Introduction
Prostate cancer is the most common non-cutaneous
cancer with a high mortality rate in American males. Previously,
studies on novel anticancer strategies have introduced prostate
cancer immunotherapy, which represents a highly attractive
therapeutic strategy for cancer treatment. Prostate stem cell
antigen (PSCA), first described by Reiter et al, is a
surface glycoprotein and has a 30% homology with stem cell
antigen-2 (1). PSCA is expressed
in 85% of prostate cancer specimens with high tissue specificity.
There is a direct correlation between the expression level of PSCA
and tumor stage, grade and bone metastasis (2). To date, studies have confirmed that
vaccination based on PSCA enhances the cellular and humoral immune
responses and inhibits the growth of PSCA-expressing tumors in mice
(3–5). Therefore, PSCA may be a potential
target for prostate cancer immunotherapy.
Heat shock protein-70 (HSP70) is a major molecular
chaperone, which assists in transport, folding and assembly of
proteins in the cytoplasm transmembrane. It is also involved in the
functions of the mitochondria, endoplasmic reticulum and nucleus.
Previous studies have demonstrated that vaccination with
HSP70-peptide complexes elicit specific antitumor responses
(6–9). These findings suggest that HSP70 is
involved in the process of antigen presentation and has potential
as a chaperone for specific antigens in vaccines.
In the present study, we successfully constructed
recombinant plasmids based on PSCA and HSP70 and obtained soluble
recombinant proteins with a purity >95%, which lays the
foundation for the development of a vaccine for prostate
cancer.
Materials and methods
Main reagents
The plasmids pET21a(+), pMD-HSP70 and pMD-PSCA were
stored in the Beijing Institute of Biotechnology, Academy of
Military Medical Sciences. Escherichia coli (E. coli) DH5α
and BL21(DE3), pMD18-T, LA Taq, T4 DNA ligase, DNA marker 2000 and
the restriction enzyme were purchased from Takara Biotechnology
Co., Ltd. (Dalian, China). A plasmid mini kit was purchased from
Promega Biotech Co., Ltd. (Beijing, China). Anti-PSCA polyclonal
antibody and fluorescein isothiocyanate (FITC)-conjugated goat
anti-rabbit IgG antibody were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Purification medium was
purchased from Qiagen (Beijing, China). All other domestic reagents
used in this study were of analytical grade. The study was approved
by the ethics committee of the Academy of Military Medical Sciences
(Beijing, China).
Construction of expression plasmids
All constructions were cloned into the pET21a(+)
vector. The human PSCA gene was amplified from the pMD-PSCA vector
with the primers 5′-CCG CAT ATG TGC TAT AGC TGC AAA GCC-3′ and
5′-CCG GAA TTC CAG GGC ATG GGC CCC GCT-3′ and cloned into the
NdeI and EcoRI sites of pET21a(+) to generate
pET21-PSCA. The amino-terminus, carboxyl-terminus and overall
length genes of HSP70 were amplified from the pMD-HSP70 vector with
the primers 5′-CCG GAA TTC ATG GCC AAA GCC GCG GCG-3′ and 5′-CCG
CTC GAG TCG CTT GTT CTG GCT GAT-3′, 5′-CCG GAA TTC CTG AAC AAG AGC
ATC AAC-3′ and 5′-CCG CTC GAG ATC TAC CTC CTC AAT GGT-3′, 5′-CCG
GAA TTC ATG GCC AAA GCC GCG GCG-3′ and 5′-CCG CTC GAG ATC TAC CTC
CTC AAT GGT-3′, respectively. Then, amplification products of
various structural domains of HSP70 were cloned into the
EcoRI and XhoI sites of pET21-PSCA to generate
pET21-PSCA-HSPN, pET21-PSCA-HSPC and pET21-PSCA-HSP. PSCA
amplification was performed for 3 min at 94°C, immediately followed
by 30 sec at 94°C, 30 sec at 55°C and 30 sec at 72°C for 30 cycles.
