Introduction
Neonatal respiratory distress syndrome (RDS) is a
syndrome in premature infants caused by developmental insufficiency
of surfactant production and structural immaturity in the lungs. It
affects ∼1% of newborn infants and is the leading cause of
mortality in preterm infants.
Pulmonary surfactant, a lipoprotein complex, is
essential for normal lung function and deficiency of surfactant
results in neonatal RDS. A number of studies have suggested a
genetic contribution to the etiology of RDS (1,2).
Surfactant protein B (SP-B) is important for optimal surfactant
function, since it is involved in the pathogenesis of pulmonary
disease (3). SP-B is one of the
hydrophobic proteins crucial to the surface-lowering properties of
surfactant. It is encoded on a relatively small gene of ∼9,500 bp
on the short arm of chromosome 2 (4). SP-B deficiency results in severe
respiratory failure in term infants shortly after birth and the
primary associated diseases are neonatal RDS and acinar dysplasia
(5). Since mutations in intron 4
of the SP-B gene are rare, few published studies illustrate the
association between mutations in intron 4 of the SP-B gene and
various diseases (3,6). To date, there have been no reports of
a mutation in intron 4 of the SP-B gene with neonatal RDS in a
Chinese population; therefore, we investigated the genetic
variability of SP-B intron 4 in an individual with RDS.
Patient and methods
Patient
At a gestational age of 42 weeks, the second child
of unrelated Chinese parents was delivered by normal vaginal
delivery at term (birthweight, 3.98 kg; Apgar score Apgar score of
6 at 1 min and 10 at 5 min). The patient was cyanosed due to
respiratory distress aged 1 h and ventilation was initiated. Serial
chest radiographs were obtained for assessment of persistent
pulmonary hypertension during the course of the disease. The
patient was treated in the intensive care unit; however, he failed
to respond to treatment and succumbed on day 16. The study was
approved by the ethics committee of General Hospital of Beijing
Military Region, Beijing, China. Written informed patient consent
was obtained from the patient’s family.
Polymerase chain reaction (PCR)
amplification
A 1-ml sample of whole blood was collected in
ethylenediamine tetraacetic acid tubes from the proband and a
newborn with necrotizing enterocolitis as a control. The genomic
DNA of the newborns was purified from the total blood using the
Wizard Genomic DNA Purification Kit® (Promega
Corporation, Madison, WI, USA) according to manufacturer’s
instructions. DNA from the patient and healthy newborn blood
samples was amplified by PCR amplification protocols, as described
by Gong et al (4). The
following oligonucleotides were used as PCR primers:
5′-CTGGTCATCGACTACTTCCA-3′; and 5′-TGTGTGTGAGAGTGAGGGTGTAAG-3′.
Lung tissue biopsy
Formalin-fixed, paraffin-embedded autopsy lung
tissue was obtained for immunohistochemical analysis of SP-B
protein expression. Antigen retrieval methods were employed
initially to ensure that a negative result was due to a lack of
protein and not due to a loss of antigenicity. Sections 5 μm
thick were cut on a rotary microtome and loaded onto
polysine-coated slides. Rabbit polyclonal anti-human SP-B antibody
(Abcam, Hong Kong, China) and a streptavidinbiotin complex (SABC)
peroxidase elite rabbit IgG kit (Wuhan Boster Biological Technology
Ltd., China) were used to detect the antigen-antibody complexes.
Sections selected for antigen retrieval were heated in sodium
citrate buffer at pH 6.0 for 15 min at 90°C. Endogenous peroxidase
was quenched, then sections were blocked with 2% normal goat serum
before incubation overnight at 4°C with the primary antibody
(dilution, 1:300). After washing with phosphate-buffered saline
(PBS), the sections were incubated for 20 min at 37°C with the
secondary antibody (goat anti-rabbit IgG marked by biotinylation).
Then, the sections were washed with PBS and incubated for 20 min at
37°C with the SABC complexes. Finally, the lung tissue was stained
with diaminobenzidine (DAB).
