Introduction
Calcium (Ca2+) oscillation refers to the
temporal and spatial undulation of Ca2+ concentration
and is indicative of synchronized electrical activities in the
neuronal network. The electrophysiological characteristics of an
epileptic attack mainly include hypersynchronous discharge of
neurons of the local or whole brain; therefore Ca2+
oscillations have always been considered as a simple mode of
electrophysiological movement of the epileptic nerves. At present,
the biggest problem in epilepsy treatment is the insensitivity to
existing exogenous substances (1).
The antiepileptic nature of adenosine (Ade) has attracted much
attention. Ade is the intermediate product of energy metabolism
widely existing in vivo, so if endogenous Ade can be
successfully induced to exert its antiepileptic effect, an
effective and new treatment method will be provided for
drug-resistant patients with refractory epilepsy. In this sense, a
study of the relationship between Ade and Ca2+
oscillations has important clinical significance. This study aims
to observe the effect of Ade on Ca2+ oscillations in the
nerve cells of primary cultured hippocampal neurons in vitro
with confocal laser scanning microscopy. The hippocampal tissue is
implicated in epilepsy and if Ade affects the electrical activities
between the neural networks it may explain the antiepileptic
mechanism of Ade.
Materials and methods
Animals
Neonatal Sprague Dawley (SD) rats (no more than 48 h
old) provided by the Experimental Animal Center of Binzhou Medical
University were randomly selected without restriction of gender.
The study was carried out in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee (IACUC) of the Affiliated Hospital of Binzhou
Medical University.
Culture of primary hippocampal
neurons
The primary hippocampal neurons were cultured
referring to related studies (2,3). The
specific steps were as follows. Neonatal SD rats were disinfected
with 75% alcohol. Their cerebral hemispheres were dissected out,
the olfactory bulbs retracted from the front with an
ophthalmological forceps perpendicular to their brainstems, and the
cerebra removed. Their cerebral cortices were opened on both sides
along their longitudinal cerebral fissures to show the semilunar
hippocampus. Each separate hippocampus could be seen on both sides.
The membrane, blood vessel and non-hippocampal formation were
carefully removed and the hippocampus was washed with Hank’s
Balanced Salt Solution (HBSS). The hippocampus was cut into 3×1 mm
pieces and added to ∼2 ml of 0.125% trypsin (Gibco-BRL, Carlsbad,
CA, USA). The mixture was gently shaken and digested for 10–15 min
in an incubator at 37°C. On completion of digestion, the pancreatin
was removed using a Pasteur pipet. The digestion was then
terminated for 5 min with 5 ml of the planting medium. The tissue
suspension was gently blown with a polished pipet ∼20 times and
filtered with a 200 mesh screen. The filtration was transferred
into the centrifuge tubes, and then centrifuged at 800 rpm for 4
min to remove the supernatant. The planting medium was added, then
the mixture was made into cell suspension through blowing, and the
cell suspension thus obtained was planted on the culture dish with
a coverslip coated with polylysine (Gibco-BRL). The planting medium
was added to each culture dish, so that the volume on each culture
dish reached 2 ml, then the culture dishes were cultured in an
incubator with 5% CO2 at 37°C. All media were replaced with
maintenance media 24 h later. Afterwards, half of the liquid volume
in the maintenance media was replaced once every three days.
Experimental group
In order to record Ca2+ oscillations, the
hippocampal neurons cultured with Krebs-Ringer solution as a basal
medium were divided into 4 groups: control group, high potassium
treament group, Ade treament group, high potassium and Ade
co-treatment group. High potassium group: neurons were treated with
60 mmol/l KCl. Ade treament group: neurons were treated with Ade at
0.1 μmol/l and 50 μmol/l, seperately. High potassium
and Ade co-treatment group: neurons were treated with 50
μmol/l Ade and 60 mmol/l KCl. Control group: neurons were
just treated with Krebs-Ringer solution.
