Introduction
There are 110 individuals with cerebral infarction
per 100,000 population in China, accounting for 60–80% of patients
with stroke (1). In 75% of the
cases, the cerebral infarction is caused by local cerebrovascular
occlusion due to acute thrombosis formation or thrombosis
metastasis from other sites. With the development of neurological
and thrombolytic research, thrombolytic therapy has become the most
effective treatment for reducing the infarction area and disability
rate, particularly in patients with moderate or severe nervous
disorders. Aggressive and reasonable thrombolytic technologies may
be used in hyper-acute and acute cerebral infarction for restoring
the blood supply in the ischemic penumbra. This rescues the nerve
cells with reversible damage and thus reduces the mortality and
disability rate of patients,. which is important for the patient,
family and society. It has been observed that if intravenous
thrombolysis using recombinant tissue-type plasminogen activator
(rTPA) is conducted within 3 h after ischemic stroke onset, the
risks of fatality and severe disability are significantly reduced,
with great improvements in the quality of life of survivors
(2). As shown in clinical studies,
intra-arterial thrombolysis has a more reliable efficacy, with a
longer therapeutic time window (3–5).
However, the recanalization time for intravenous and intra-arterial
thrombolysis is at least 1–2 h (6–8),
rarely <1 h. An exception has been reported by Farkas et
al(9), in which the average
recanalization time was 54 min for 17 patients treated with
intra-arterial rTPA thrombolysis. In addition, the recanalization
rate of intra-arterial thrombolysis remains unsatisfactory.
Further reduction of the recanalization time relies
on mechanically assisted thrombolysis, i.e., the combination of
thrombolytic injection and mechanical thrombus disruption or
removal. The latter may further reduce the thrombolytic dosage and
increase the contact area of the thrombolytic agent with the
thrombus, resulting in safer intra-arterial thrombolysis and a
higher recanalization rate (10–14).
Therefore, an understanding of the factors influencing
recanalization and the identification of the most favorable
mechanically assisted thrombolysis methods are particularly
important. In this study, mechanically assisted intra-arterial
urokinase thrombolysis was conducted in 28 patients with acute
cerebral infarction between January 2009 and October 2012. The
clinical efficacy and safety of mechanically assisted thrombolysis
in the treatment of acute cerebral infarction were assessed.
Patients and methods
Patients
According to the cerebral infarction diagnosis
standard (Fourth National Cerebrovascular Diseases Conference,
China) (15) and Guideline of
Cerebrovascular Disease Prevention and Treatment in China (People’s
Health Publishing House, 2007) (16), 28 patients (20 males and 8 females)
diagnosed with acute cerebral infarction between January 2009 and
October 2012 were enrolled in this study. The patients were aged
33–78 years old, with an average age of 57.6 years. There were 23
cases with a thrombus in the internal carotid artery system and 5
cases with a thrombus in the vertebral-basilar artery system. A
total of 20 cases were treated within 3–6 h after thrombosis onset
and 8 cases were treated within 6–8 h after thrombosis onset. The
cases of combined vascular stenosis (>50%), hypertension,
diabetes, atrial fibrillation, transient ischemia attack (TIA) and
cerebral infarction history were 7 (25.0%), 16 (57.1%), 6 (21.4%),
3 (10.7%), 8 (28.6%) and 3 (10.7%), respectively (Table II). The study was supported by
Ningbo Medical Technology project (No. 2006058) has been approved
by the ethics committee of the Affiliated Yinzhou Hospital, Ningbo,
China. Informed consent was obtained from the patient or the
patient’s family.
 | Table IIIndices in patients with
recanalization and non-recanalization (mean ± SD). |
Table II
Indices in patients with
recanalization and non-recanalization (mean ± SD).
| Indices | Recanalization
(n=23) | Non-recanalization
(n=5) | P-value |
|---|
| Age (years) | 55.26±12.01 | 66.60±8.62 | 0.040 |
| Disease onset time
(min) | 239.35±75.70 | 408.00±27.75 | 0.000 |
| Urokinase dosage
(U) | 69.35±21.65 | 73.00±15.65 | 0.725 |
| Preoperative NIHSS
score | 11.09±5.45 | 16.60±9.18 | 0.082 |
Mechanically assisted thrombolysis
The preoperative routine examinations, including CT
scanning, blood coagulation test, blood sugar test and
electrocardiogram were performed on patients prior to thrombolysis,
and the routine preoperative preparation was conducted. The
cerebral angiography and arterial thrombolysis were then conducted
immediately. The CT scanning results were ready within 24 h. The
time between emergency visit and treatment initiation was ≤45
min.