Results
Clinical findings
The patient was cyanosed due to respiratory distress
aged 1 h and ventilation was initiated. Clinical manifestations,
including lividity of the skin, progressive dyspnea with continous
groaning, inhaling three concave sign, were discovered in the
physical examination. Following conception, the pregnant mother of
the proband had no premature rupture, infection or diabetes. The
proband had no intrauterine problems or meconium aspiration
syndrome during the pregnancy and intrapartum. Full blood count and
liver function tests were normal. Blood gas analysis revealed
hypercapnemia and hypoxemia, which indicated progressive hypoxic
respiratory failure. Routine blood and reactive protein tests were
normal. The patient demonstrated pulmonary hypertension and
congenital heart disease confirmed by B-mode ultrasonic diagnostic
equipment. Serological screenings for enterovirus, adenovirus,
influenza types A and B, Chlamydia trachomatis, respiratory
syncytial virus (RSV), cytomegalovirus (CMV), toxoplasmosis and
human immunodeficiency virus (HIV) were all negative. No bacteria
was observed by blood culture analysis.
Radiological findings
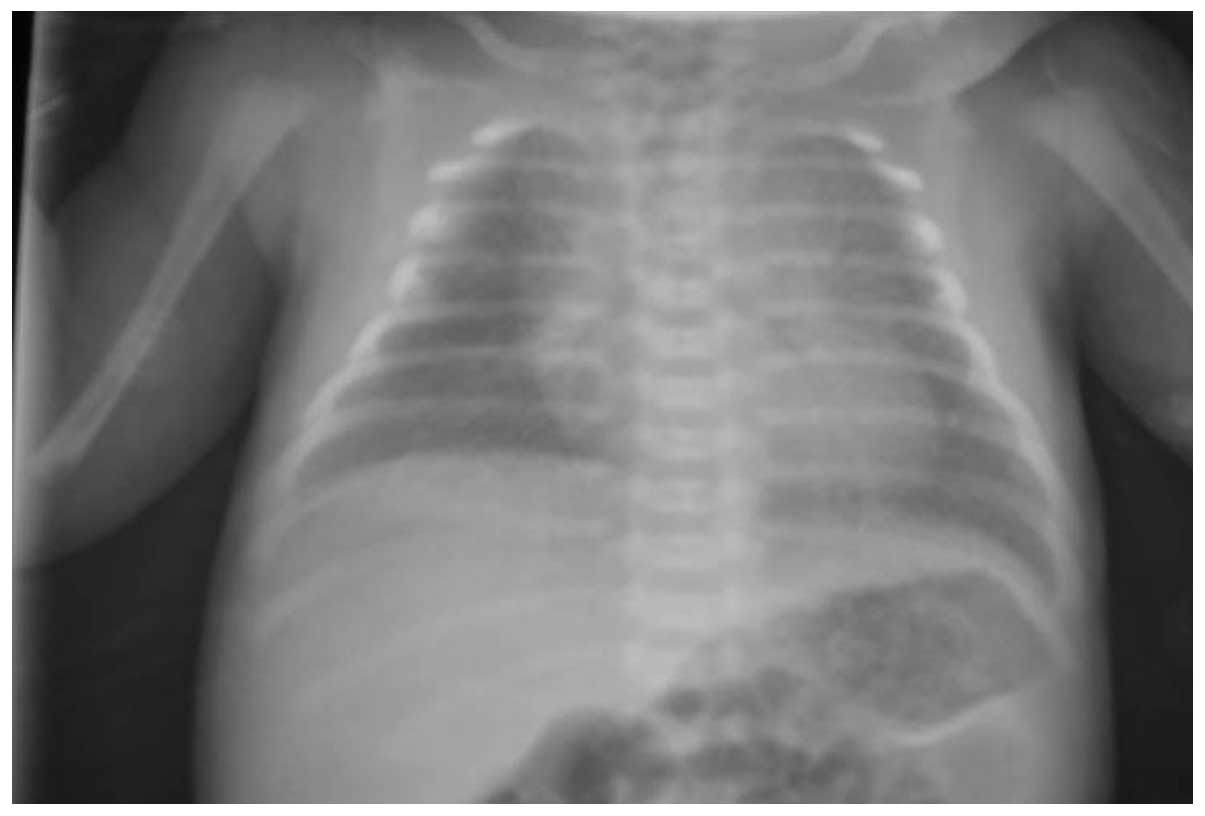
The initial chest X-ray indicated hyper-inflation
and diffuse opacification and air bronchogram of the lungs
(Fig. 1), which is suggestive of
neonatal RDS. A series of therapies, including high-frequency
oscillatory ventilation (HFOV), penicillin for preventing
infection, intravenous nutrition and oxygen therapy were
administered. Serial chest X-rays revealed serious change
throughout this period. The pathogenetic condition of the patient
worsened and the chest X-ray indicated white lung.
