Introduction
Astrocytic tumors are the most common tumors of the
central nervous system (CNS) and are categorized into diffuse
astrocytomas [World Health Organization (WHO) grade II], anaplastic
astrocytomas (WHO grade III) and glioblastomas (WHO grade IV)
(1). More than 51,000 individuals
are diagnosed with a primary brain tumor in the United States each
year, and for those with astrocytoma, ∼75% succumb to the disease
within 5 years of diagnosis (2).
Although surgery, radiation and chemotherapy have improved the
length of survival, astrocytoma mortality remains high.
Particularly, the overall survival rate of glioblastoma patients
was only 17.7% at one year, and 3.3% at two years (3,4).
Therefore, novel strategies to treat astrocytoma, particularly
glioblastoma, are urgently required. However, the mechanisms of
malignant progression of astrocytic tumors have not been completely
resolved.
Podocalyxin (PODXL) is a highly glycosylated and
sialylated transmembrane protein, and a CD34 ortholog normally
expressed on hematopoietic stem cells, hemangioblasts, vascular
endothelial cells, podocytes and a subset of neural progenitors
(5). Recently, increased PODXL
expression has been associated with a subset of aggressive cancers
including acute myeloid and lymphoid leukemia, myeloid sarcomas, as
well as certain breast, liver, pancreatic and kidney tumors
(5,6). The clinical significance of PODXL in
cancer progression has been investigated in numerous tumor types,
including breast, colon and uterine carcinoma. In uterine
endometrioid adenocarcinoma, PODXL expression is correlated with
tumor grade (7), while its
overexpression is an independent indicator of poor outcome in
breast and colorectal carcinoma (8,9).
PODXL also reportedly leads to increased in vitro migration
and invasion, increased matrix metalloproteinase (MMP) expression,
and increased activation of phosphatidylinositol 3-kinase (PI3K) in
breast and prostate cancer cells (10). Thus, PODXL may play a critical role
in cancer development and aggressiveness. A recent study reported
that PODXL expression was detected on the surface of 42.9% of
anaplastic astrocytoma samples and 54.8% of glioblastoma samples,
suggesting that PODXL may be associated with the malignant
progression of astrocytic tumors (11). However, the role of PODXL in
astrocytoma progression remains to be fully elucidated. In the
present study, the effect of PODXL on astrocytoma cell invasion and
survival against a chemotherapy agent was investigated.
Materials and methods
Cells lines, plasmids and reagents
The human astrocytoma cell lines SW1783 and U-87
were purchased from the American Tissue Culture Collection (ATCC,
Rockville, MD, USA). Human full length PODXL cDNA was subcloned
into pcDNA 3.1 expression vector. Human PODXL shRNA plasmid
(RHS3979-98487921) and pLKO.1 empty plasmid (RHS4080) were
purchased from Open Biosystems, Inc. (Huntsville, AL, USA).
Anti-PODXL (3D3; 39-3800) antibody was purchased from Life
Technologies (Carlsbad, CA, USA). Anti-MMP-9 (sc-13520), anti-Akt
(ser473; sc-24500) and anti-P-Akt (ser473; sc-101629) antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). All the secondary antibodies were purchased from Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). The
DeadEnd™ Fluorometric TUNEL system was purchased from Promega
(Madison, WI, USA). SuperFect® transfection reagent was
purchased from Qiagen (Valencia, CA, USA). Temozolomide, LY294002
(LY) and all the chemicals of reagent grade were purchased from
Sigma (St. Louis, MO, USA).
Transfection and lentiviral
transduction
The PODXL expression construct was transfected into
SW1783 and U-87 cells using SuperFect® transfection
reagent according to the manufacturer’s instructions. Pools of
stable transductants were generated via selection with G418 (800
μg/ml) using the manufacturer’s protocol. Lentiviral
transduction was performed in the SW1783 and U-87 cells. Pools of
stable transductants were generated via selection with puromycin (5
μg/ml).
In vitro cell invasion assay
Transwell® cell invasion assays (Corning
Life Sciences, Lowell, MA, USA) were performed. Briefly,
Transwell® cell culture chambers with 8-μm pore
size (BD Biosciences, Bedford, MA, USA) for 24-well plates were
coated with 50 μl Matrigel (BD Biosciences; 10 mg/ml;
diluted 1:3 in RPMI-1640). The SW1783 and U-87 cells were seeded in
the upper chamber at 5×105 cells/well in RPMI-1640
serum-free medium. Complete medium (600 ml) was added to the lower
chamber. The cells were treated with LY (50 μM) and allowed
to migrate for 24 h followed by fixation and staining with crystal
violet. The invasive cells were counted in 10 random fields/chamber
under a microscope. Each experiment was repeated three times in
triplicate.
