Introduction
Propofol is a widely used general anesthetic with a
high incidence of injection pain when administered intravenously
(1). However, the anti-nociceptive
properties of propofol have been demonstrated in several studies
(2–6). A previous study demonstrated that
propofol decreases nerve excitability of primary sensory afferents
(7). Local injection of propofol
produces a dose-dependent anti-nociceptive effect on the early and
late phases of the formalin test (2). Another study identified that
co-injection of propofol inhibits the pain behavior evoked by bee
venom (6). Intraperitoneal
injection of propofol in mice inhibits the acetic acid-induced
writhing response through a spinal mechanism (4).
The capsaicin receptor, transient receptor potential
vanilloid subtype-1 (TRPV1) plays an important role in pain
signaling and is essential for the development of inflammatory
thermal hyperalgesia in mice (8–10).
Activation of TRPV1 is vital in visceral pain. TRPV1 antagonists or
depletion of TRPV1 neurons by neonatal capsaicin injection inhibit
writhing caused by acetic acid (11–13).
A series of factors, including endothelin-1, prostaglandins and
bradykinin, sensitize the response of TRPV1 mainly through the
protein kinase A (PKA) or protein kinase C (PKC) pathways to
phosphorylate TRPV1 (14–16). Propofol increases the sensitivity
of dorsal root ganglion neurons to capsaicin through transient
receptor potential ankyrin receptor subtype-1 (TRPA1) and
PKCε-mediated phosphorylation of TRPV1 (17,18).
Intraperitoneal co-injection of propofol may increase visceral
nociception induced by TRPV1 agonists via sensitization of TRPV1,
which is paradoxic to the anti-nociceptive effects of propofol.
Therefore, we investigated the effects of intraperitoneal
co-injection of propofol on noceception induced by acetic acid and
capsaicin.
Materials and methods
Animals
Male C57BL/6J mice, weighing 20–22 g, were purchased
from the Center for Laboratory Animals, Sun Yat-Shen University
(Guangzhou, China). The mice were housed at room temperature
(22±1°C) on a 12/12-h light (8am–8pm)/dark (8pm–8am) cycle and had
free access to rodent chow and water. The experimental procedures
and the animal use and care protocols were approved by the
Committee on Ethical Use of Animals of Guangdong General Hospital
(Guangzhou, China). The procedures also followed the animal use and
care guidelines of the National Institutes of Health. All efforts
were made to reduce the number of animals used and to minimize
animal suffering.
Drugs and chemicals
Propofol (2,6-diisopropylphenol) and capsaicin were
purchased from Sigma (St. Louis, MO, USA) and dissolved in
dimethylsulfoxide (DMSO) as stock solution. Ice acetic acid was
purchased from Hangzhou Chemical Reagents Co., Ltd. (Zhejiang,
China). All drugs were diluted in normal saline prior to use.
Nociception induced by acetic acid and
capsaicin
Mice were acclimatized to the testing environment
(clear Plexiglas box) for 30 min. Care was taken while handling the
animals in order to minimize stress. Mice were injected
intraperitoneally using a 27 gauge needle, in the left lower
quadrant of the abdomen with 0.01 ml/g body weight of a 0.6% acetic
acid solution plus 5 mM propofol or 200 μM capsaicin plus 5 mM
propofol (n=10 for each group). The final concentration of DMSO in
the solution for intraperitoneal injection was adjusted to 2.5% for
acetic acid or 4.5% for capsaicin. Following injection, the animals
were returned to the chamber and their subsequent nociceptive
behavior observed. The number of writhing movements (‘writhing’
consists of contractions of the abdomen, twisting and turning of
the trunk, arching of the back and extension of the hind limbs)
were counted for 30 min after injection of acetic acid. The amount
of time spent recumbent (either lying on the stomach or sitting in
a hunched position with head down, not moving) was recorded for 10
min after injection of capsaicin (19).
Capsaicin treatment in neonatal mice
To demonstrate the role of TRPV1 on the effects of
propofol, male neonatal mice (aged 2 days) were anesthetized with
sevoflurane and injected subcutaneously with capsaicin, a
TRPV1-depleting agent (50 mg/kg) or the vehicle [10% ethanol, 10%
Tween-80 and 80% phosphate-buffered saline (PBS)] as described
previously (20). Animals were
included in the study 6 weeks after the administration of capsaicin
or the vehicle. The effects of capsaicin were expected to cause
depletion of TRPV1 neurons and was verified by the eye-wiping test.