Ca2+ oscillation records by
laser scanning confocal microscopy
The fluorescence intensity produced by a fluorescent
probe Fluo-3/AM (Biotium Company, San Francisco, CA, USA) is
proportional to the intracellular free Ca2+
concentration. Therefore, the hippocampal neurons were carried in
Krebs-Ringer solution (final concentration of 6 μmol/l) of
Fluo-3/AM in an incubator with 5% CO2 at 37°C for ∼30
min, followed by quick flushing three times and then suspending in
the recording solution for delipidation with Fluo-3/AM for ∼15 min
(4). When the above steps were
completed, the cells growing on 30-mm special slides were directly
placed in the matching stainless steel tank, followed by image
acquisition with an inverted fluorescence microscope (Olympus
FV500, Japan). A 485-nm wavelength was selected to activate
Fluo-3/AM. Here, a pre-cooled camera was used to acquire the
necessary images, which were acquired and analyzed using the
Fluoview Tiempo time course software. After adjusting to the
desired concentration, Ade was administered with the perfusion
device. Ca2+ oscillations in hippocampal neurons were
recorded as follows: before addition of Ade, the oscillation was
shot for ∼3 min; after addition of Ade, the oscillation was
continuously shot for ∼12 min.
Quantitative analysis method of
Ca2+ oscillations (2)
Data were obtained from fluorescence images by
analyzing the average fluorescence intensity in the pixel region of
∼3×3 in the center of the hippocampal neuronal cell bodies which
had pyramidal shapes, well developed branches and good adherence.
ΔF/F0, the relative fluorescence intensity change of
Fluo-3 shows the intracellular Ca2+ concentration
change, and is used to reflect the amplitude of Ca2+
oscillation. ΔF is the Ca2+ concentration at the moment
of t and F0 is the average baseline value obtained
within the unit time of t±10 sec. Ca2+ oscillation is
defined when ΔF/F0 is dramatically increased by more
than 20%. Ca2+ oscillation frequency and amplitude are
obtained from calculating the frequency and average amplitude
within the fixed unit time of 2 min, and are used as the
statistical record of the experimental data.
Statistical analysis
Data were analyzed using SPSS 11.0 (SPSS, Inc.,
Chicago, IL, USA) statistical software. Measured data were
expressed as the means ± SEM using the paired t-test. P<0.05 was
considered to indicate a statistically significant result.
Results
Identification of the hippocampal
neurons
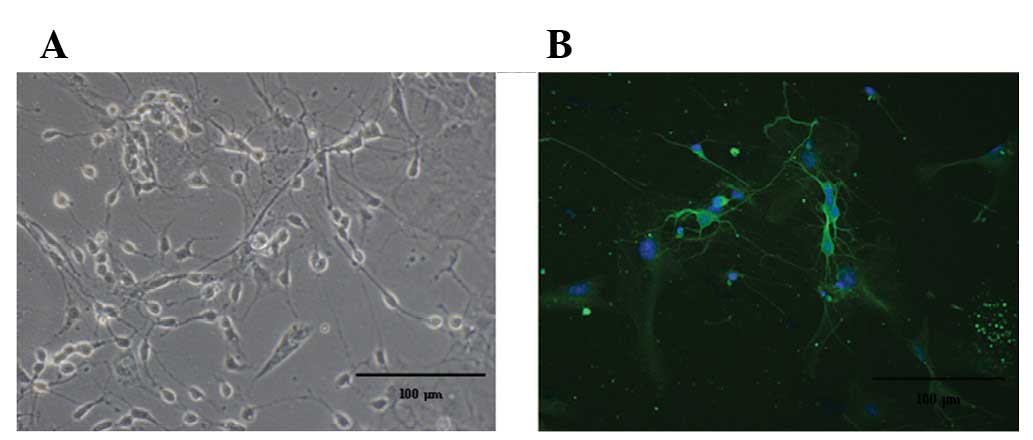
Hippocampal neurons were observed with a high power
microscope after 7–9 days using indirect immunofluorescence
staining with Tubulin. Green represents neuronal matter and blue
represents the nerve cell nucleus (Fig. 1).