Western blot analysis
Immunoblotting was performed with the respective
antibodies. Briefly, cells were dissolved in 250 μl 2X SDS
loading buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 25% glycerol,
0.01% bromophenol blue and 5% 2-mercaptoethanol), and incubated at
95°C for 10 min. Equal amount of proteins for each sample were
separated by 10% SDS-polyacrylamide gel and blotted onto a
polyvinylidene difluoride microporous membrane (Millipore,
Billerica, MA, USA). Membranes were incubated for 1 h with a 1/1000
dilution of the primary antibody (1/10000 for 3D3 PODXL blotting),
and then washed and revealed using secondary antibodies with
horseradish peroxidase conjugate (1/5000, 1 h). Peroxidase was
revealed using an ECL kit (GE Healthcare, Piscataway, NJ, USA).
Proteins were quantified before being loaded onto the gel, and
equal loading of extracts was verified by Ponceau coloration.
Measurement of apoptosis by TUNEL
(terminal deoxynucleotidyl-transferase-mediated nick-end labeling)
assay
The TUNEL assay was performed using the DeadEnd™
Fluorometric TUNEL system following the instructions provided by
Promega. Cells were treated with temozolamide (100 μM) in
the presence or absence of LY (50 μM) for up to 8 h.
Apoptotic cells exhibited a strong nuclear green fluorescence that
was detected using a standard fluorescein filter. All the cells
stained with DAPI exhibited a strong blue nuclear fluorescence. The
slides were observed under a fluorescent microscope with relative
apoptotic cells determined by counting the TUNEL-positive cells in
five random fields (magnification, ×100) for each sample.
Statistical analysis
Statistical analyses were performed with SPSS for
Windows 10.0. Data values were expressed as the mean ± standard
deviation (SD). Comparisons of means among multiple groups were
performed with one-way ANOVA followed by post hoc pairwise
comparisons using the least significant difference method. The
significance level of this study was set at a two-tailed
P=0.05.
Results
Effect of PODXL overexpression and
knockdown on astrocytoma cell invasion and MMP-9 expression
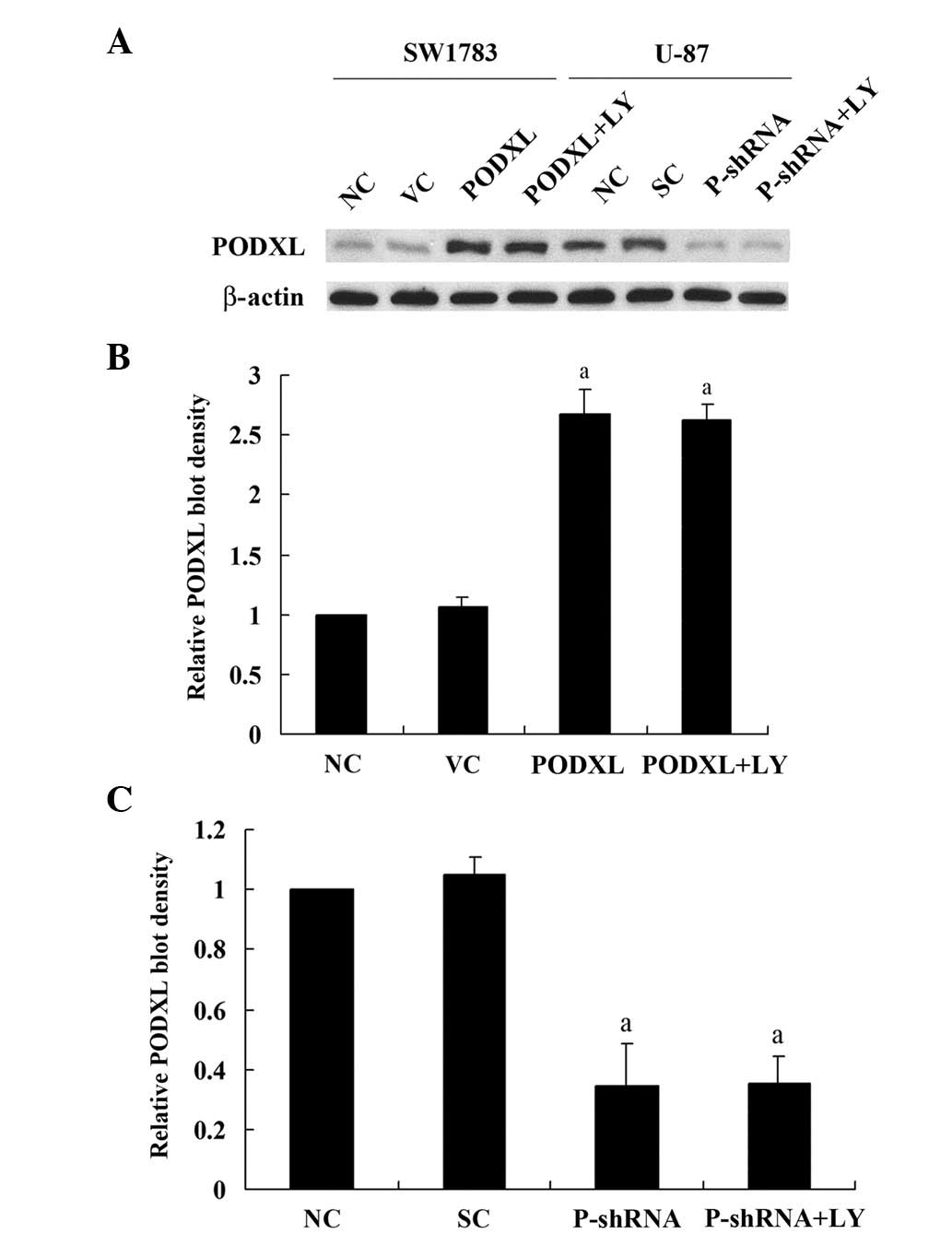
As shown in Fig. 1,
SW1783 (grade III astrocytoma) cells had a relatively low
constitutive expression of PODXL compared with U-87 (grade IV
astrocytoma; gliobalstoma) cells. Thus, to investigate the
functional role of PODXL in astrocytoma cells, we stably