For this test, capsaicin (0.01%, 20 μl) was sprayed into the eye
and the number of wiping movements that occurred within 1 min was
counted. The animal was considered to be desensitized to TRPV1 by
neonatal capsaicin treatment when the animal wiped its eyes no more
than five times.
Statistical analysis
Minitab 16 for Windows (Minitab Inc, PA, USA) was
used to carry out statistical analyses. All data are presented as
mean ± standard deviation (SD). Data were statistically evaluated
by analysis of variance followed by Bonferroni’s test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Intraperitoneal injection of acetic
acid
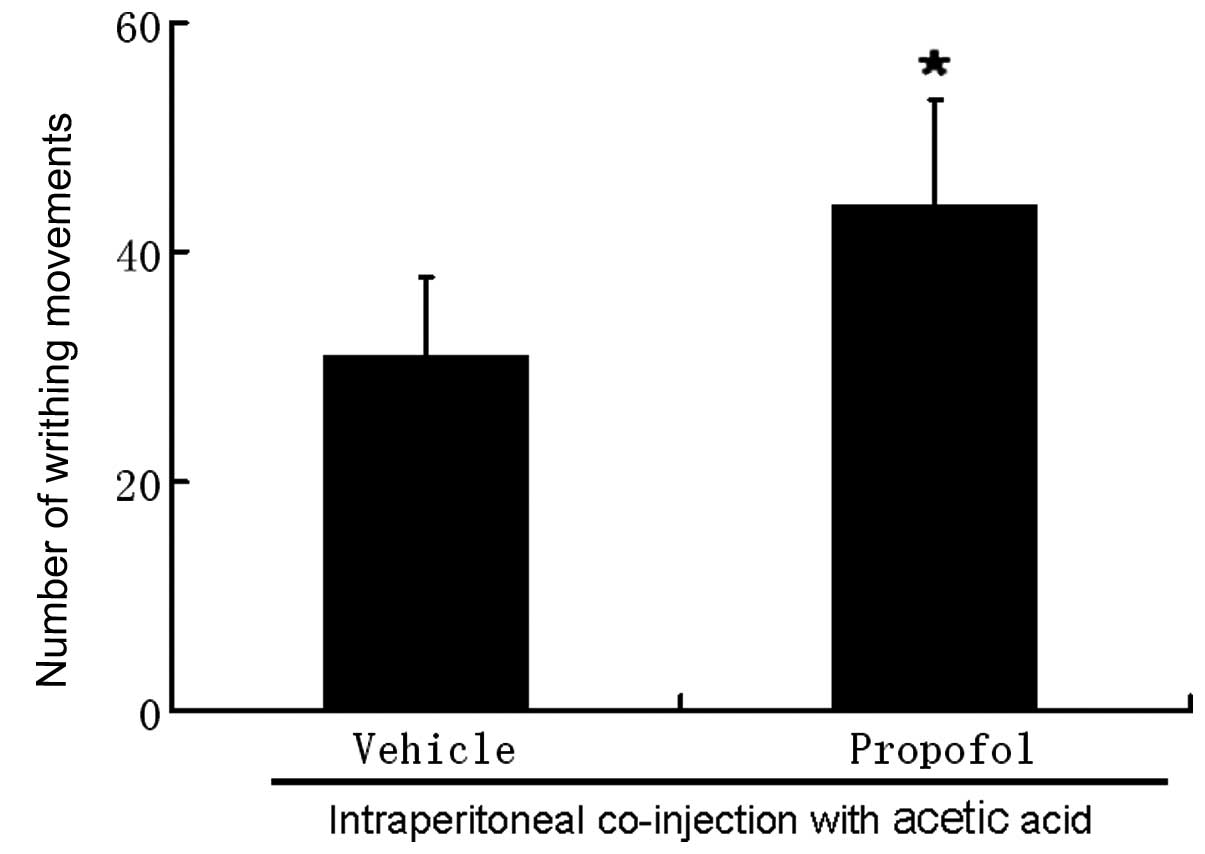
Intraperitoneal injection of propofol at 5 mM did
not cause pain-related behavior in mice. Intraperitoneal injection
of 0.6% acetic acid evoked an average of 31±6.9 writhing responses
with a latency <2 min. We counted the number of writhing
movements for 30 min as the majority of the writhing responses
occurred within 30 min of injection with acetic acid.
