possible
residual disease on imaging.
Induction chemotherapy and toxicity
The majority of patients received their intended
treatment (Table III). Induction
chemotherapy was administered over a median of 48.2 days (range,
13.2–53.7), with some variability in the number of pre-planned
cycles. The most common planned regimen was eight cycles (not
including the loading dose of 400 mg/m2 cetuximab, which
all patients received the week prior to starting the three-drug
regimen); however, the range was from 5 to 8 pre-planned cycles.
The dose intensity for all treated patients was high and the number
of doses withheld or reduced for all three agents was low (Table III).
 | Table IIIInduction chemotherapy. |
Table III
Induction chemotherapy.
| Parameter | Carboplatin | Paclitaxel | Cetuximab |
|---|
| Number of doses
administered | | | |
| Median | 7 | 8 | 8 |
| Range | 2–8 | 2–8 | 0–8 |
| Dose intensity,
(%) | | | |
| Total doses
expected | 229 | 229 | 229 |
| Total doses
administered | 211 (92.1) | 212 (92.6) | 195 (85.2) |
| Total doses
held | 18 (7.9) | 17 (7.4) | 34 (14.8) |
| Total doses
reduced | 0 (0) | 21 (9.2) | 2 (0.9) |
Overall, induction chemotherapy was well tolerated
(Table IV). There were no observed grade 5 events. The only grade
3–4 toxicities included rash (6.7%), neutropenia without fever
(6.7%) and infusion reactions (3.3%). The most common grade 1–2
toxicities included rash (77%), nausea (37%), fatigue (37%), anemia
(17%), alopecia (27%) and electrolyte abnormalities (13%). No renal
toxicity, hearing loss or febrile neutropenia were observed. Three
patients (10%) were hospitalized during induction chemotherapy; two
for non-neutropenic infections and the third for neutropenia
without fever.
The majority of patients’ body weights remained
stable throughout induction chemotherapy; 3 weeks after induction,
23% of patients gained >5% of their initial body weight (10%
gained >10%). Twenty-one patients experienced pain secondary to
their cancer at the start of treatment and 15 of these patients
(71%) had significant reduction or complete resolution of their
cancer pain and/or dysphagia during their induction course.
Response to induction chemotherapy
All patients were evaluated by imaging with CT, MRI
and/or PET, physical examination and NPL prior to induction
chemotherapy initiation. All were evaluated by physical examination
and NPL post-induction, and 18 had imaging with MRI or PET. Using
the medical record documentation of physical examination and NPL,
and applying the RECIST criteria whenever possible, 9 patients
(30%) had a complete clinical or radiographical response to
induction chemotherapy, 20 patients (67%) had a partial response
and 1 patient (3%) had stable disease. No patient experienced
disease progression during induction chemotherapy.
Chemoradiotherapy
Twenty-nine patients (97%) received
chemoradiotherapy 3–4 weeks post-induction chemotherapy. All had
completed their full treatment at the time of analysis. One patient
went onto surgery due to prior head and neck radiation for
lymphoma.
Chemoradiotherapy and toxicity
Fig. 1 summarizes
the treatment regimens of the 29 patients who completed
chemoradiotherapy. Two patients received their chemoradiotherapy at
an outside institution and thus their data were only partially
analyzed. The median radiation dose administered was 7040 cGy
(range, 2600–7040 cGy). Twenty-five of the 27 patients received
100% of their intended dose, one patient received 94% and one
patient received only 37%. The median radiation course length was
6.7 weeks (range, 5.4–9.7 weeks). One patient (with a course length
of 9.7 weeks) had a treatment interruption of 3 weeks due to
non-compliance, although the patient ultimately received 100% of
the intended radiation dose. Another patient (with a course length
of 5.4 weeks) experienced several multiple day treatment
interruptions and ultimately only received 2600 cGy (37%) of the
intended dose due to non-compliance, despite multiple attempts to
re-establish follow-up. The remaining 27 patients completed their
radiation on schedule without interruption.
The initial planned concurrent chemotherapy regimen
was changed in 9 patients (30%) due to toxicities (Fig. 1). The most common toxicities that
required alteration of the regimen were hematological cytopenias
and hearing loss. Table IV details the toxicity data for combined
chemoradiotherapy. The most common grade 3 and 4 toxicities
included mucositis, anorexia, odynophagia, neutropenia and
thrombosis (67, 63, 41, 19 and 7%, respectively).
Weight loss was common during chemoradiotherapy
compared with the induction phase of treatment; 59% of patients
lost >5% of their baseline body weight and 22% lost >10% of
their baseline body weight.