Ca2+ oscillation records
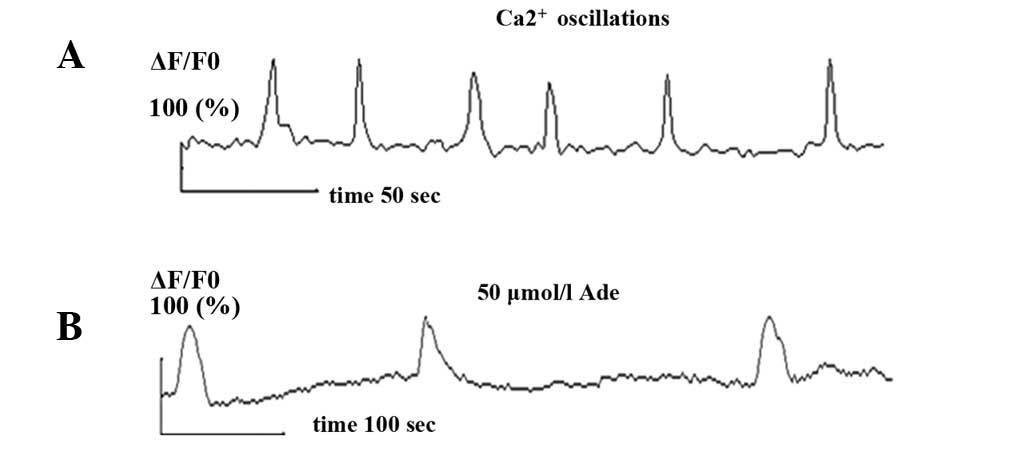
Spontaneous synchronized Ca2+
oscillations were observed in primary cultured hippocampal neurons
using confocal laser technology. Ca2+ oscillation
frequency and amplitude were 1.2±0.12/min (0.02±0.002 Hz) and
1.87±0.17, respectively (Fig. 2A),
which was consistent with literature (4).
Effect of Ade on Ca2+
oscillations
Once dissolved in Krebs-Ringer solution to achieve
the desired concentration, Ade could be tested for its effect on
mature hippocampal neurons. Krebs-Ringer solution (1 ml) was
prepared in a dish and observed for ∼3 min, which was taken as the
control Ade intervention. The solution was then replaced by 1 ml
freshly prepared Ade solution for continual recording and neurons
maintained in 1 ml of Krebs-Ringer solution were used as a control.
Following the addition of Ade, spontaneous Ca2+
oscillation frequency was measured at various times. The results
showed that 0.1 μmol/l Ade had no significant effect on
Ca2+ oscillations but 50 μmol/l Ade inhibited the
spontaneous synchronized Ca2+ oscillation frequency and
amplitude (n=12, P<0.05). Its frequency was reduced from
1.2±0.2/min (0.02±0.003 Hz) before addition of Ade to 0.3±0.05/min
(0.005±0.001 Hz) and its amplitude was decreased from 1.87±0.17
before addition of Ade to 1.1±0.07 (Fig. 2B).
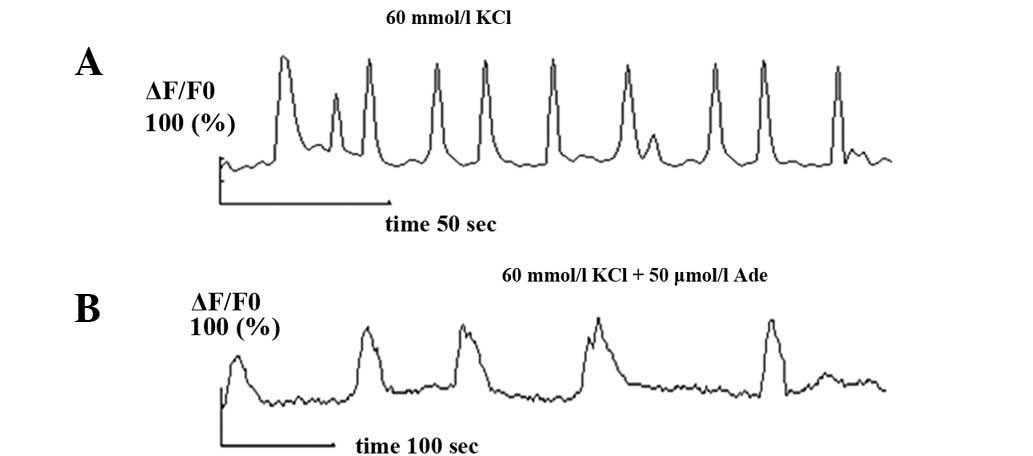
After perfusion of high potassium recording
solution, the observed confocal results showed that Ca2+
oscillations were significantly enhanced in respect of frequency
and amplitude. After recording for 3 min, the Ade (50
μmol/l) intervention began and Ca2+ oscillation
frequency and amplitude were inhibited again (n=7, P<0.05). The
frequency was reduced from 2.39±0.22/min (0.04±0.003 Hz) before
addition of Ade to 0.44±0.13/min (0.01±0.002 Hz) and its amplitude
was decreased from 2.45± 0.27 before addition of Ade to 1.12±0.08
(Fig. 3).