Co-administration of propofol 5 mM with acetic acid increased the
number of writhing movements to 42.8±9.2 (P<0.05, Fig. 1).
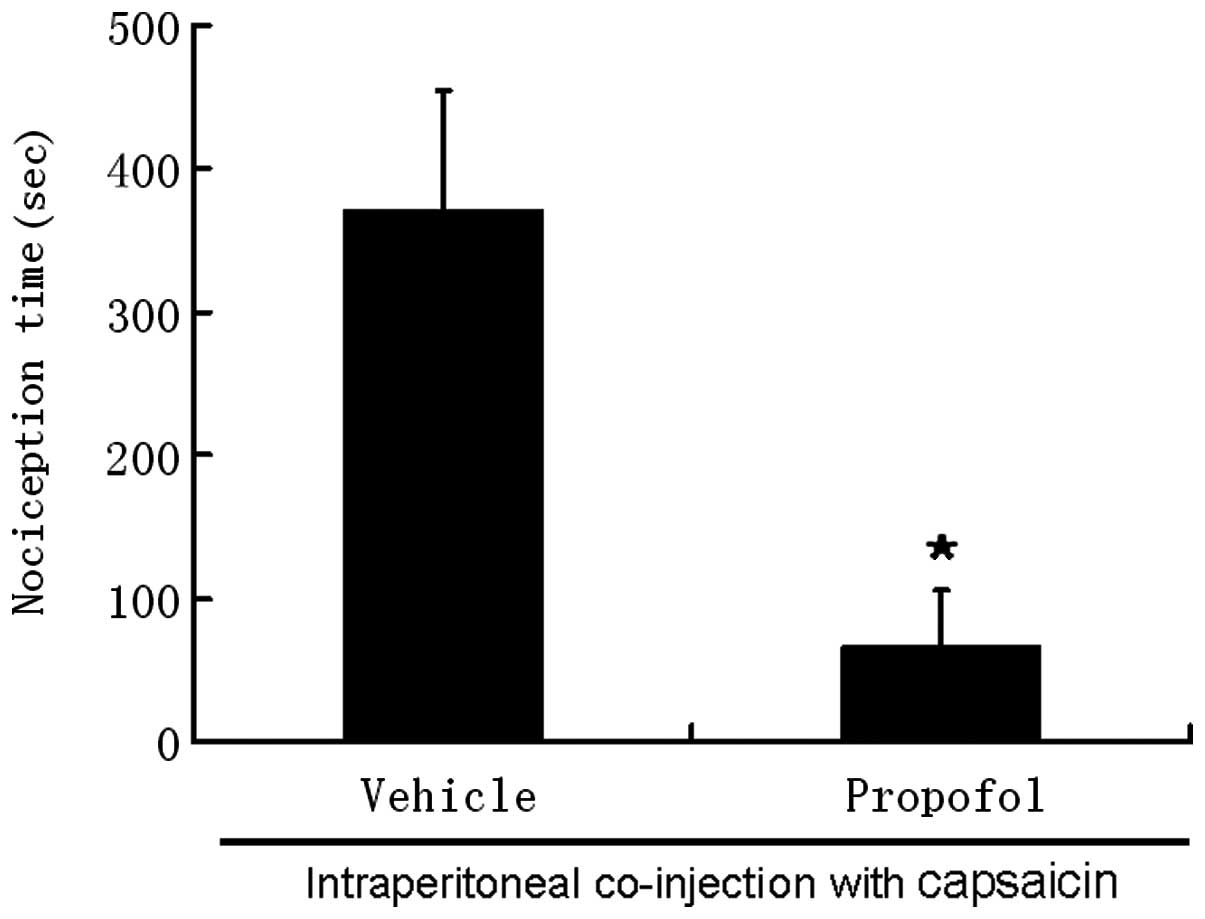
Intraperitoneal injection of
capsaicin
Intraperitoneal injection of 200 μM capsaicin evoked
pronounced nociceptive behaviors within 10 sec of injection. These
behaviors disappeared 10 min after injection. The average
nociception time induced by 200 μM capsaicin was 371±82.7 sec. The
nociception time was sharply reduced to 67±39 sec following
co-administration of capsaicin with propofol (P<0.05; Fig. 2).