Genetic findings
To determine the genetic factors causing RDS, a
sample of whole blood was collected for gene analysis. DNA from the
patient and healthy newborn blood samples was amplified by PCR
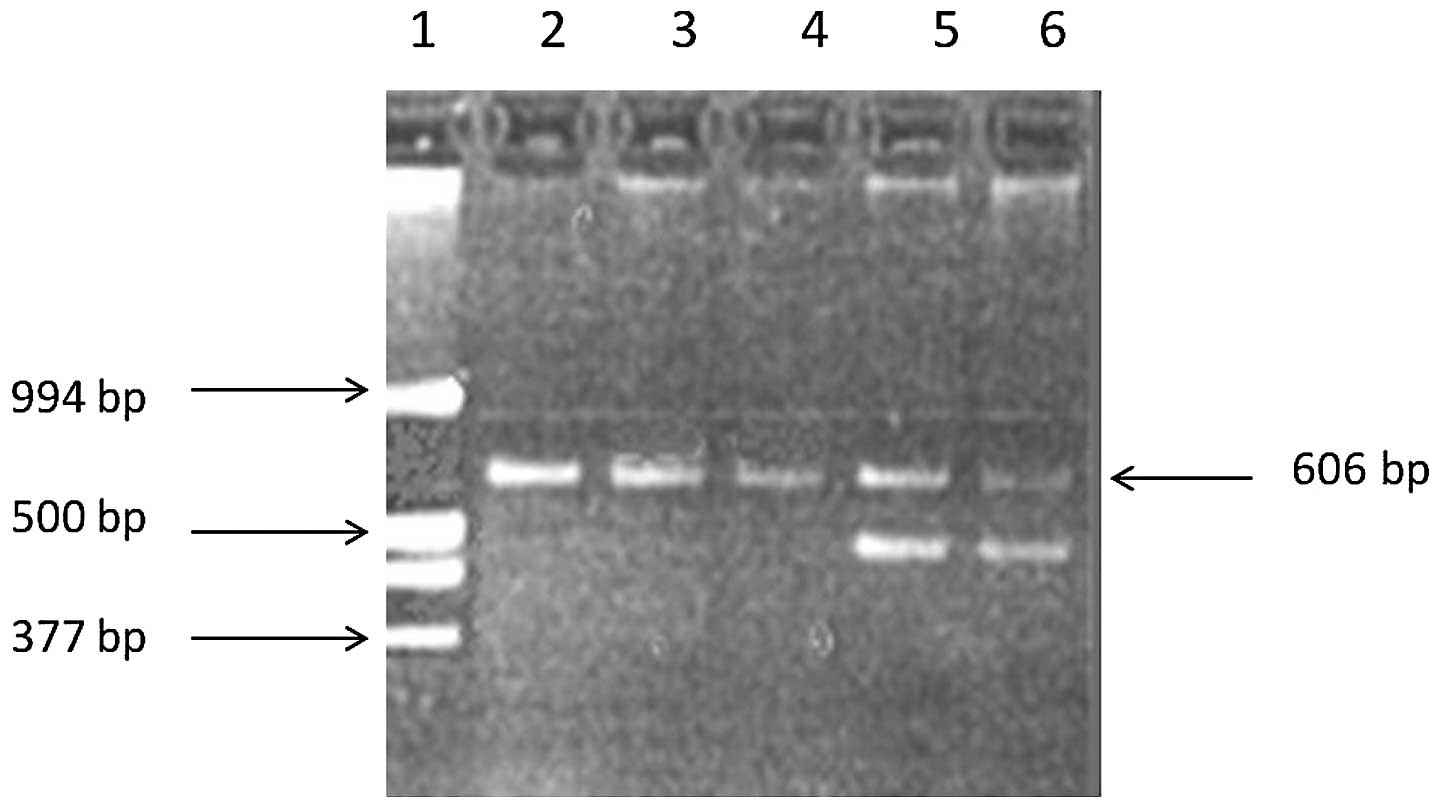
amplification protocols. The control with 606 bp bands was
considered to be wild-type and the proband with an additional band
smaller than 606 bp was considered to have a variant allele, the
121del2 mutation in intron 4 of the SP-B gene (Fig. 2). In this case, the family
requested that palliative therapy be administered and the infant
succumbed on day 16.