transfected SW1783 cells with PODXL expression vector to
overexpress PODXL, and stably transduced U-87 cells with
PODXL-shRNA to knock down PODXL. Compared with the controls, PODXL
was overexpressed by >2.5-fold in SW1783 cells, and the
endogenous PODXL level was knocked down ∼70% in U-87 cells.
Selective PI3K inhibitor LY showed no effect on PODXL expression in
either cell line.
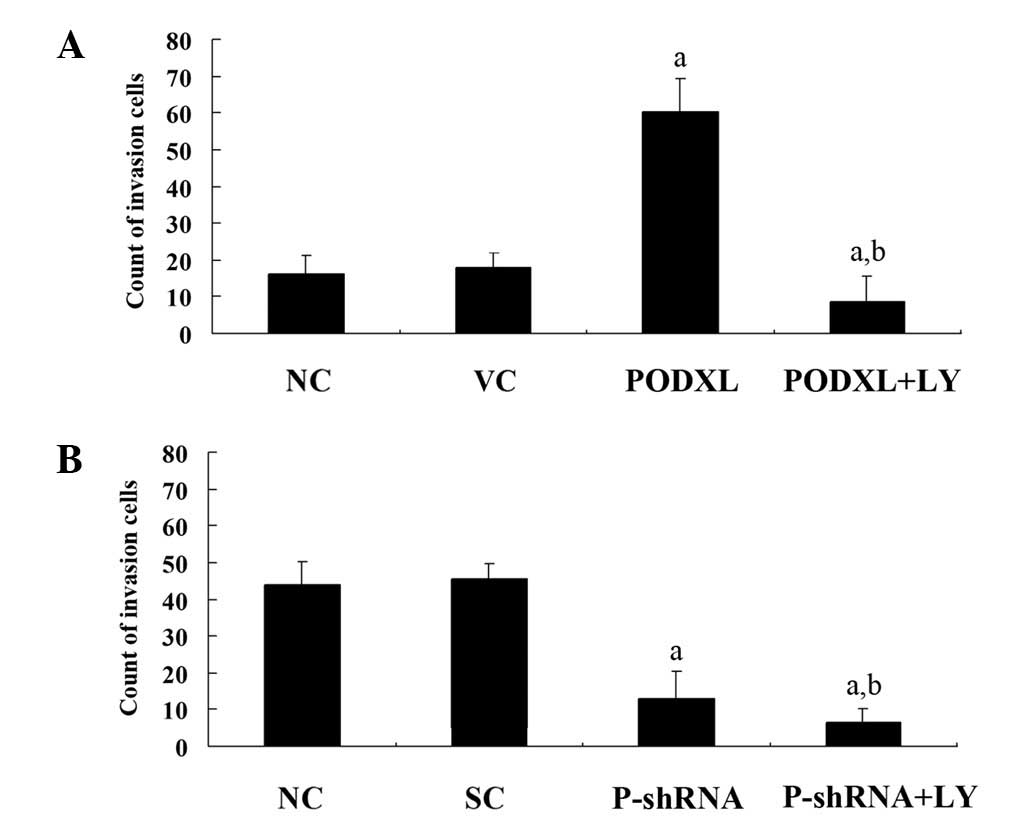
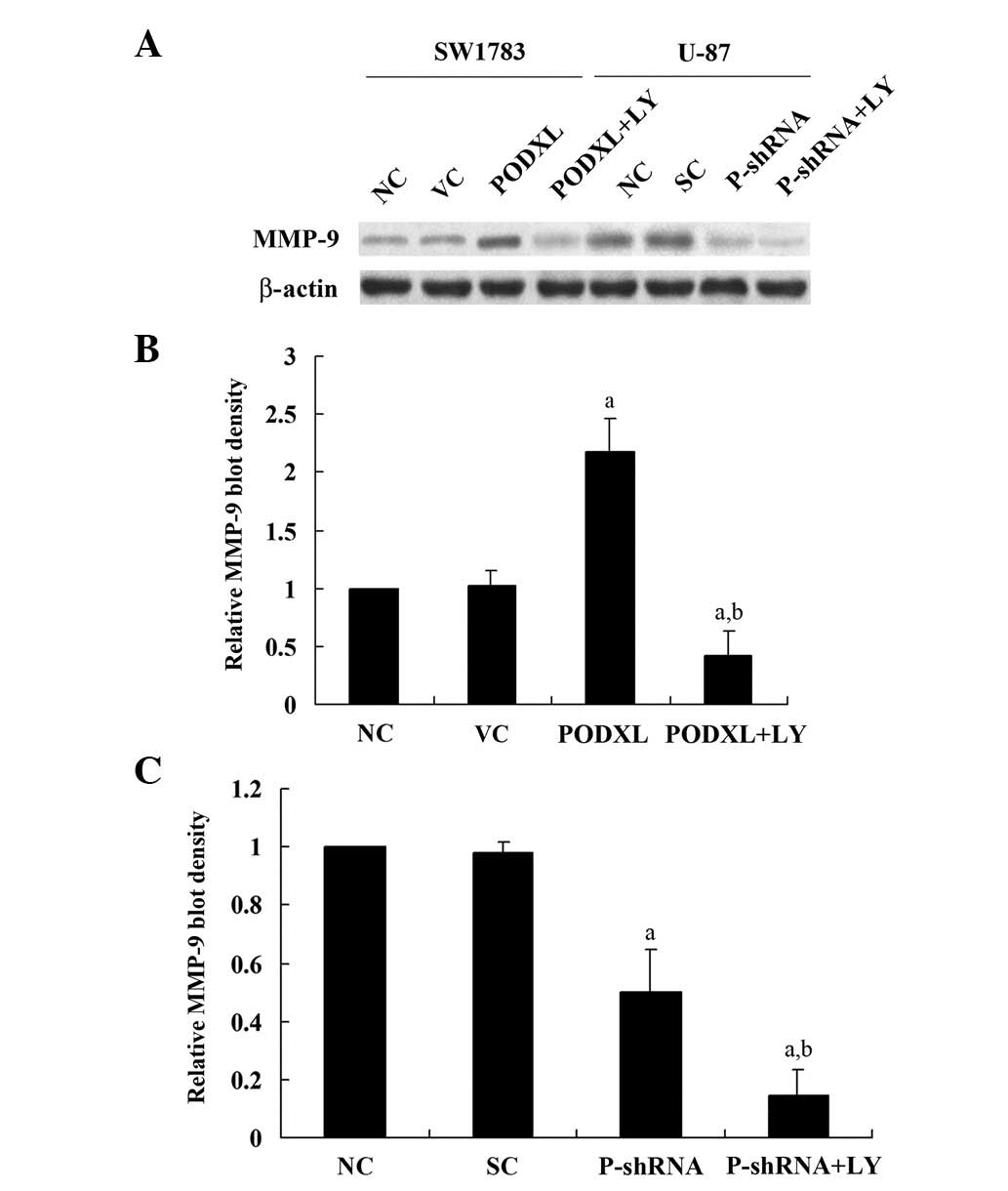
PODXL has been reported to promote tumor cell
invasion through MMPs (10). To
investigate the effect of PODXL on astrocytoma cell invasion, we
performed in vitro cell invasion assays and examined the
MMP-9 expression level in the two cell lines. As shown in Fig. 2, PODXL overexpression in SW1783
cells increased cell invasion by ∼4-fold compared with that of the
controls, and this increase was eradicated by LY. By contrast,
PODXL knockdown in U-87 cells decreased cell invasion by ∼3-fold
compared with the controls, and this was further decreased by LY
treatment. Similar trends were observed with MMP-9 expression
(Fig. 3). These results suggest
that PODXL promotes astrocytoma cell invasion, potentially by
upregulating MMP-9 expression in a PI3K-dependent manner.