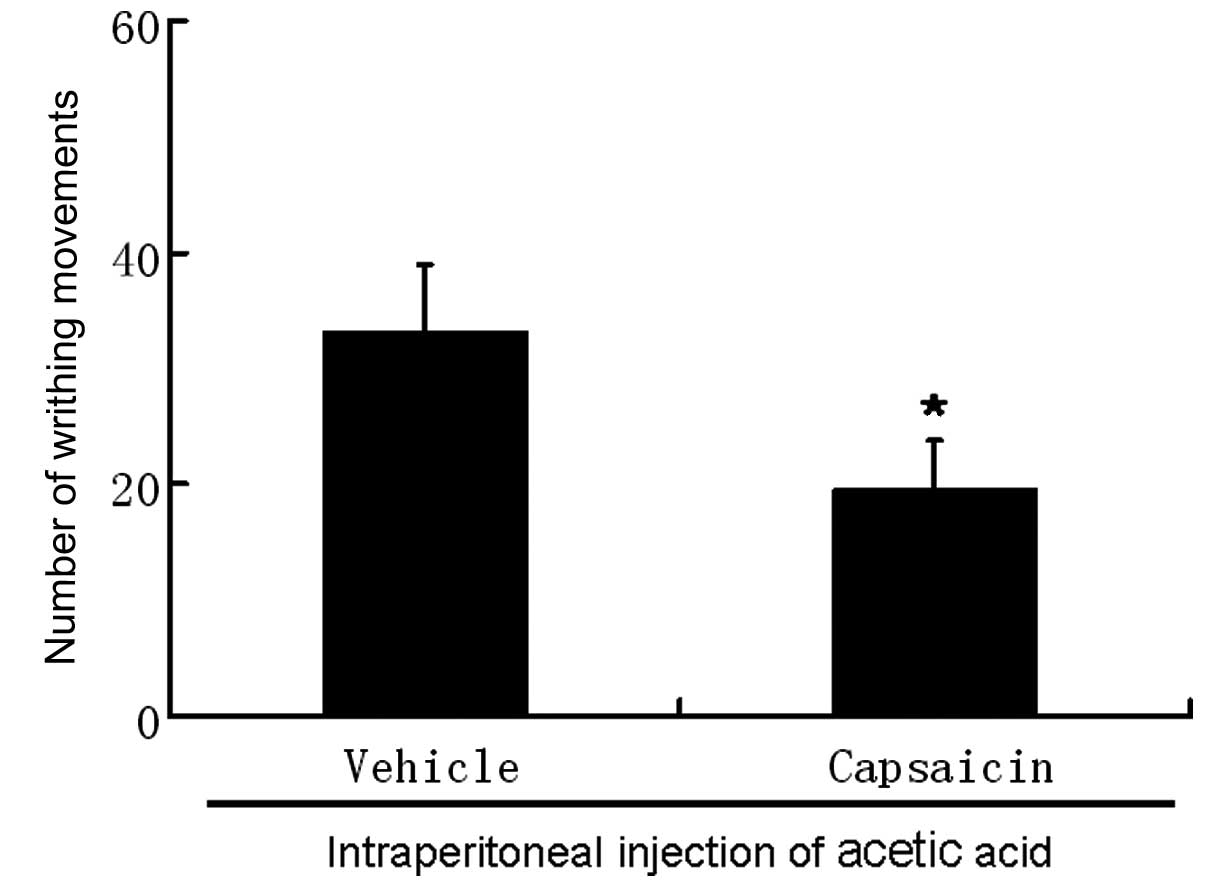
Contribution of C-fiber activation
To determine the contribution of C-fiber activation
to the effects of propofol in the algogens-induced nociceptive
behavior in mice, neonatal animals (aged 2 days) were
subcutaneously treated with capsaicin (50 mg/kg) and underwent
acetic acid or capsaicin-induced nociception when they became
adults. Mice treated with capsaicin did not spend any time
recumbent following intraperitoneal injection of capsaicin and
demonstrated a marked reduction in the number of writhing movements
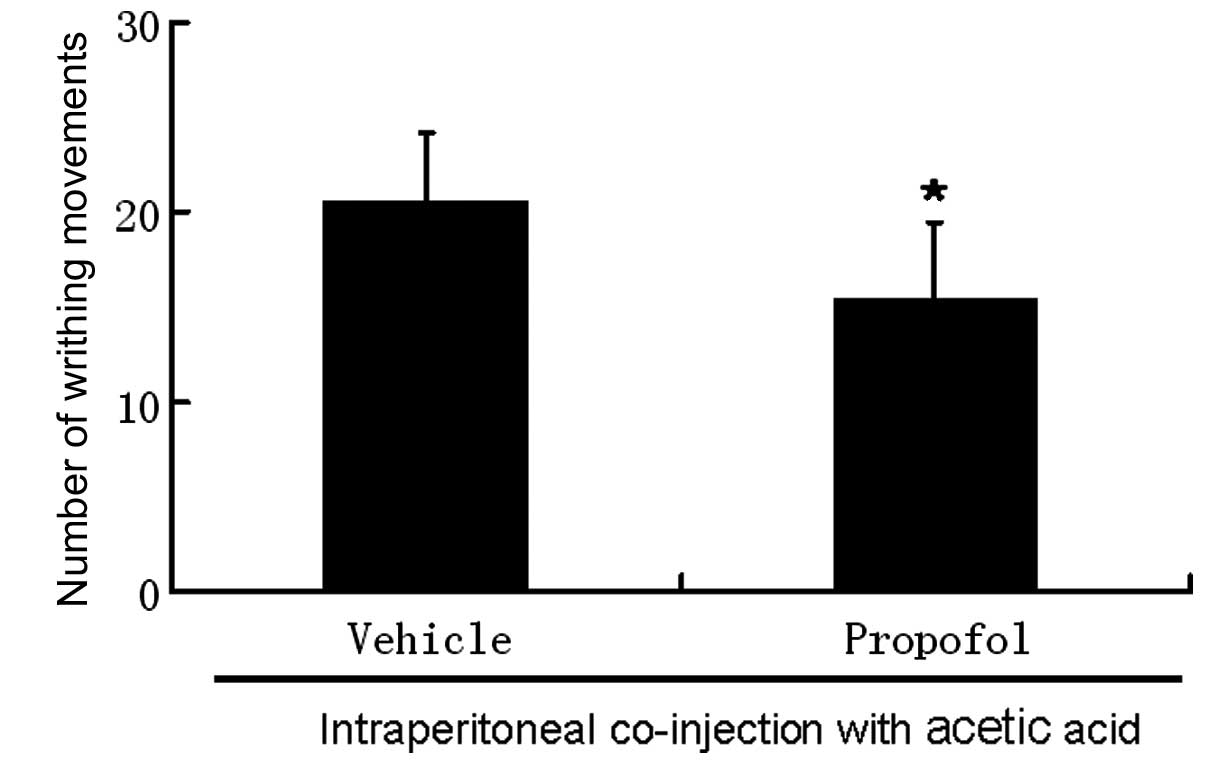
(20±4.1) compared with vehicle-treated animals (33±5.8; Fig. 3) following intraperitoneal
injection of acetic acid. Co-administration of propofol reduced the
number of writhing movements induced by acetic acid to 16±3.9
compared to that without propofol (21±3.6; Fig. 4) in capsaicin-treated mice.
Discussion
In this study, co-injection of propofol had a
pro-nociceptive effect on the writhing response induced by acetic
acid. The writhing response to intraperitoneal acetic acid is
mainly mediated by TRPV1 and was sharply inhibited following
neonatal treatment with capsaicin in this study and in previous
research (13). TRPV1 is a
non-selective cation channel with a preference for calcium that is
directly activated by capsaicin, protons, cations, noxious
temperatures and other endogenous ligands, including lipid
metabolites (21). Various agents,
including endothelin-1 and bradykinin in the ‘inflammatory soup’,
act together to lower the activation threshold of TRPV1 (14–16).
Propofol often induces pain when injected into peripheral small
veins. Bradykinin and prostaglandins are involved in the pain
following injection of propofol (22,23).
Propofol restores the sensitivity of TRPV1 receptors following
agonist-induced desensitization and attenuates agonist-induced
desensitization via PKCε and the TRPA1-dependent pathway in mouse
dorsal root ganglion (DRG) sensory neurons (17,18).