HSPN, HSPC and HSP were incubated for 3 min at 94°C, followed by 30
sec at 94°C, 30 sec at 55°C and 90 sec at 72°C for 30 cycles,
respectively. An additional extension step was performed for 10 min
at 72°C. DNA sequencing was performed to confirm that all
constructs had the desired sequence and open reading frame. All
plasmids were transformed into DH5α-competent E. coli.
Plasmid DNA copies were amplified in liquid culture and purified
using a plasmid mini kit.
Expression of recombinant fusion
proteins
A single transformed BL21(DE3) colony was inoculated
into 10 ml Luria-Bertani (LB) medium supplemented with ampicillin
(100 g/ml) followed by agitation at 250 rpm overnight at 37°C.
Then, 3 ml culture was transferred to 300 ml fresh LB medium in a
1,000 ml shake flask. The culture was grown at 37°C with 250 rpm
agitation until the optical density at 600 nm (OD600) reached 1.2.
Then, induction was performed with the addition of 0.3 ml 1 M
isopropyl β-D-1-thiogalactopyranoside (IPTG). At 5 h after
induction, a 1 ml sample was collected. The pellet was resuspended
in 100 μl ddH2O, mixed with 2X sodium dodecyl
sulfate (SDS) loading buffer [0.0625 M Tris-HCl (pH 6.8), 2% SDS,
25% glycerol, 5%-mercaptoethanol and 0.01% bromphenol blue] and
heated at 95°C for 4 min. Following centrifugation at 10,000 × g
for 5 min, 20 μl supernatant was analyzed by
SDS-polyacrylamide gel electrophoresis (PAGE) and stained by
Coomassie blue R-250.
Lysis of cells and purification of the
recombinant fusion proteins
Following induction for 5 h, cells were harvested by
centrifugation at 10,000 × g for 15 min from 4,000 ml culture,
resuspended in 100 ml 20 mM lysis buffer (20 mM
NaH2PO4, 0.5 mM NaCl and 10 mM imidazole; pH
7.4) and lysed with a high pressure homogenizer (pressure, 900 bar;
one time). The supernatant collected following centrifugation at
15,000 × g for 20 min was loaded in a nickel-nitrilotriacetic acid
(Ni-NTA) resin column pre-equilibrated with 20 mM lysis buffer. The
column was washed with equilibration buffer and when the absorbance
at 280 nm was <0.01, the column was eluted using 50 mM imidazole
in 20 mM equilibration buffer followed by a linear gradient of
50–500 mM imidazole in 20 mM equilibration buffer. The ammonium
sulfate powder was added to the above elution liquid containing the
eluted target protein to a concentration of 1 M. The above elution
was loaded in to the Phenyl-Sepharose Fast Flow (FF) column
pre-equilibrated with 20 mM phosphate-buffered saline (PBS; pH 7.4)
supplemented with 1 M ammonium sulfate. The column was washed with
equilibration buffer and when the absorbance at 280 nm was below
0.01, the column was eluted using a linear gradient of 1-0 M
ammonium sulfate in 20 mM phosphate buffer (pH 7.4). The elution
peak from the Phenyl-Sepharose FF column was loaded in to the
HiLoad™ 26/60 Superdex 75 and washed with 20 mM phosphate buffer
(pH 7.4); then the target protein peak was collected. The purified
recombinant proteins were collected and analyzed by SDS-PAGE
analysis. The final purity of products was determined by means of
size exclusion-high-performance liquid chromatography
(SE-HPLC).
SDS-PAGE analysis
SDS-PAGE was performed using 12% resolution gel on
the MiniProtean 3 system (Bio-Rad, Hercules, CA, USA). Briefly, the
whole E. coli cell lysate was loaded on the gel. Then, the
gel was electrophoresed at 150 V for 1 h and stained with Coomassie
brilliant blue R-250. Low molecular weight protein markers were
rabbit phosphorylase B (97.2 kDa), bovine serum albumin (66.4 kDa),
rabbit actin (44.3 kDa), bovine carbonic anhydrase (29.0 kDa),
soybean trypsin inhibitor (20.1 kDa) and hen egg white lysozyme
(14.3 kDa).