Discussion
Ade is the precursor and product of adenine
nucleotide metabolism. Energy is used throughout the human body and
this endogenous purine nucleoside is widely distributed in tissues
all over the body. It is involved in the regulation of a variety of
physiological functions by activating Ade receptors (A1, A2a, A2b,
and A3) (5,6). In the central nervous system, Ade is
a normal component of the extracellular fluid of neurons and has a
low physiological level (0.03–0.3 μmol/l). During an
epileptic attack, however, the Ade concentration is increased by
6–31 times. Some researchers have reported that Ade secretion and
Ade receptor expression were significantly decreased in the
refractory epilepsy model (7).
Some studies have also shown that transplantation of adenosine
kinase (ADK)-knockout glial cells which secrete Ade was able to
terminate an epileptic attack in animal models, suggesting that
endogenous Ade exerts a natural antiepileptic effect. Knowledge of
the target area and mechanism of action of Ade has important
significance for further research on how to activate Ade to fully
exert its antiepileptic effect. Jackisch et al found that
the hippocampal neurons in the central nervous system are
particularly sensitive to the effect of Ade (4), so a preliminarily discussion of the
antiepileptic mechanism of Ade with primary cultured hippocampal
neurons as the model is appropriate.
Ca2+ oscillation is the temporal and
spatial undulation of Ca2+. The formation of
Ca2+ oscillations within the neuron network is modified
through extracellular Ca2+ influx in combination with
the release and assimilation of intracellular Ca2+
stores. Ca2+ oscillation is an ubiquitous phenomenon in
the nervous system tissues and Ca2+ can rapidly diffuse
in several ways, including gap junctions. Its frequency and
amplitude changes code neural network information and play an
important role in synaptic plasticity and neuronal transfer
(11). Koizumi and Inoue (12) reported that under physiological
conditions, synchronous primary Ca2+ oscillations exist
in the hippocampal neurons of rats; such Ca2+
oscillation is closely related to the synapse stimuli and can
trigger excitation. Some experiments proved that the
synchronization of neuronal electrical activity marked by the
Ca2+ oscillation is crucial to the propagation of
epileptiform discharges (13). The
release of epileptiform activity starts with the intrinsic burst
discharge in neurons, wherein the excessive influx of
Ca2+ passing through the Ca2+ channels and
intracellular Ca2+ store release plays an important
role. At the same time, Ca2+ overload phenomenon exists
in neurons during an epileptic attack. Calcium is known as the
basic promoter of the excitatory toxic action of epilepsy. In the
central nervous system, temporal and spatial undulation of
Ca2+ at a certain frequency and amplitude is known as
the Ca2+ oscillation. In primary cultured hippocampal
neurons, synchronous primary Ca2+ oscillation is
associated with dynamic changes of the membrane potential. The
synergistic effect of NMDA receptors and voltage-dependent L-type
Ca2+ channel activation allows the Ca2+ to
move from the intracellular to the extracellular area, thereby
modifying the shape of Ca2+ oscillation every time
(14). High-level Ade is likely to
affect the intracellular Ca2+ oscillation by regulating
the Ca2+ channel current in neurons and decrease
Ca2+ oscillation frequency and amplitude, so as to
further play a role in inhibiting the pathological processes such
as synchronized electrical activity between neurons and peripheral
excitatory neurotransmitter release during epileptic attack,
finally reduce the excitability of neural networks in the central
nervous system, and inhibit epileptic attack. As a result,
spontaneous synchronized Ca2+ oscillations are
considered the electro-physiological basis of epileptic activity
(13,15).