Acetic acid-induced visceral nociception is reduced by ∼70% in
knockout mice lacking B1 and B2 receptors (24). Propofol may increase the
nociceptive response to acetic acid through sensitization of TRPV1
or kinin receptors. However, it is hard to interpret the same dose
of propofol ameliorating the response to capsaicin.
Several studies reported that the systemic
administration of a subhypnotic dosage of propofol produces
hyperalgesic effects (25–27) and a large dosage, which results in
a loss of the right reflex, produces analgesic effects (25). Intraperitoneal administration of a
subhypnotic dose of propofol or microinjection of propofol into the
lateral ventricle or ventrolateral periaqueductal gray matter
produces significant hyperalgesia assessed by the hot-plate test
and formalin test in conscious rats (25), while intrathecal injection of
propofol demonstrated an analgesic effect (25). It was concluded that propofol
induces hyperalgesia through a superspinal mechanism.
Intraperitoneal injection of propofol in mice results in
anti-nociceptive effects in the hot-plate test and acetic acid
induces the writhing response through a spinal
N-methyl-D-aspartic acid (NMDA) and
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)
mechanism (4). Co-injection of
propofol inhibits the pain behavior evoked by bee venom (6). Generally speaking, the dosage and
route of administration determine the hyperalgesic or analgesic
effect of propofol. In contrast to previous reports, we identified
that co-injection of propofol exerts hyperalgesic and
anti-nociceptive effects. It increases the number of writhing
movements induced by acetic acid and decreases the nociception time
evoked by capsaicin. Our results suggest that TRPV1 is involved in
the effect of propofol in inhibiting the writhing response evoked
by acetic acid in neonatal capsaicin-treated animals.
TRPA1 mediates propofol-induced pain behavior caused
by intranasal propofol and flexor reflex response following
intra-arterial propofol (23,28).
Propofol evokes an inward current only in TRPA1-expressing neurons
(28,29) and fails to evoke this current in
HEK293 cells transfected with TRPV1 (28). Propofol does not alter the response
of recombinant rat TRPV1 to capsaicin (30). However, there is conflicting
evidence on the alteration of TRPV1 by propofol. A previous study
identified that propofol increases the intracellular calcium
concentration in HEK 293 cells transfected with Wistar rat TRPV1
cDNA (31). Co-application of
capsaicin and propofol results in even smaller currents than when
capsaicin is applied alone in HEK 293 cells transfected with human
TRPV1 cDNA (32). These studies
suggest that propofol has a functional interaction with TRPV1.
Through direct binding to TRPV1, certain agonists
also act as potentiation mediators; for example, mild acidification
will lower the temperature threshold and enlarge the heat- or
capsaicin-induced current (33,34).
Chick TRPV1 is not sensitive to capsaicin; however, high
concentrations of capsaicin enhance the proton-evoked current
(35). When the binding site is
overlapped, the response is inhibited due to competitive binding,
as cations act at TRPV1 proton binding residues E600 and E648
(34,36). Divalent cations, including Mg,
sensitize TRPV1 at 1–10 mM Mg and block the response to protons at
5–10 mM Mg (36). Propofol
possibly acts on the receptor inside the cell membrane or
hydrophobic regions of the receptor penetrating the lipid bilayers,
since propofol is lipophilic and permeable to the cell membrane
(37,38). Propofol enhanced the writhing
response to acetic acid and reduced the nociceptive response to
capsaicin in the current study, which suggests that propofol binds
to TRPV1 at the capsaicin binding site, which is a hydrophobic
region of the receptor.
Propofol is a widely used general anesthetic and the
mechanism of general anesthesia induced by propofol is still under
investigation. In the current study, in vivo results suggest
that propofol binds to TRPV1 at the site of the capsaicin binding
pocket. Further investigation is required to explore how propofol
binds to ion channels.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (No. 30700791).
References
|
1
|
Smith I, White PF, Nathanson M and
Gouldson R: Propofol. An update on its clinical use.
Anesthesiology. 81:1005–1043. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guindon J, LoVerme J, Piomelli D and
Beaulieu P: The antinociceptive effects of local injections of
propofol in rats are mediated in part by cannabinoid CB1 and CB2
receptors. Anesth Analg. 104:1563–1569. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nadeson R and Goodchild CS:
Antinociceptive properties of propofol: involvement of spinal cord
gamma-aminobutyric acid(A) receptors. J Pharmacol Exp Ther.