Surgical outcomes
The one patient undergoing primary surgery instead
of chemoradiotherapy for definitive therapy had tumor invading the
submucosa on microscopic pathology (primary site; tonsil). Four
other patients underwent neck dissections after they completed
therapy due to concern for residual disease on imaging. Three of
these patients had no evidence of disease on pathological
examination. Pathological examination of one patient revealed
microscopic foci of residual squamous cell carcinoma at a single
lymph node level.
Overall response
Of the 30 patients evaluated, all had completed
their full treatment at the time of analysis. Of those 30, 29
underwent imaging post chemoradiotherapy, although the timing and
type of imaging was variable. PET/CT was the most commonly used to
follow treatment effect over time. In total, 27/30 patients
underwent their first PET/CT between 2.5 months to 1 year after
chemoradiation terminated. Applying the RECIST criteria to PET/CT,
MRI or clinical exam, 19 (63%) had complete clinical or
radiographical responses at 3–6 months post-treatment, and 18 of
these patients continued to demonstrate no evidence of disease at
their time of last follow-up (range, 5.0–38.7 months). One of these
19 patients sustained a local recurrence 7.5 months after
completion of chemoradiation and has since succumbed to progression
of disease. In addition, another one of the 19 patients succumbed
to non-cancer-related issues. Of the 30 patients, 11 (37%) had
partial responses at the end of full treatment with induction
chemotherapy and concurrent chemoradiation. Eight of these 11
patients have since succumbed to progression of their disease. Of
the p16-positive patients, 8/10 (80%) had a complete response. Of
the p16-negative patients, 1/3 (33%) experienced a complete
response.
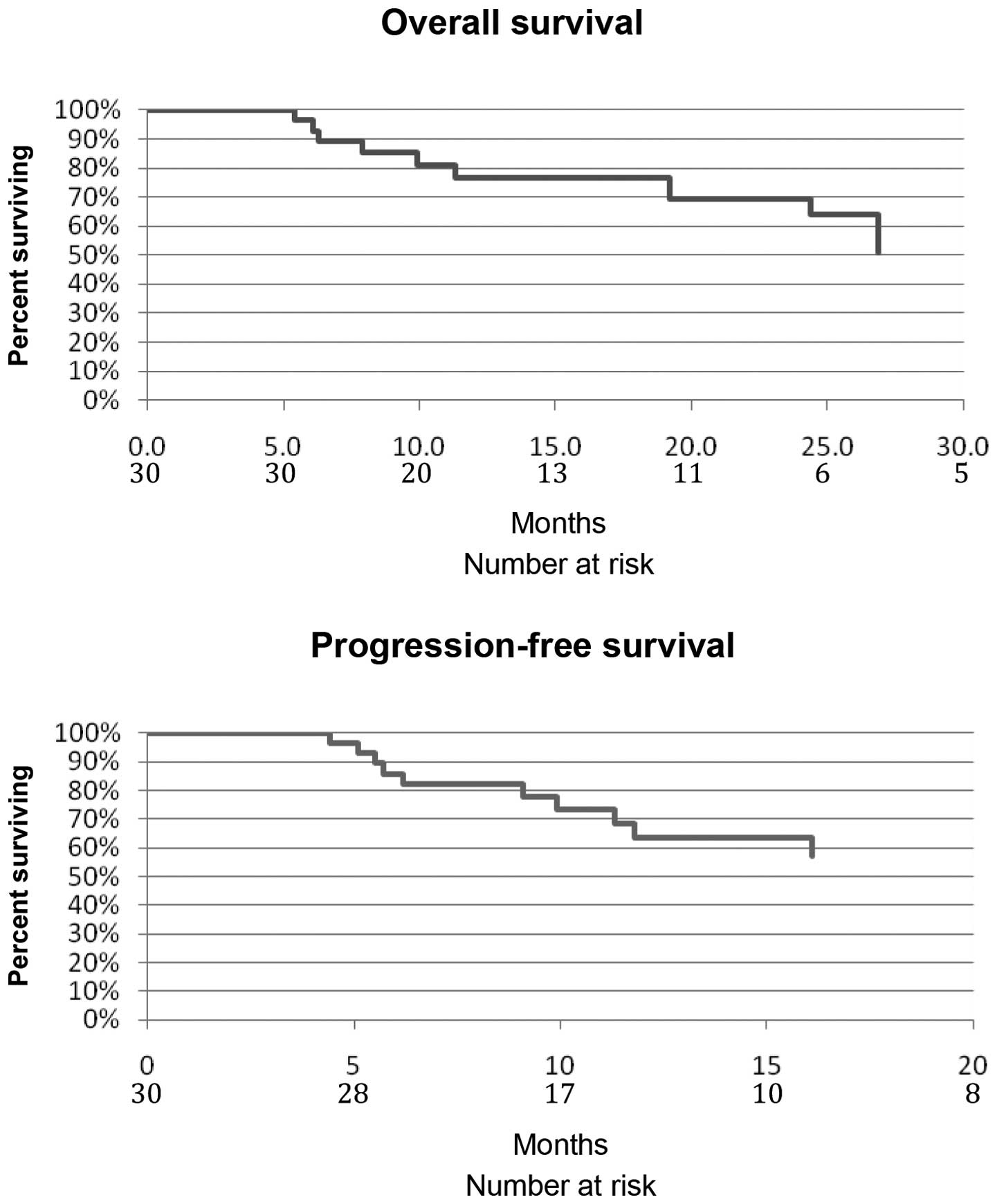
OS and PFS
OS and PFS are still immature since median follow-up
is only 13.7 months (range, 5.0–38.7 months). To date, 9 (30%)
patients have experienced progressive disease and 9 (30%) patients
have succumbed to disease. Of the 9 patients with progressive
disease, 4 patients had a local recurrence (primary site; oral
cavity, tonsil, supraglottic larynx, hypopharynx), 2 had metastatic
recurrence (primary site; larynx, oral cavity) and 3 had local and
metastatic recurrence (primary site; all three with tonsil). Of the
9 mortalities, 7 were attributed to disease progression. The eighth
patient succumbed to unclear reasons after their last imaging
examination demonstrated continued regression of disease. The ninth
patient succumbed to injuries sustained in a motor vehicle accident
while in a complete response (CR). Kaplan-Meier curves of PFS and
OS are depicted in Fig. 2.
Discussion
The TAX 323 and TAX 324 studies revealed the high
toxicity and compromised feasibility of TPF-induction chemotherapy.
While some patients may elect to accept short-term toxicities if
they are associated with a higher rate of long-term cure,
experience with this regimen has demonstrated that toxicity is
capable of translating into long-term harm, with many patients