Pathological findings
Immunohistochemical characterization and
pathological analyses were obtained post-mortem. Pathological
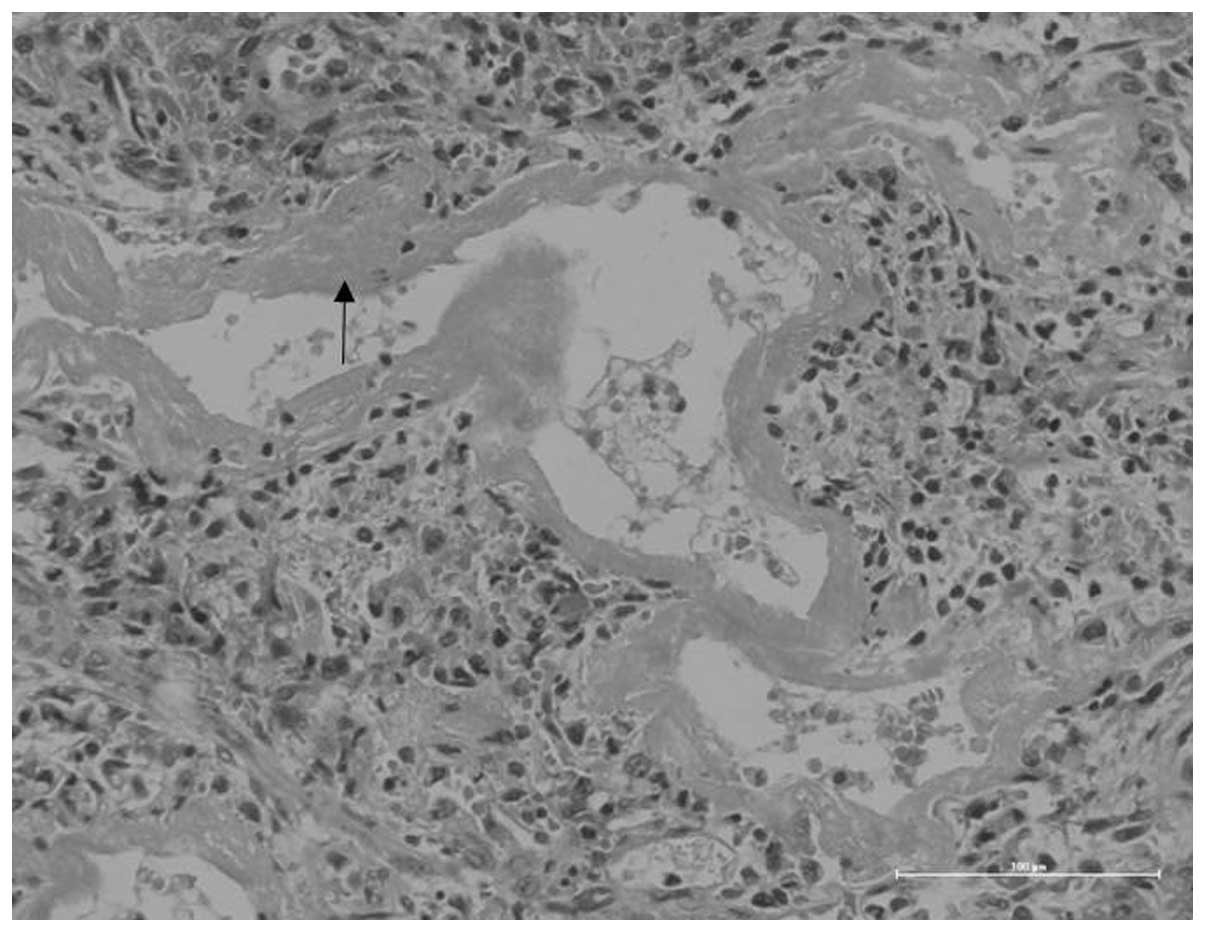
changes of the lung tissue were observed with hematoxylin and eosin
(HE) staining. This revealed eosinophilic material called the
hyaline membrane, which was attached to the wall of the respiratory