Identification of recombinant fusion
proteins with western blotting
Following electrophoresis at 150 V for 1 h, the
target protein was transferred to a polyvinylidene difluoride
(PVDF) membrane using a Bio-Rad semidry apparatus with transfer
buffer [10 mM CAPS buffer (pH 11), 10% methanol]. The PVDF
membrane, wetted in 100% methanol, was soaked in transfer buffer
before being placed on the PAGE gel. After being coated with
anti-PSCA monoclonal antibody, the membrane was incubated at 4°C
for 24 h. Then, it was washed and blocked with blocking buffer [3%
bovine serum albumin V in Tris-buffered saline with 0.05% Tween-20
(TBST); pH 7.2]. Following three TBST washes for 5 min, the
membrane was incubated for 1 h at 37°C with FITC-conjugated goat
anti-rabbit IgG antibody followed by coloration with
3,3-diaminobenzidine (DAB) tetrahydrochlorate using a DAB kit
according to the manufacturer’s instructions.
Results
Construction of expression plasmids
The PSCA gene and various structural domains of
HSP70 were amplified by PCR with the respective primers. Then, the
PSCA was cloned into pET21a(+) with the amino-terminus,
carboxyl-terminus and overall length of HSP70, by enzyme digestion
to construct the recombinant plasmids pET21-PSCA-HSPN,
pET21-PSCA-HSPC and pET21-PSCA-HSP, respectively. The recombinant
plasmids containing the target gene sequences were further analyzed
by restriction enzymatic pattern and finally confirmed to be in