This study first observed spontaneous synchronized
Ca2+ oscillations in primary cultured hippocampal
neurons using confocal laser scanning microscopy. Based on this,
the effect of Ade on Ca2+ oscillations was further
investigated. Low-level Ade does not have a significant effect on
Ca2+ oscillations in neurons but Ade at a concentration
of 50 μmol/l causes the spontaneous Ca2+
oscillation frequency and amplitude in hippocampal neurons to be
decreased. Ade may influence intra-cellular Ca2+
oscillations by regulating the balance between the release and
assimilation of the spontaneous intracellular Ca2+
stores in neurons. This effect shows some dependence on the Ade
concentration. The high extracellular potassium environment allows
the intracellular and extracellular potassium concentration
gradient to be decreased, the negative value of the resting
potential to be reduced, the threshold potential gap to be
shortened and finally results in abnormal rise of the cell
excitability. Potassium and Ca2+ are mutually
antagonistic, so the influx of potassium is reduced and the
Ca2+ influx is increased correspondingly, which can
further trigger the electrical imbalance. Therefore, a high
potassium solution was used in this study as the depolarization
stimuli to activate voltage-gated Ca2+ channels. This
was to facilitate the extracellular Ca2+ influx,
accelerate Ca2+ circulation in the cytosol and
Ca2+ stores and simulate the epileptic discharge model
to observe the effect of Ade. The results showed that Ade at
certain concentrations plays a role in inhibiting the high
potassium-induced Ca2+ oscillations. When the Ade is
above the physiological level, it will decrease the Ca2+
oscillation frequency and amplitude and is likely to further
inhibit the pathological processes such as synchronized electrical
activity between neurons and peripheral excitatory neurotransmitter
release during epileptic attack, thereby finally reducing the
excitability of neural network in the central nervous system, which
is also consistent with the research findings of Brundege and
Dunwiddie (16) and Phillis and Wu
(17).
Ade has several receptors including A1, A2a, A2b and
A3, which are all distributed throughout the central nervous
system, as indicated by autoradiography. Research has shown that
activated A3 receptors stabilize Ca2+ transport within
the sarcoplasmic reticulum of myocardial cells and studies on the
protective effect of the A3 receptor on the central nervous system,
have also been reported (18). Ade
at certain concentrations may, therefore, regulate intracellular
Ca2+ oscillations by acting on A3 receptors, thereby
eventually antagonizing the synchronized electrical activity
between neurons during an epileptic attack.
As the most important member of the Ade receptor
family, Ade receptor A1 with high affinity is highly expressed in
the cerebral cortex, hippocampus and other sites. Pék and Lutz
(19) proved through studies on
the cerebral hypoxia model that through acting on A1 receptors, Ade
allows the potassium conductance in the cell membrane to be
increased and hyperpolarized and extracellular Ca2+
influx to be reduced, thereby inhibiting the excitability of
neurons. Many studies have shown that Ade can inhibit the release
of glutamate. Flint et al (20) recorded Ca2+ oscillations
mediated by metabotropic glutamate receptors in embryonic cortical
slices. Sohn et al (21)
further pointed out that such short-term Ca2+ waves are
mediated by metabotropic glutamate receptor 5. It was further
hypothesized that Ade may open Ca2+ channels by acting
on A1 receptors at certain concentrations. This results in
hyperpolarization, inhibition of the excitatory effect of glutamate
and regulation of the spatiotemporal changes of intracellular
Ca2+ concentration. This eventually antagonizes the
synchronized excitatory electrical activity between neurons during
an epileptic attack. Proteomic mass spectrometry analysis in this
study showed that when epilepsy is induced by pentrazole, there is
no expression of glutamine synthetase in the cerebral cortex of Ade
Al receptor gene knockout mice, compared with wild-type controls.
Glutamine synthetase is the key enzyme in the glutamate/glutamine
pathway. The Ade A1 receptor is likely to have important relevance
with the activation of glutamine synthetase and acceleration of the
metabolic hydrolysis of excitatory neurotransmitter glutamate in
the brain, which also corroborates the results in this study to a
certain extent.
To understand the action mechanism of Ade, it is
necessary to further study the neuronal membrane surface receptor
characteristics and intracellular and extracellular complex
signaling pathways. This research provides a basis and lays
preliminary research foundation for new clinical therapy and
preventive approaches against epilepsy.