Effect of PODXL overexpression and
knockdown on astrocytoma cell survival against temozolamide-induced
apoptosis
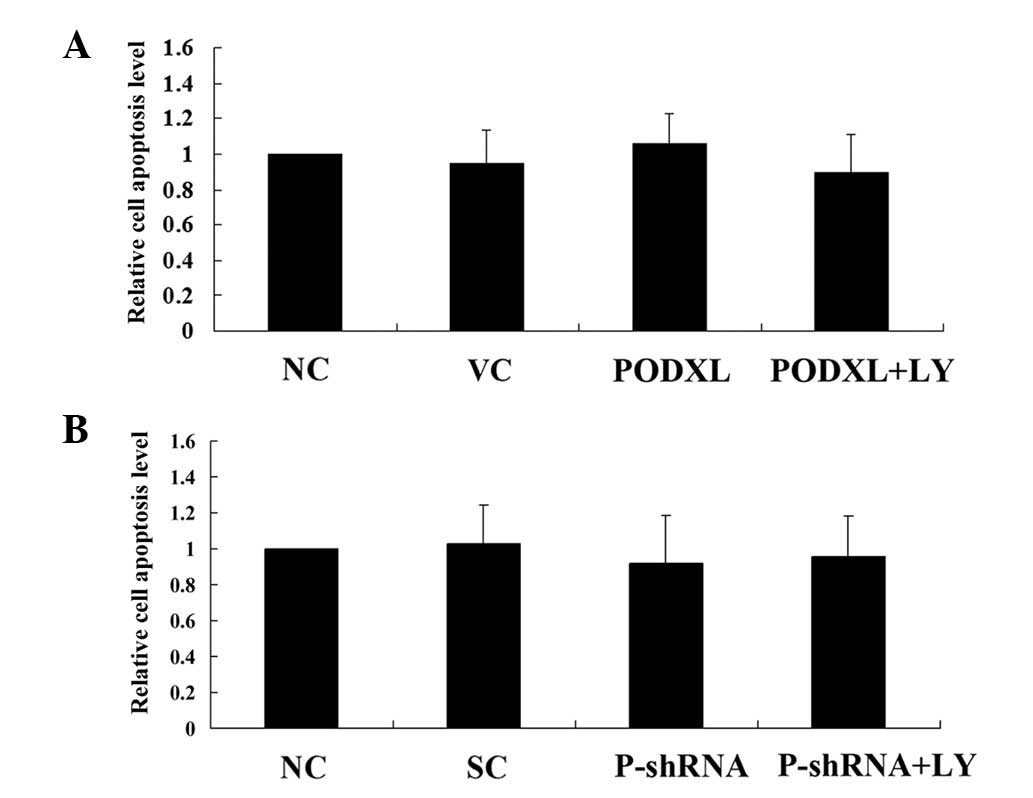
PODXL reportedly promotes the metastatic potential
of tumor cells. Since tumor cell survival is critical for
metastasis (12), we next examined
the effect of PODXL on astrocytoma cell survival against apoptotic
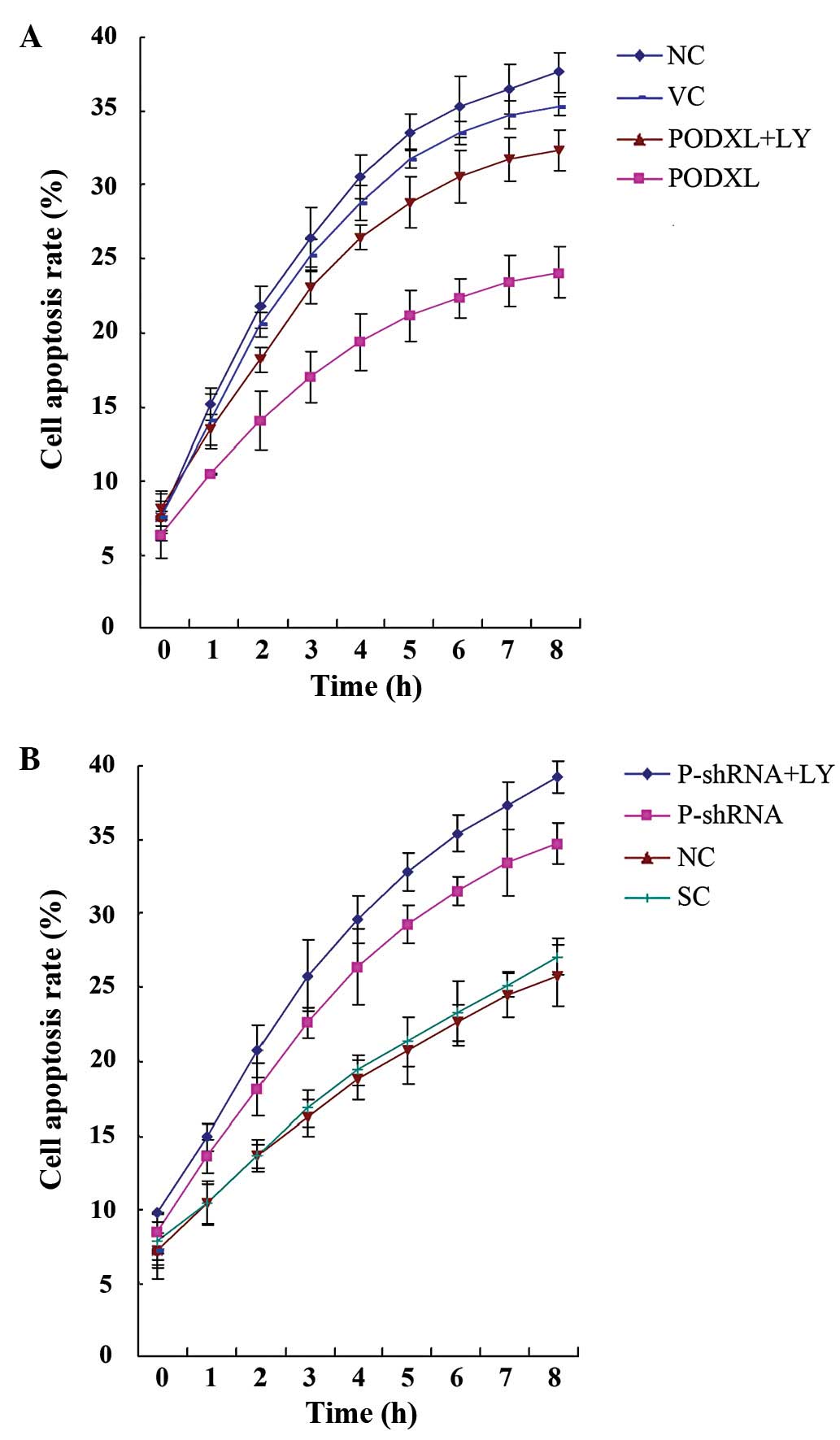
stress. As shown in Fig. 4, PODXL
overexpression and knockdown with or without LY treatment did not
significantly alter the apoptosis rate of astrocytoma cells in
normal culture conditions. Then, the cells were treated with 100
μM of temozolomide, an apoptosis-inducing chemotherapeutic
agent used to treat high-grade astrocytoma. In SW1783 cells treated
with temozolamide, PODXL overexpression significantly reduced cell
apoptosis compared with that in the controls, and this reduction
was reversed by LY (Fig. 5). In
U-87 cells, PODXL knockdown significantly increased cell apoptosis
in the presence of temozolamide. LY treatment further increased the
apoptosis in the PODXL-knocked down cells (Fig. 5).These results suggest that PODXL
promotes astrocytoma cell survival against temozolamide in a
PI3K-dependent manner.
Effect of PODXL overexpression and