full accord with sequences issued by GenBank [PSCA cDNA (GenBank
original accession no. AF04F3498) and HSP70 cDNA (no.
NM005345)].
Expression of the recombinant fusion
proteins
When the OD600 of the culture reached 1.2, E.
coli cells harboring expression plasmids were treated with IPTG
at a final concentration of 1 mM. The expression of proteins
corresponding to the predicted size were induced in the presence of
IPTG. The proteins PSCA-HSPC and PSCA-HSP expressed in E.
coli were confirmed to exist in soluble form by SDS-PAGE
analysis (Fig. 1).
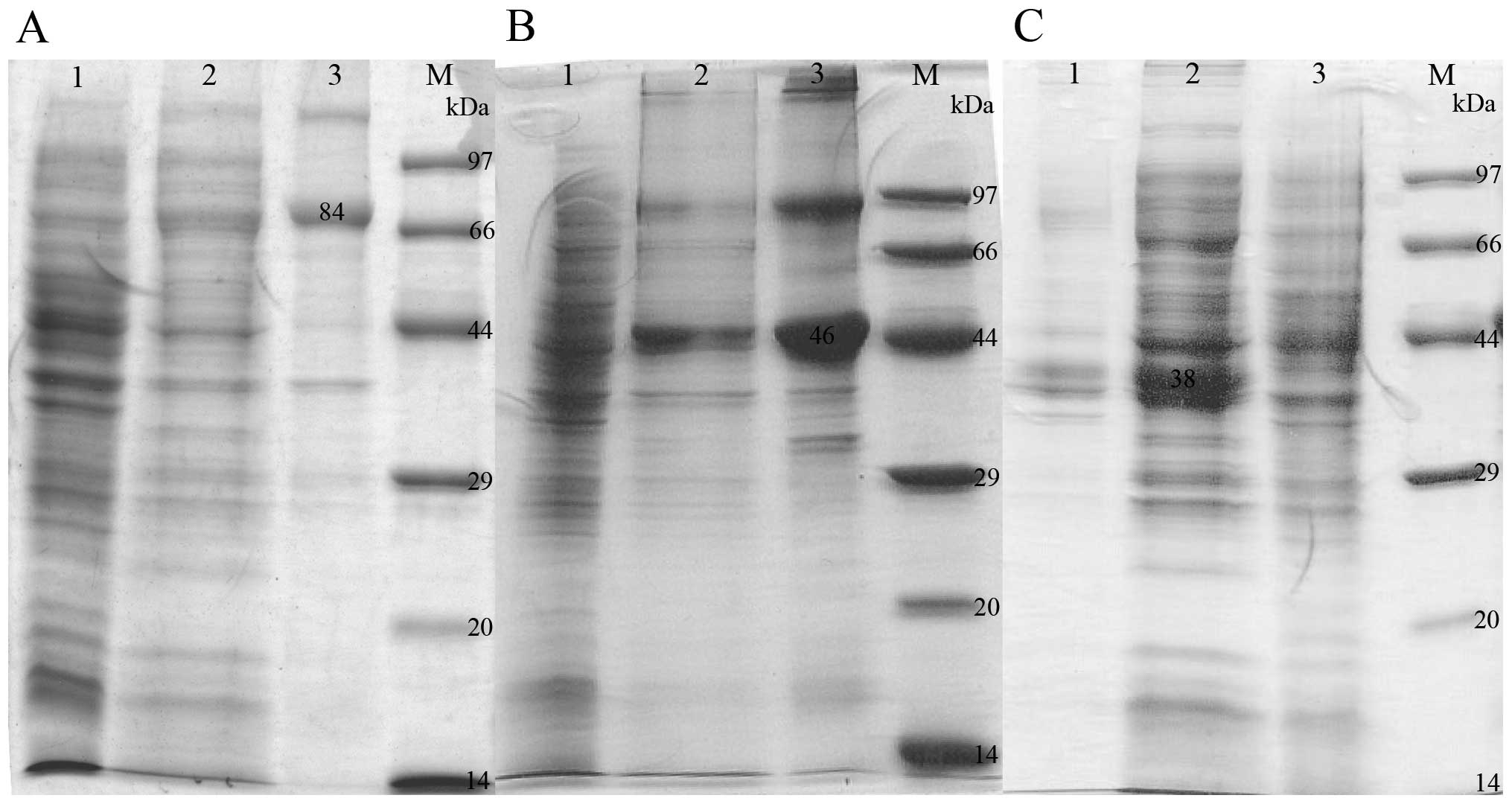 | Figure 1SDS-PAGE analysis of recombinant
fusion proteins. (A) Lane 1, uninduced BL21(DE3)/pET21-PSCA-HSP;
lane 2, induced BL21(DE3)/pET21-PSCAHSP, supernatant of the
bacterial lysate; lane 3, induced BL21(DE3)/pET21-PSCA-HSP, pellet
of the bacterial lysate; lane M, low molecular protein marker
(97.2, 66.4, 44.3, 29.0, 20.1 and 14.3 kDa). (B) Lane 1, uninduced
BL21(DE3)/pET21-PSCA-HSPN; lane 2, induced BL21(DE3)/
pET21-PSCA-HSPN, super-natant of the bacterial lysate; lane 3,
induced BL21 (DE3)/pET21-PSCA-HSPN, pellet of the bacterial lysate;
lane M, low molecular protein marker (97.2, 66.4, 44.3, 29.0, 20.1
and 14.3 kDa). (C) Lane 1, induced BL21(DE3)/pET21-PSCA-HSPC,
pellet of the bacterial lysate; lane 2, induced
BL21(DE3)/pET21-PSCA-HSPC, supernatant of the bacterial lysate;
lane 3, uninduced BL21(DE3)/pET21-PSCA-HSPC; lane M, low molecular
protein marker (97.2, 66.4, 44.3, 29.0, 20.1 and 14.3 kDa).
SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel
electrophoresis; PSCA, prostate stem cell antigen; HSP, heat shock
protein. |
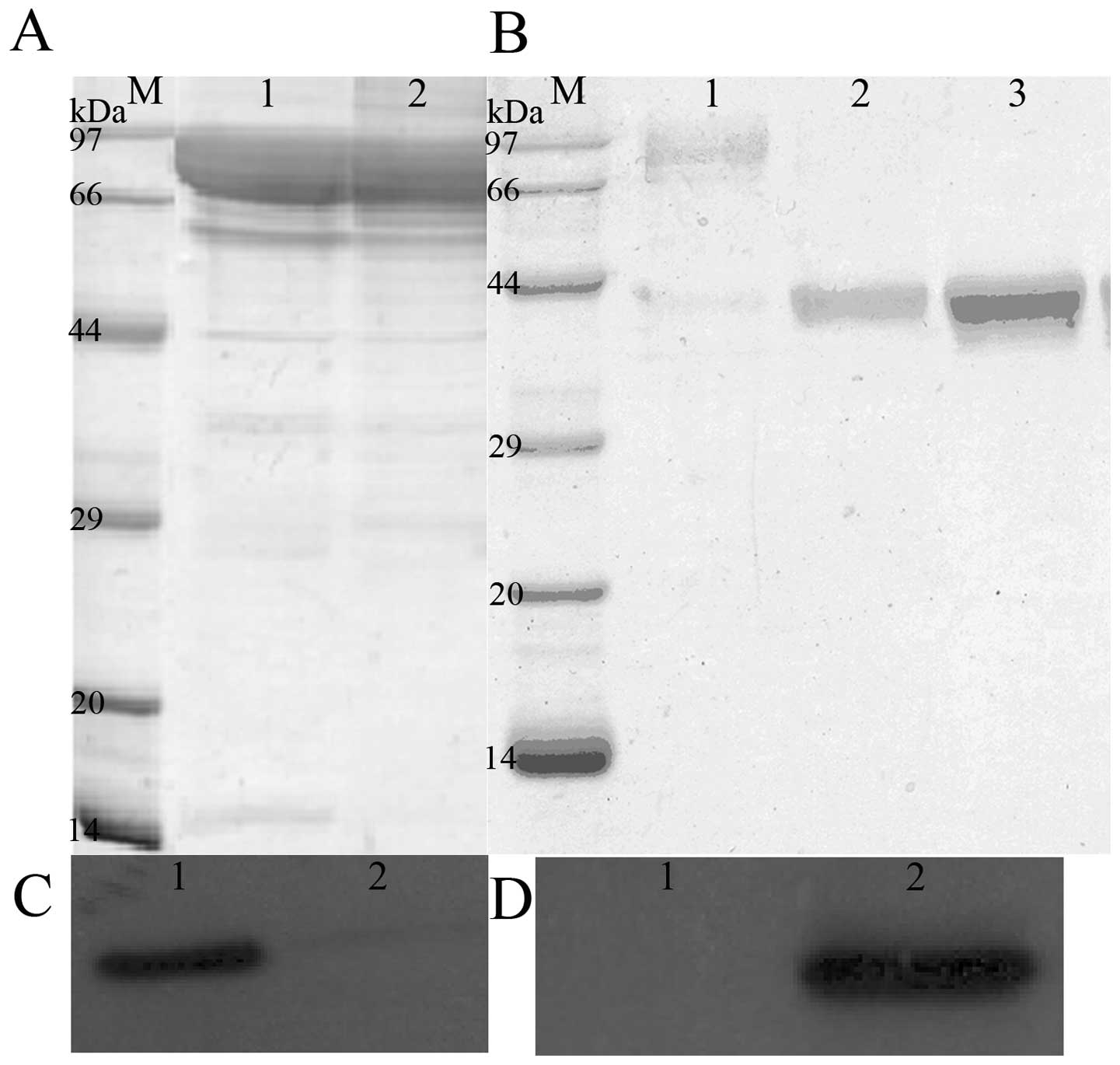
Purification and identification of
recombinant fusion proteins
The cell paste was collected following induction.
Recombinant PSCA-HSPC (Fig. 2A)
and PSCA-HSP (Fig. 2B) were
purified from the supernatant of the bacteria lysate following
treatment with a combination of Ni-NTA resin, Phenyl-Sepharose FF
and HiLoad™ 26/60 Superdex 75. A purity up to 95% was achieved, as
confirmed by SDS-PAGE.