References
|
1
|
Schmidt D and Löscher W: Drug resistance
in epilepsy: putative neurobiologic and clinical mechanisms.
Epilepsia. 46:858–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhu SQ, Qi L, Rui YF, Li RX, He XP and Xie
ZP: Astragaloside IV inhibits spontaneous synaptic transmission and
synchronized Ca2+ oscillations on hippocampal neurons. Acta
Pharmacol Sin. 29:57–64. 2008.PubMed/NCBI
|
|
3
|
Kaech S and Banker G: Culturing
hippocampal neurons. Nat Protoc. 1:2406–2415. 2006. View Article : Google Scholar
|
|
4
|
Jackisch R, Strittmatter H, Kasakov L and
Hertting G: Endogenous adenosine as a modulator of hippocampal
acetylcholine release. Naunyn Schmiedebergs Arch Pharmacol.
327:319–325. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dragunow M and Faull RL: Neuroprotective
effects of adenosine. Trends Pharmacol Sci. 9:193–194. 1988.
View Article : Google Scholar
|
|
6
|
Etherington LA and Frenguelli BG:
Endogenous adenosine modulates epileptiform activity in rat
hippocampus in a receptor subtype-dependent manner. Eur J Neurosci.
19:2539–2550. 2004. View Article : Google Scholar
|
|
7
|
Rebola N, Porciúncula LO, Lopes LV, et al:
Long-term effect of convulsive behavior on the density of adenosine
A1 and A2A receptors in the rat cerebral cortex. Epilepsia.
46:159–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boison D: Adenosine and epilepsy: from
therapeutic rationale to new therapeutic strategies.
Neuroscientist. 11:25–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boison D: Adenosine as a modulator of
brain activity. Drug News Perspect. 20:607–611. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boison D: Adenosine augmentation therapies
(AATs) for epilepsy: prospect of cell and gene therapies. Epilepsy
Res. 85:131–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bacci A, Verderio C, Pravettoni E and
Matteoli M: Synaptic and intrinsic mechanisms shape synchronous
oscillations in hippocampal neurons in culture. Eur J Neurosci.
11:389–397. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koizumi S and Inoue K: Inhibition by ATP
of calcium oscillations in rat cultured hippocampal neurones. Br J
Pharmacol. 122:51–58. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santini CC and Tyrrell AM: The
manipulation of calcium oscillations by harnessing
self-organisation. Biosystems. 94:153–163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hou XY, Zhang GY, Yan JZ, Chen M and Liu
Y: Activation of NMDA receptors and L-type voltage-gated calcium
channels mediates enhanced formation of Fyn-PSD95-NR2A complex
after transient brain ischemia. Brain Res. 955:123–132. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Traub RD and Jefferys JG: Are there
unifying principles underlying the generation of epileptic
afterdischarges in vitro? Prog Brain Res. 102:383–394. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brundege JM and Dunwiddie TV: Role of
adenosine as a modulator of synaptic activity in the central
nervous system. Adv Pharmacol. 39:353–391. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phillis JW and Wu PH: The role of
adenosine and its nucleotides in central synaptic transmission.
Prog Neurobiol. 16:187–239. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chelardoni S, Carnicelli V, Frascarelli S,
et al: Effects of A1 adenosine receptor stimulation on the
expression of genes involved in calcium homeostasis. J Mol Cell
Cardiol. 39:964–971. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pék M and Lutz PL: Role for adenosine in
channel arrest in the anoxic turtle brain. J Exp Biol.
200:1913–1917. 1997.PubMed/NCBI
|
|
20
|
Flint AC, Dammerman RS and Kriegstein AR:
Endogenous activation of metabotropic glutamate receptors in
neocortical development causes neuronal calcium oscillations. Proc
Natl Acad Sci USA. 96:12144–12149. 1999. View Article : Google Scholar
|
|
21
|
Sohn JW, Yu WJ, Lee D, Shin HS, Lee SH and
Ho WK: Cyclic ADP ribose-dependent Ca2+ release by group
1 metabotropic glutamate receptors in acutely dissociated rat
hippocampal neurons. PLoS One. 6:e266252011.PubMed/NCBI
|