knockdown on the PI3K/Akt survival signaling pathway in astrocytoma
cells
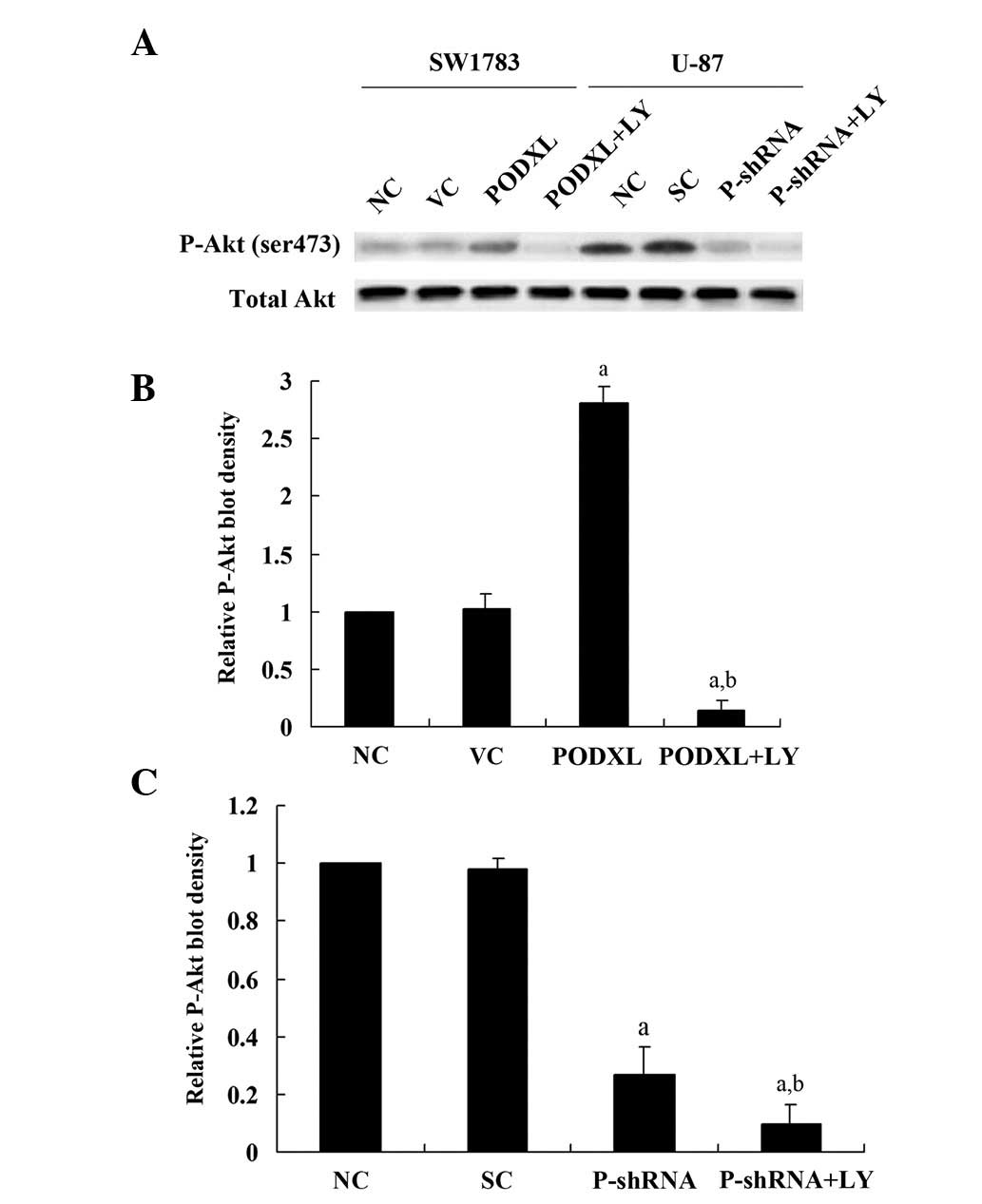
Since PODXL showed a protective effect on
astrocytoma cells against temozolomide-induced apoptotic stress in
a PI3K-dependent manner (Fig. 5),
we investigated the effect of PODXL on the PI3K/Akt survival
signaling pathway in astrocytoma cells. In SW1783 cells, PODXL
overexpression significantly increased phosphorylation at serine
473 (ser473) of Akt, which is required for full activation of Akt
(Fig. 6). LY treatment totally
eradicated the effect of PODXL over-expression. In U-87 cells,
PODXL knockdown decreased phosphorylation at serine 473 (ser473) of
Akt by >3-fold compared with the control level (Fig. 6), which was further decreased by LY
treatment. Taken together, these findings suggest that PODXL
enhances the activation of the PI3K/Akt signaling pathway and,
thereby, promotes astrocytoma cell survival against apoptotic
stress.
Discussion
PODXL reportedly increases the aggressive phenotype
of numerous types of cancer, including acute myeloid and lymphoid
leukemia, myeloid sarcomas, as well as certain breast, liver,
pancreatic and kidney tumors (13,14).
To the best of our knowledge, the effect of PODXL on astrocytoma
cell invasion and survival against chemotherapy agent was
investigated for the first time in the present study
We examined several astrocytoma cell lines and found
that while PODXL was amply expressed in U-87 cells, it was
expressed at a low level in SW1783 cells. Thus, overexpression and
knockdown of PODXL were respectively performed in the two cell
lines.
Sizemore et al (10) reported that PODXL overexpression
increased the in vitro invasive potential of breast and
prostate cancer cells and led to increased MMP-9 expression and
enhanced PI3K activity in the cells. Similar results in astrocytoma
cells were found in the present study. Additionally, our findings
that the selective PI3K inhibitor LY eradicated the effect of PODXL
overexpression and extended the effect of PODXL knockdown, suggest
that PODXL promotes invasion and MMP-9 expression in astrocytoma
cells by a PI3K-dependent mechanism.
Besides invasion potential, cell viability against
apoptotic stress is an additional important characteristic of
metastatic tumor cells (12). To
the best of our knowledge, the effect of PODXL on astrocytoma cell
viability/survival against chemotherapeutic agent-induced apoptotic
stress was investigated for the first time in the present study.