either not able to proceed to definitive therapy or with severe
compromises to planned chemoradiotherapy regimens. Furthermore,
high toxicity of TPF has not translated into an increased response.
As a result, the treating clinicians at the University of
Pennsylvania switched from TPF to carboplatin/paclitaxel/cetuximab
in May 2008, when induction chemotherapy was chosen.
The weekly regimen was well tolerated and the low
rate of toxicity translated into excellent feasibility for the
total treatment plan. The majority of patients received all
intended cycles of induction chemotherapy, with high-dose
intensity. The majority of patients’ body weights remained stable
throughout induction, with 30% gaining in body weight. Grade 3 and
4 toxicity occurred in <7% of patients. Only a few
hospitalizations occurred during induction and a high proportion of
patients experienced relief of cancer pain and dysphagia.
Additionally, all 30 patients were able to advance to definitive
therapy with the majority able to receive the full, intended
radiation dose on schedule and without treatment interruptions.
The excellent feasibility observed in our studies
likely reflects the low toxicity of the induction regimen as well
as the positive secondary effects from clinical responses. For
patients restaged after induction chemotherapy, all but one patient
had at least a partial response, with 30% obtaining complete
response prior to initiation of chemoradiation. Relief of dysphagia
and odynophagia likely resulted in the high rates of observed body
weight stability, which in turn may have enabled our patients to
more readily begin and complete definitive local-regional therapy.
Definitive therapy in our cohort was fairly aggressive, as 74% of
patients initiated definitive radiation with concurrent high-dose
cisplatin. Of those patients, 70% completed their course with
cisplatin (30% with other concurrent chemotherapy). As concurrent
chemoradiation is the therapy best shown to increase cure rates
compared with radiation alone (19), preservation of the intended
treatment plan and the ability to receive the full dose of
radiation likely contribute to overall outcomes.
Although still immature, our overall PFS and OS
curves are inferior to those reported by Kies et al
(16). This likely reflects the
higher average stage of our patient population. In addition, to
avoid bias, we included all patients receiving induction therapy,
regardless of baseline PFS or other demographic variables. The
higher average smoking history of our patient population may also
contribute since tobacco history influences prognosis independent
of HPV status (20).
Based on the extremely favorable outcomes reported
by Kies et al for HPV+ patients, we chose to evaluate the
treatment outcomes of our HPV+ patients. Although HPV
data were only available for 13 of our patients (43%), the 80%
complete RR observed in those with p16 positivity, in contrast to
the 33% complete RR observed in those with p16 negativity, supports
the findings of improved treatment response in the HPV-positive
subset which has been previously reported (21–23).