bronchioles, alveolar ducts and alveolus, and was also observed
within the alveolar spaces. The lobes of the lungs had varying
degrees of atelectasis and pneumonedema. These manifestations
suggest a diagnosis of neonatal RDS (Fig. 3). Our results reveal
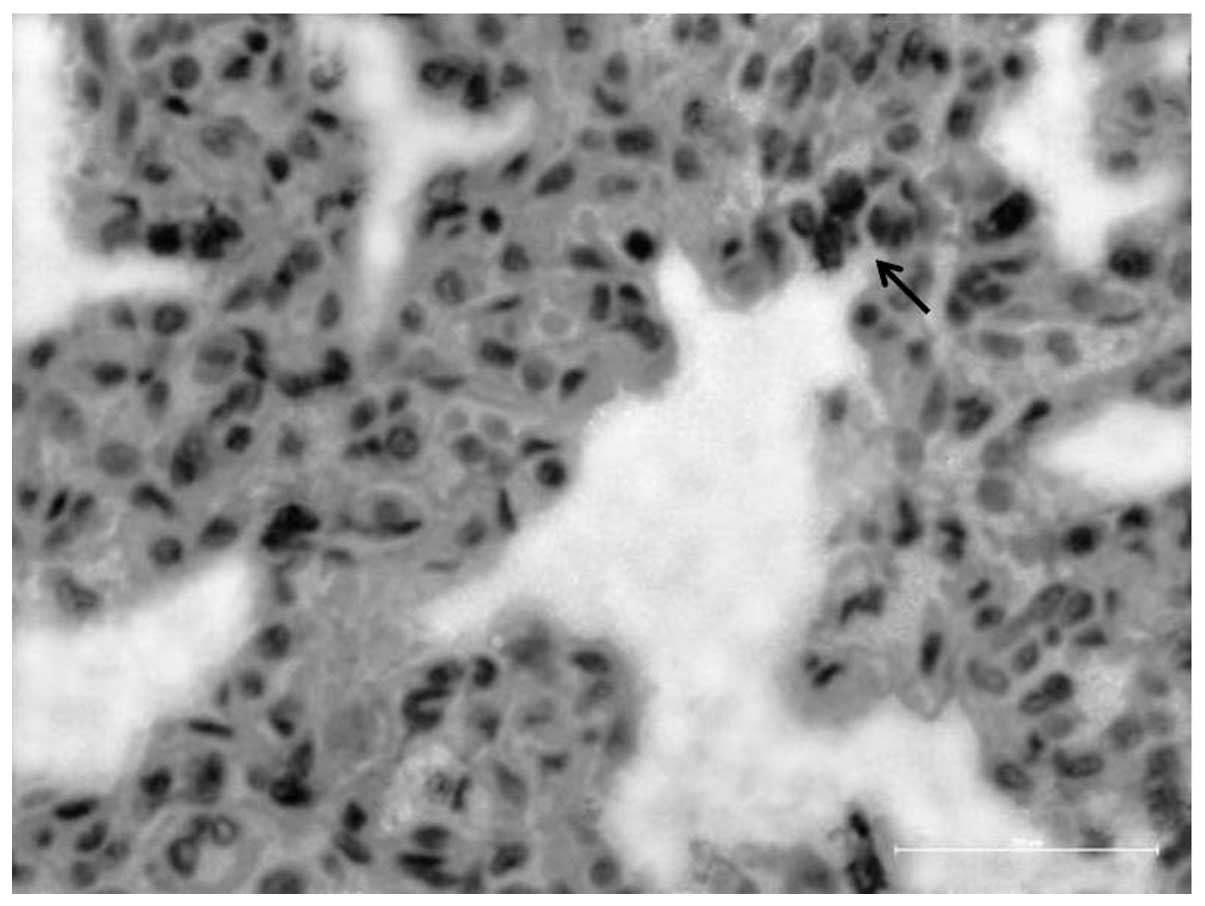
rarely-expressed SP-B-positive cells in the lung tissue (Fig. 4).