282:1181–1186. 1997.PubMed/NCBI
|
|
4
|
Xu AJ, Duan SM and Zeng YM: Effects of
intrathecal NMDA and AMPA receptors agonists or antagonists on
antinociception of propofol. Acta Pharmacol Sin. 25:9–14.
2004.PubMed/NCBI
|
|
5
|
Antognini JF, Wang XW, Piercy M and
Carstens E: Propofol directly depresses lumbar dorsal horn neuronal
responses to noxious stimulation in goats. Can J Anaesth.
47:273–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun YY, Li KC and Chen J: Evidence for
peripherally antinociceptive action of propofol in rats: behavioral
and spinal neuronal responses to subcutaneous bee venom. Brain Res.
1043:231–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neukom L, Vastani N, Seifert B, Spahn DR
and Maurer K: Propofol decreases the axonal excitability in rat
primary sensory afferents. Life Sci. 90:343–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caterina MJ and Julius D: The vanilloid
receptor: a molecular gateway to the pain pathway. Annu Rev
Neurosci. 24:487–517. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caterina MJ, Leffler A, Malmberg AB,
Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI
and Julius D: Impaired nociception and pain sensation in mice
lacking the capsaicin receptor. Science. 288:306–313. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Davis JB, Gray J, Gunthorpe MJ, Hatcher
JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, et al:
Vanilloid receptor-1 is essential for inflammatory thermal
hyperalgesia. Nature. 405:183–187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Urban L, Campbell EA, Panesar M, Patel S,
Chaudhry N, Kane S, Buchheit K, Sandells B and James IF: In vivo
pharmacology of SDZ 249–665, a novel, non-pungent capsaicin
analogue. Pain. 89:65–74. 2000.
|
|
12
|
Rigoni M, Trevisani M, Gazzieri D,
Nadaletto R, Tognetto M, Creminon C, Davis JB, Campi B, et al:
Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are
blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J
Pharmacol. 138:977–985. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikeda Y, Ueno A, Naraba H and Oh-ishi S:
Involvement of vanilloid receptor VR1 and prostanoids in the
acid-induced writhing responses of mice. Life Sci. 69:2911–2919.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamamoto H, Kawamata T, Ninomiya T, Omote
K and Namiki A: Endothelin-1 enhances capsaicin-evoked
intracellular Ca2+ response via activation of endothelin
a receptor in a protein kinase Cepsilon-dependent manner in dorsal
root ganglion neurons. Neuroscience. 137:949–960. 2006.PubMed/NCBI
|
|
15
|
Moriyama T, Higashi T, Togashi K, Iida T,
Segi E, Sugimoto Y, Tominaga T, Narumiya S and Tominaga M:
Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive
mechanism of prostaglandins. Mol Pain. 1:32005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuang HH, Prescott ED, Kong H, Shields S,
Jordt SE, Basbaum AI, Chao MV and Julius D: Bradykinin and nerve
growth factor release the capsaicin receptor from PtdIns(4,5)
P2-mediated inhibition. Nature. 411:957–962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wickley PJ, Yuge R, Russell MS, Zhang H,
Sulak MA and Damron DS: Propofol modulates agonist-induced
transient receptor potential vanilloid subtype-1 receptor
desensitization via a protein kinase Cepsilon-dependent pathway in
mouse dorsal root ganglion sensory neurons. Anesthesiology.
113:833–844. 2010. View Article : Google Scholar
|
|
18
|
Zhang H, Wickley PJ, Sinha S, Bratz IN and
Damron DS: Propofol restores transient receptor potential vanilloid
receptor subtype-1 sensitivity via activation of transient receptor
potential ankyrin receptor subtype-1 in sensory neurons.
Anesthesiology. 114:1169–1179. 2011. View Article : Google Scholar
|
|
19
|
Wang X, Miyares RL and Ahern GP:
Oleoylethanolamide excites vagal sensory neurones, induces visceral
pain and reduces short-term food intake in mice via capsaicin
receptor TRPV1. J Physiol. 564:541–547. 2005. View Article : Google Scholar
|
|
20
|
Rashid MH, Inoue M, Kondo S, Kawashima T,
Bakoshi S and Ueda H: Novel expression of vanilloid receptor 1 on
capsaicin-insensitive fibers accounts for the analgesic effect of
capsaicin cream in neuropathic pain. J Pharmacol Exp Ther.