Identification of recombinant fusion
proteins with western blotting
After the proteins were transferred to the PVDF
membranes, the PVDF membranes were treated successively with
anti-PSCA monoclonal antibody and FITC-conjugated goat anti-rabbit
IgG antibody. Western blotting confirmed the presence of PSCA
(Fig. 2C and D).
Discussion
The PSCA gene encodes a 123 amino acid protein.
Studies have identified a low-expression of the protein in normal
prostate and a high-expression in prostate, bladder and pancreatic
cancer (10,11). Further studies demonstrated that
the antibody of PSCA inhibits the growth of prostate cancer
(12,13). However, is is difficult to express
PSCA in a soluble condition in E. coli due to its
complicated spatial structure, which makes it difficult to develop
the vaccine for prostate cancer treatment. Therefore, a method to
enhance the soluble expression of PSCA and elevate the
immunogenicity of PSCA must be established. If assisted with a
suitable adjuvant molecule, the immunogenicity and solubility of
PSCA may be enhanced, which may improve the potency of protein
vaccines based on PSCA.
The function of HSP70 in immunoadjuvant therapy has
been identified and a number of studies have confirmed that
vaccination with HSP70-peptide complexes and HSP70-antigen fusion
proteins reconstituted in vitro with genetic recombination
elicit antitumor immune responses (14–17).
In addition, according to the results of our pre-experiment, HSP70
increases the potency of a DNA vaccine based on PSCA with strong
cellular and humoral immune responses, which inhibit the growth of
PSCA-expressing tumors and prolong the survival time of vaccinated
mice (18). Moreover, coupling
antigens to the amino-terminus of HSP70 may induce stronger immune
responses than coupling to the carboxyl-terminus (18). HSP70 has two functional domains:
the carboxyl-terminus is a peptide-binding domain, which provokes
the production of cytokines and the amino-terminus is the ATPase
domain, which is not capable of provoking the generation of
cytokines. This terminal inhibits the secretion of interleukin
(IL)-10 and transforming growth factor (TGF)-β (19). The antibody titres of anti-HSP70 or
anti-HSPC generated by HSPC are lower compared with those generated
by HSP70 (20).
In the present study, we presented evidence that
human HSP70 enhances the solubility of PSCA. PSCA-HSPC and PSCA-HSP
were successfully expressed to a high level in E. coli in
soluble form and it is convenient for purification. PSCA-HSPN
existed in insoluble form. Following three steps of purification, a
purity of greater than 95% of the recombinant fusion proteins was
obtained. Western blotting revealed that the PSCA and recombinant
fusion proteins obtained via purification had the same
immunological characteristics.
In conclusion, the present study confirmed the
potency of human HSP70 as a molecular chaperone for a recombinant
protein vaccine, which lays the foundation for the development of
vaccines for prostate cancer and further clinical research.
References
|
1
|
Reiter RE, Gu Z, Watabe T, et al: Prostate
stem cell antigen: A cell surface maker overexpressed in prostate
cancer. Proc Natl Acad Sci USA. 95:1735–1740. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu Z, Thomas G, Yamashiro J, et al:
Prostate stem cell antigen (PSCA) expression increases with high
gleason score, advanced stage and bone metastasis in prostate
cancer. Oncogene. 19:1288–1296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krupa M, Canamero M, Gomez CE, Najera JL,
Gil J and Esteban M: Immunization with recombinant DNA and modified
vaccinia virus Ankara (MVA) vectors delivering PSCA and STEAP1
antigens inhibits prostate cancer progression. Vaccine.
29:1504–1513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garcia-Hernandez Mde L, Gray A, Hubby B,
Klinger OJ and Kast WM: Prostate stem cell antigen vaccination
induces a long-term protective immune response against prostate
cancer in the absence of autoimmunity. Cancer Res. 68:861–869.