Discussion
SP-B is essential for maintaining normal surface
tension at the air-liquid interface in the alveolus of the lung.
The absence of SP-B results in loss of life and dysfunction of SP-B
compromises lung function (7,8).
Evidence indicates that SP-B exhibits an anti-inflammatory function
in the lung (9). The SP-B gene
consists of 11 exons (10) and
maps on chromosome 2 (11). It
encodes a 42 kDa precursor protein (12). The SP-B precursor undergoes several
post-translational processing steps to produce a mature protein of
8 kDa, with the last two protein processing steps being type
II-cell-specific (13,14).
Neonatal RDS in the newborn is a major cause of
neonatal mortality and morbidity. There have been a number of
preliminary investigations into the genetic susceptibility of
neonatal RDS (1,15). The effect of hereditary factors on
neonatal RDS was previously studied. Length variants of intron 4 in
the human SP-B gene are associated with several pulmonary diseases.
Previous results indicate that intron 4 length variants affect SP-B
mRNA splicing and that this may contribute to lung disease
(6). Polymorphisms and mutations
in the SP-B gene are associated with the pathogenesis of
respiratory distress (15).
Several studies have demonstrated that deletion variants of intron
4 affect SP-B gene expression (3,4,6);
however, SP-B intron 4 variant frequencies have no detectable
association with RDS in Brazilian and Finnish populations (1,16).
These studies suggest that population, race and the geographical
environment affect gene distribution and alignment. In the present
study, the association between an SP-B intron 4 variant and
neonatal RDS was investigated.
In this study, the patient demonstrated typical
clinical, radiological and pathological manifestations of neonatal
RDS. The immunohistochemical results of autopsy lung tissue
suggested SP-B protein deficiency, and the results of gene analysis
indicated that an SP-B intron 4 variant caused SP-B protein
deficiency. The onset of progressive hypoxic respiratory failure in
the patient was characteristic of RDS. The chest X-ray of the
proband revealed hyperinflation, diffuse opacification and air
bronchogram in the lungs, indicating a diagnosis of neonatal RDS.
The histological appearance was quite dramatic since SP-B-positive
cells are rarely observed in lung tissues. Gene analysis revealed
that the proband had the 121del2 mutation in SP-B gene intron 4,
which is consistent with the study by Gong et al (4). The present study suggests the
following points: i) the patient had the 121del2 mutation in intron
4 of the SP-B gene; ii) the patient had partial SP-B protein
deficiency and iii) the 121del2 mutation in intron 4 of the SP-B
gene may cause partial SP-B deficiency, eventually leading to
almost irreversible hypoxic respiration. Absence of SP-B may be the
most common finding in neonatal RDS; however, it is not identified
in all cases. Numerous treatment options have been used for SP-B
deficiency; however, treatments involving vigorous surfactant
replacement or lavage using cardiopulmonary bypass are
unsuccessful. Gene transfer therapy holds future promise for the
eventual cure of this usually single gene defect.
In summary, previous studies demonstrated an
association between RDS and SP-B gene polymorphism. The
polymorphism and mutation of surfactant proteins are helpful for
the understanding of the susceptibility to neonatal RDS. However,
the association between SP-B gene polymorphism and RDS was
previously unclear (17). Our
study demonstrates an association between an SP-B intron 4 variant
and neonatal RDS in an individual patient. A larger study is
required to confirm this finding.
Acknowledgements
This study was funded by the National
Natural Science Foundation of China (30871397).
References
|
1
|
Lyra PP, Diniz EM, Abe-Sandes K, Angelo
AL, Machado TM and Cardeal M: Surfactant protein B gene
polymorphism in preterm babies with respiratory distress syndrome.