In contrast to the high rates of treatment adherence
reported in the present study, other previously published studies
combining induction TPF with aggressive chemoradiation resulted in
high toxicity and a considerably lower feasibility. For example, in
the SWOG 0216 phase II study, cisplatin was used concurrently with
definitive radiation following TPF induction (2). For the 74 patients in this trial,
there was an 85% rate of grade 3–4 toxicity during induction
chemotherapy as well as two mortalities during induction and a
further two during subsequent chemoradiotherapy. Sixty-one patients
(82.4%) completed induction chemotherapy and began concurrent
chemoradiotherapy; 50 (68%) completed chemoradiotherapy. The design
of the two major TPF trials conceded this limitation; in TAX 323
(6), no chemotherapy was added to
definitive radiation and in TAX 324 (7), weekly, low-dose carboplatin (AUC 1.5)
was administered. Even so, substantial toxicities and treatment
delays during chemoradiation were observed in those receiving TPF
induction.
The DeCIDE and PARADIGM trials sought to address
whether the addition of induction chemotherapy to definitive
chemoradiotherapy is able to improve survival. However, both trials
were flawed at inception. In each trial, patients in the induction
arms were treated with chemoradiotherapy regimens that are not
generally considered as standard of care. Patients in DeCIDE
received fluorouracil/hydroxyurea/docetaxel with split course
radiotherapy and patients in PARADIGM received weekly carboplatin
with standard XRT or weekly docetaxel with accelerated boost
radiotherapy. No patient on the investigational arms received bolus
cisplatin or cetuximab, the chemoradiotherapy regimens supported by
phase III trial results and recommended by NCCN guidelines
(11). Patients in the control
arms also did not receive standard chemoradiotherapy. By contrast,
in DeCIDE, they received the same regimen of split-course
radiotherapy concurrent with fluorouracil/hydroxyurea/docetaxel
used in the experimental arm and in PARADIGM, they received two
(not the standard three) courses of cisplatin. Both studies
terminated early due to poor accrual and also failed to meet their
primary endpoints. In light of the negative results of the DeCIDE
and PARADIGM trials, the feasibility and efficacy of the
alternative weekly carboplatin/paclitaxel/cetuximab regimen outside
of a clinical trial setting is of increased interest to the head
and neck oncologist faced with clinical care decisions for the most
locally advanced patients.
Despite the number of limitations associated with
DeCIDE and PARADIGM, we nonetheless feel that the key problem was
the choice of induction chemotherapy regimen. TPF is insufficiently
active, too toxic and not feasible in combination with standard of
care chemoradiotherapy. We believe that our off-protocol experience
with carboplatin, paclitaxel and cetuximab lends support to the
prospective experiences of Kies and Wanebo, that this regimen,
outside of a formal clinical trial, is tolerable and very
efficacious. As a result of this experience, we are prospectively
studying induction chemotherapy regimens based on this platform. If
our ongoing phase II study (24)
is positive, we hope to evaluate induction chemotherapy in a phase
III study in which both arms receive identical standard of care
chemoradiotherapy.
Acknowledgements
Dr Corey Langer is currently a member
of the Advisory Boards of BMS, Imclone and Lilly, and a former
member of their Speaker’s Bureaus. Dr Jared Weiss has received
research funding from Celgene, GSK and Astellas.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Chandana SR and Conley BA: Neoadjuvant
chemotherapy for locally advanced squamous cancers of the head and
neck: current status and future prospects. Curr Opin Oncol.
21:218–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cohen EE, Lingen MW and Vokes EE: The
expanding role of systemic therapy in head and neck cancer. J Clin
Oncol. 22:1743–1752. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pfister DG, Ang KK, Brizel D, et al: NCCN
Practice Guidelines in Oncology, Head and Neck Cancers, Version
2.2011. National Comprehensive Cancer Network®; 2011
|
|
5
|
Brockstein B, Haraf DJ, Rademaker AW, et
al: Patterns of failure, prognostic factors and survival in
locoregionally advanced head and neck cancer treated with
concomitant chemoradiotherapy: a 9-year, 337-patient,
multi-institutional experience. Ann Oncol. 15:1179–1186. 2004.
|
|
6
|
Posner MR, Hershock DM, Blajman CR, et al
TAX 324 Study Group: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vermorken JB, Remenar E, van Herpen C, et
al EORTC 24971/TAX 323 Study Group: Cisplatin, fluorouracil, and
docetaxel in unresectable head and neck cancer. N Engl J Med.