Temozolomide alkylates/methylates DNA, which damages DNA and
triggers the death of tumor cells (15). Borges et al (16) showed that the IC50 of
temozolomide on glioblastoma cells was >300 μM. Thus, in
the present study, we used a relatively small concentration of
temozolomide (100 μM) to induce apoptotic stress without
killing most of the cells. Our results showed that PODXL knockdown
significantly increased cell apoptosis in the presence of
temozolamide, suggesting that PODXL may be a potential target for
overcoming chemoresistance in astrocytomas, particularly,
glioblastomas. However, it still remains unclear whether PODXL
knockdown would impact astrocytoma cell survival against other
types of chemotherapy agents. As a result, further studies with
more types of chemotherapy agents and astrocytoma cell lines are
required.
In conclusion, we demonstrated that PODXL promotes
astrocytoma cell invasion, potentially through upregulating MMP-9
expression in a PI3K-dependent manner. Additionally, PODXL was
shown to promote astrocytoma cell survival against
temozolomide-induced apoptotic stress by enhancing the activation
of the PI3K/Akt survival signaling pathway. This study provides
novel insights into the molecular mechanisms underlying astrocytoma
progression, cell survival and chemoresistance.
References
|
1
|
Kleihues P, Burger PC, Collines VP,
Newcomb EW, Ohagi H and Cavenee WK: Astrocytic tumors.
Glioblastoma. International Agency for Research on Cancer Press;
Lyons: pp. 29–39. 2000
|
|
2
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohgaki H, Dessen P, Jourde B, et al:
Genetic pathways to glioblastoma: a population-based study. Cancer
Res. 64:6892–6899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reardon DA, Rich JN, Friedman HS and
Bigner DD: Recent advances in the treatment of malignant
astrocytoma. J Clin Oncol. 24:1253–1265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nielsen JS and McNagny KM: The role of
podocalyxin in health and disease. J Am Soc Nephrol. 20:1669–1676.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Riccioni R, Calzolari A, Biffoni M, et al:
Podocalyxin is expressed in normal and leukemic monocytes. Blood
Cells Mol Dis. 37:218–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yasuoka H, Tsujimoto M, Inagaki M, et al:
Clinicopathological significance of podocalyxin and phosphorylated
ezrin in uterine endometrioid adenocarcinoma. J Clin Pathol.
65:399–402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Larsson A, Johansson ME, Wangefjord S, et
al: Overexpression of podocalyxin-like protein is an independent
factor of poor prognosis in colorectal cancer. Br J Cancer.
105:666–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Somasiri A, Nielsen JS, Makretsov N, et
al: Overexpression of the anti-adhesin podocalyxin is an
independent predictor of breast cancer progression. Cancer Res.
64:5068–5073. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sizemore S, Cicek M, Sizemore N, Peng Ng K
and Casey G: Podocalyxin increases the aggressive phenotype of
breast and prostate cancer cells in vitro through its interaction
with ezrin. Cancer Res. 67:6183–6191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayatsu N, Kato Kaneko M, Mishima K,
Nishikawa R, Matsutani M, Price JE and Kato Y: Podocalyxin
expression in malignant astrocytic tumors. Biochem Biophys Res
Commun. 374:394–398. 2008. View Article : Google Scholar
|
|
12
|
Hopkin K, Edwards P, Harris A, Klausner R,
Peters G, Selby P and Stanley M: Cancer. Molecular Biology of the
Cell. Alberts B, Johnson A, Lewis J, Martin R, Keith R and Peter W:
4th edition. Garland Science; New York: pp. 1324–1325. 2002
|
|
13
|
Favreau AJ, Cross EL and Sathyanarayana P:
miR-199b-5p directly targets PODXL and DDR1 and decreased levels of
miR-199b-5p correlate with elevated expressions of PODXL and DDR1
in acute myeloid leukemia. Am J Hematol. 87:442–446. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu YH, Lin WL, Hou YT, et al: Podocalyxin
EBP50 ezrin molecular complex enhances the metastatic potential of
renal cell carcinoma through recruiting Rac1 guanine nucleotide
exchange factor ARHGEF7. Am J Pathol. 176:3050–3061. 2010.
View Article : Google Scholar
|
|
15
|
Khasraw M and Lassman AB: Advances in the
treatment of malignant gliomas. Curr Oncol Rep. 12:26–33. 2010.
View Article : Google Scholar
|
|
16
|
Borges KS, Castro-Gamero AM, Moreno DA, et
al: Inhibition of Aurora kinases enhances chemosensitivity to
temozolomide and causes radiosensitization in glioblastoma cells. J
Cancer Res Clin Oncol. 138:405–414. 2012. View Article : Google Scholar : PubMed/NCBI
|