304:940–948. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szallasi A, Cortright DN, Blum CA and Eid
SR: The vanilloid receptor TRPV1: 10 years from channel cloning to
antagonist proof-of-concept. Nat Rev Drug Discov. 6:357–372.
2007.PubMed/NCBI
|
|
22
|
Nakane M and Iwama H: A potential
mechanism of propofol-induced pain on injection based on studies
using nafamostat mesilate. Br J Anaesth. 83:397–404. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ando R and Watanabe C: Characteristics of
propofol-evoked vascular pain in anaesthetized rats. Br J Anaesth.
95:384–392. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cayla C, Labuz D, Machelska H, Bader M,
Schäfer M and Stein C: Impaired nociception and peripheral opioid
antinociception in mice lacking both kinin B1 and B2 receptors.
Anesthesiology. 116:448–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang QY, Cao JL, Zeng YM and Dai TJ: GABAA
receptor partially mediated propofol-induced hyperalgesia at
superspinal level and analgesia at spinal cord level in rats. Acta
Pharmacol Sin. 25:1619–1625. 2004.PubMed/NCBI
|
|
26
|
Petersen-Felix S, Arendt-Nielsen L, Bak P,
Fischer M and Zbinden AM: Psychophysical and electrophysiological
responses to experimental pain may be influenced by sedation:
comparison of the effects of a hypnotic (propofol) and an analgesic
(alfentanil). Br J Anaesth. 77:165–171. 1996. View Article : Google Scholar
|
|
27
|
Goto T, Marota JJA and Crosby G:
Pentobarbitone, but not propofol, produce pre-emptive analgesia in
the rat formalin model. Br J Anaesth. 72:662–667. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matta JA, Cornett PM, Miyares RL, Abe K,
Sahibzada N and Ahern GP: General anesthetics activate a
nociceptive ion channel to enhance pain and inflammation. Proc Natl
Acad Sci USA. 105:8784–8789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patwardhan A, Edelmayer R, Annabi E, Price
T, Malan P and Dussor G: Brief report: receptor specificity defines
algogenic properties of propofol and fospropofol. Anesth Analg.
115:837–840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hirota K, Smart D and Lambert DG: The
effects of local and intravenous anesthetics on recombinant rat VR1
vanilloid receptors. Anesth Analg. 96:1656–1660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsutsumi S, Tomioka A, Sudo M, Nakamura A,
Shirakura K, Takagishi K and Kohama K: Propofol activates vanilloid
receptor channels expressed in human embryonic kidney 293 cells.
Neurosci Lett. 312:45–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fischer MJ, Leffler A, Niedermirtl F,
Kistner K, Eberhardt M, Reeh PW and Nau C: The general anesthetic
propofol excites nociceptors by activating TRPV1 and TRPA1 rather
than GABAA receptors. J Biol Chem. 285:34781–3492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tominaga M, Caterina MJ, Malmberg AB,
Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI and Julius
D: The cloned capsaicin receptor integrates multiple pain-producing
stimuli. Neuron. 21:531–543. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jordt SE, Tominaga M and Julius D: Acid
potentiation of the capsaicin receptor determined by a key
extracellular site. Proc Natl Acad Sci USA. 97:8134–8139. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jordt SE and Julius D: Molecular basis for
species-specific sensitivity to ‘hot’ chili peppers. Cell.
108:421–430. 2002.
|
|
36
|
Ahern GP, Brooks IM, Miyares RL and Wang
XB: Extracellular cations sensitize and gate capsaicin receptor
TRPV1 modulating pain signaling. J Neurosci. 25:5109–5116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Orser BA, Bertlik M, Wang LY and MacDonald
JF: Inhibition by propofol (2,6 di-isopropylphenol) of the
N-methyl-D-aspartate subtype of glutamate receptor in cultured
hippocampal neurones. Br J Pharmacol. 116:1761–1768. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Franks NP and Lieb WR: Molecular and
cellular mechanisms of general anaesthesia. Nature. 367:607–614.
1994. View
Article : Google Scholar : PubMed/NCBI
|