Braz J Med Biol Res. 44:66–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yin XJ, Li LH, Wang Y, Xie L and Feng ZC:
A study on expression of surfactant protein B in neonatal
respiratory distress syndrome. Chin J Neonatol. 26:336–339.
2011.
|
|
3
|
Floros J, Veletza SV, Kotikalapudi P,
Krizkova L, Karinch AM, Friedman C, Buchter S and Marks K:
Dinucleotide repeats in the human surfactant protein-B gene and
respiratory-distress syndrome. Biochem J. 305:583–590.
1995.PubMed/NCBI
|
|
4
|
Gong MN, Wei Z, Xu LL, Miller DP, Thompson
BT and Christiani DC: Polymorphism in the surfactant protein-B
gene, gender and the risk of direct pulmonary injury and ARDS.
Chest. 125:203–211. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hugosson CO, Salama HM, Al-Dayel F,
Khoumais N and Kattan AH: Primary alveolar capillary dysplasia
(acinar dysplasia) and surfactant protein B deficiency: a clinical,
radiological and pathological study. Pediatr Radiol. 35:311–316.
2005. View Article : Google Scholar
|
|
6
|
Lin Z, Thomas NJ, Wang Y, Guo X, Seifart
C, Shakoor H and Floros J: Deletions within a CA-repeat-rich region
of intron 4 of the human SP-B gene affect mRNA splicing. Biochem J.
389:403–412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clark JC, Weaver TE, Iwamoto HS, Ikegami
M, Jobe AH, Hull WM and Whitsett JA: Decreased lung compliance and
air trapping in heterozygous SP-B-deficient mice. Am J Respir Cell
Mol Biol. 16:46–52. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clark JC, Wert SE, Bachurski CJ, Stahlman
MT, Stripp BR, Weaver TE and Whitsett JA: Targeted disruption of
the surfactant protein B gene disrupts surfactant homeostasis,
causing respiratory failure in newborn mice. Proc Natl Acad Sci U S
A. 92:7794–7798. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Epaud R, Ikegami M, Whitsett JA, Jobe AH,
Weaver TE and Akinbi HT: Surfactant protein B inhibits
endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol.
28:373–378. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pilot-Matias TJ, Kister SE, Fox JL, Kropp
K, Glasser SW and Whitsett JA: Structure and organization of the
gene encoding human pulmonary surfactant proteolipid SP-B. DNA.
8:75–86. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vamvakopoulos NC, Modi WS and Floros J:
Mapping the human pulmonary surfactant-associated protein B gene
(SFTP3) to chromosome 2p12→p11.2. Cytogenet Cell Genet. 68:8–10.
1995.PubMed/NCBI
|
|
12
|
Jacobs KA, Phelps DS, Steinbrink R, Fisch
J, Kriz R, Mitsock L, Dougherty JP, Taeusch HW and Floros J:
Isolation of a cDNA clone encoding a high molecular weight
precursor to a 6-kDa pulmonary surfactant-associated protein. J
Biol Chem. 262:9808–9811. 1987.PubMed/NCBI
|
|
13
|
Weaver TE: Synthesis, processing and
secretion of surfactant proteins B and C. Biochim Biophys Acta.
1408:173–179. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hawgood S, Derrick M and Poulain F:
Structure and properties of surfactant protein B. Biochim Biophys
Acta. 1408:150–160. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lyra PP, Vaz FAC, Moreira PE, Hoffmann JW,
DeMello DE and Diniz EMA: Comparison of surfactant protein B
polymorphisms of healthy term newborns with preterm newborns having
respiratory distress syndrome. Braz J Med Biol Res. 40:779–789.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramet M, Haataja R, Marttila R, Floros J
and Hallman M: Association between the surfactant protein A (SP-A)
gene locus and respiratory-distress syndrome in the Finnish
population. Am J Hum Genet. 66:1569–1579. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Haataja R, Marttila R, Uimari P, Lofgren
J, Ramet M and Hallman M: Respiratory distress syndrome: evaluation
of genetic susceptibility and protection by transmission
disequilibrium test. Hum Genet. 109:351–355. 2001. View Article : Google Scholar : PubMed/NCBI
|