357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beitler JJ and Cooper JS: Seduction by
induction? J Clin Oncol. 27:9–10. 2009. View Article : Google Scholar
|
|
9
|
Haddad RI and Posner MR: Induction
chemotherapy in head and neck cancer. J Clin Oncol. 27:e52–e53.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen EW, Karrison T, Kocherginsky M,
Huang C, Agulnik M, Mittal B, Yunus F, Samant S, Brockstein B, Raez
L, Mehra R, Kumar P, Ondrey F, Seiwert T, Villaflor V, Haraf D and
Vokes E: DeCIDE: A phase III randomized trial of docetaxel (D),
cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC)
in patients with N2/N3 locally advanced squamous cell carcinoma of
the head and neck (SCCHN). J Clin Oncol. 30(Suppl): abstr 5500.
2012.PubMed/NCBI
|
|
11
|
Haddad RI, Rabinowits G, Tishler RB,
Adkins D, Khuri FR, Clark J, Lorch JH, Limaye SA, Wirth LJ, O’Neill
A, Riley S and Posner MR: The PARADIGM trial: A phase III study
comparing sequential therapy (ST) to concurrent chemoradiotherapy
(CRT) in locally advanced head and neck cancer (LANHC). J Clin
Oncol. 30(Suppl): abstr 5501. 2012.
|
|
12
|
Haraf DJ, Rosen FR, Stenson K, et al:
Induction chemotherapy followed by concomitant TFHX
chemoradiotherapy with reduced dose radiation in advanced head and
neck cancer. Clin Cancer Res. 9:5936–5943. 2003.PubMed/NCBI
|
|
13
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonner JA, Harari PM, Giralt J, et al:
Radiotherapy plus cetuximab for locoregionally advanced head and
neck cancer: 5-year survival data from a phase 3 randomised trial,
and relation between cetuximab-induced rash and survival. Lancet
Oncol. 11:21–28. 2010.PubMed/NCBI
|
|
15
|
Vermorken JB, Mesia R, Rivera F, et al:
Platinum-based chemotherapy plus cetuximab in head and neck cancer.
N Engl J Med. 359:1116–1127. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kies MS, Holsinger FC, Lee JJ, et al:
Induction chemotherapy and cetuximab for locally advanced squamous
cell carcinoma of the head and neck: results from a phase II
prospective trial. J Clin Oncol. 28:8–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wanebo HJ and Ghebremichael M, Burtness B,
Spencer S, Ridge J, Forastiere A and Ghebremichael M: Phase II
evaluation of cetuximab (C225) combined with induction paclitaxel
and carboplatin followed by C225, paclitaxel, carboplatin, and
radiation for stage III/IV operable squamous cancer of the head and
neck (ECOG, E2303). J Clin Oncol (ASCO Annual Meeting Proceedings
Part I) (Suppl). (18S): 60152007.
|
|
18
|
Seiwert TY, Haraf DJ, Cohen EE, et al: A
randomized phase II trial of cetuximab-based induction chemotherapy
followed by concurrent cetuximab, 5-FU, hydroxyurea, and
hyperfractionated radiation (CetuxFHX), or cetuximab, cisplatin,
and accelerated radiation with concomitant boost (CetuxPX) in
patients with locoregionally advanced head and neck cancer (HNC). J
Clin Oncol. 29:2011.(suppl; abstr 5519).
|
|
19
|
Forastiere AA, Goepfert H, Maor M, et al:
Concurrent chemotherapy and radiotherapy for organ preservation in
advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adelstein DJ, Moon J, Hanna E, et al:
Docetaxel, cisplatin, and fluorouracil induction chemotherapy
followed by accelerated fractionation/concomitant boost radiation
and concurrent cisplatin in patients with advanced squamous cell
head and neck cancer: A Southwest Oncology Group phase II trial
(S0216). Head Neck. 32:221–228. 2010.
|
|
21
|
Ang KK, Harris J, Wheeler R, et al: Human
papillomavirus and survival of patients with oropharyngeal cancer.
N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fakhry C, Westra WH, Li S, et al: Improved
survival of patients with human papillomavirus-positive head and
neck squamous cell carcinoma in a prospective clinical trial. J
Natl Cancer Inst. 100:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lassen P, Eriksen JG, Hamilton-Dutoit S,
Tramm T, Alsner J and Overgaard J: Effect of HPV-associated
p16INK4A expression on response to radiotherapy and survival in
squamous cell carcinoma of the head and neck. J Clin Oncol.
27:1992–1998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weiss J: Induction Chemotherapy for
Locally Advanced Squamous Cell Carcinoma of the Head and Neck.
http://clinicaltrials.gov/ct2/show/NCT01412229?term=jared+weiss&rank=2uri.
Accessed December 7, 2012.
|