2008.PubMed/NCBI
|
|
5
|
Ahmad S, Casey G, Cronin M, Rajendran S,
Sweeney P, Tangney M and O’Sullivan GC: Induction of effective
antitumor response after mucosal bacterial vector mediated DNA
vaccination with endogenous prostate cancer specific antigen. J
Urol. 186:687–693. 2011. View Article : Google Scholar
|
|
6
|
Srivastava PK and Udono H: Heat shock
protein-peptide complexes in cancer immunotherapy. Curr Opin
Immunol. 6:728–732. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Srivastava PK, Udono H, Blachere NE and Li
Z: Heat shock proteins transfer peptides during antigen processing
and CTL priming. Immunogenetics. 39:93–98. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blachere NE and Srivastava PK: Heat shock
protein-based cancer vaccines and related thoughts on
immunogenicity of human tumors. Semin Cancer Biol. 6:349–355. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matsueda S, Kobayashi K, Nonaka Y, Noguchi
M, Itoh K and Harada M: Identification of new prostate stem cell
antigen-derived peptides immunogenic in HLA-A2(+) patients with
hormone-refractory prostate cancer. Cancer Immunol Immunother.
53:479–489. 2004.PubMed/NCBI
|
|
10
|
Bahrenberg G, Brauers A, Joost HG and
Jakse G: PSCA expression is regulated by phorbol ester and cell
adhesion in the bladder carcinoma cell line RT112. Cancer Lett.
168:37–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Argani P, Rosty C, Reiter RE, et al:
Discovery of new markers of cancer through serial analysis of gene
expression: prostate stem cell antigen is overexpressed in
pancreatic adenocarcinoma. Cancer Res. 61:4320–4324.
2001.PubMed/NCBI
|
|
12
|
Dannull J, Diener PA, Prikler L, et al:
Prostate stem cell antigen is a promising candidate for
immunotherapy of advanced prostate cancer. Cancer Res.
60:5522–5528. 2000.PubMed/NCBI
|
|
13
|
Saffran DC, Raitano AB, Hubert RS, Witte
ON, Reiter RE and Jakobovits A: Anti-PSCA mAbs inhibit tumor growth
and metastasis formation and prolong the survival of mice bearing
human prostate cancer xenografts. Proc Natl Acad Sci USA.
98:2658–2663. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blachere NE, Li Z, Chandawarkar RY, et al:
Heat shock protein-peptide complexes, reconstituted in vitro,
elicit peptide-specific cytotoxic T lymphocyte response and tumor
immunity. J Exp Med. 186:1315–1322. 1997. View Article : Google Scholar
|
|
15
|
Srivastava PK: Immunotherapy for human
cancer using heat shock protein-peptide complexes. Curr Oncol Rep.
7:104–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI
and Mizzen LA: Immunotherapy of a human papillomavirus (HPV) type
16 E7-expressing tumour by administration of fusion protein
comprising Mycobacterium bovis bacille Calmette-Guérin (BCG)
hsp65 and HPV16 E7. Clin Exp Immunol. 121:216–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu DW, Tsao YP, Kung JT, Ding YA, Sytwu
HK, Xiao X and Chen SL: Recombinant adeno-asseciated virus
expressing human papilomavirus type 16 E7 peptide DNA fused with
heat shock protein DNA as a potential vaccine for cervical cancer.
J Virol. 74:2888–2894. 2000. View Article : Google Scholar
|
|
18
|
Zhang X, Yu C, Zhao J, et al: Vaccination
with a DNA vaccine based on human PSCA and HSP70 adjuvant enhances
the antigen-specific CD8+ T-cell response and inhibits
the PSCA+ tumors growth in mice. J Gene Med. 9:715–726.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu X, Zhao X, Burkholder WF, Gragerov A,
Ogata CM, Gottesman ME and Hendrickson WA: Structural analysis of
substrate binding by the molecular chaperone DnaK. Science.
272:1606–1614. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Kelly CG, Singh M, McGowan EG,
Carrara AS, Bergmeier LA and Lehner T: Stimulation of
TH1-polarizing cytokines, C-C chemokines, maturation of dendritic
cells and adjuvant function by the peptide binding fragment of heat
shock protein 70. J Immunol. 169:2422–2429. 2002. View Article : Google Scholar
|