Systemic heparinization was performed following a
puncture to the femoral artery using Seldinger technology. The
aortic arch and cerebral angiography were conducted under local
anesthesia to determine the infarction site (the angiography was
often conducted on the aortic arch and suspected sites according to
clinical indicators to save time). After the infarction site was
confirmed, heparin (40 IU/kg) was administered to the patient. For
patients with thrombus formation in the internal carotid artery or
vertebral-basilar artery, the thrombosis treatments were as
follows: i) for 5 patients with complete vascular occlusion, a
0.035-inch guidewire and angiography catheter were used for
thrombus disruption. After the guidewire had passed the thrombus
site, the thrombolysis was conducted and the thrombus was removed
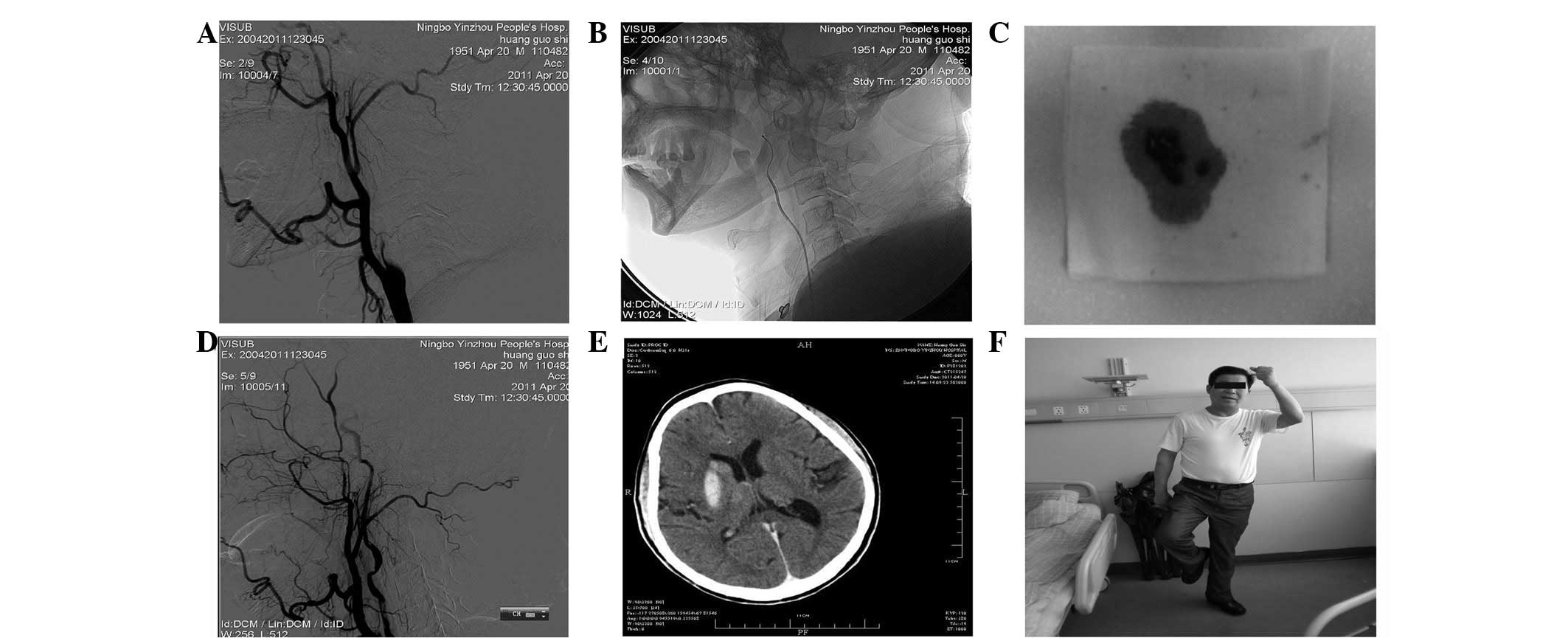
using a 5-ml injector (Figs. 1 and
2). ii) For patients with severe
internal carotid artery stenosis (hemodynamic infarction), a dose
of 400,000 units of urokinase was administered for 1 h of
thrombolysis. If the stenosis site did not change, the first
stent-assisted revascularization was conducted (1 case). If the
stenosis was eased, the thrombolysis was continued until the total
amount of urokinase reached 1,000,000 units. Then 2–3 weeks of
routine anticoagulation therapy was performed, followed by
re-examination. The second stent-assisted revascularization was
conducted on 1 case.
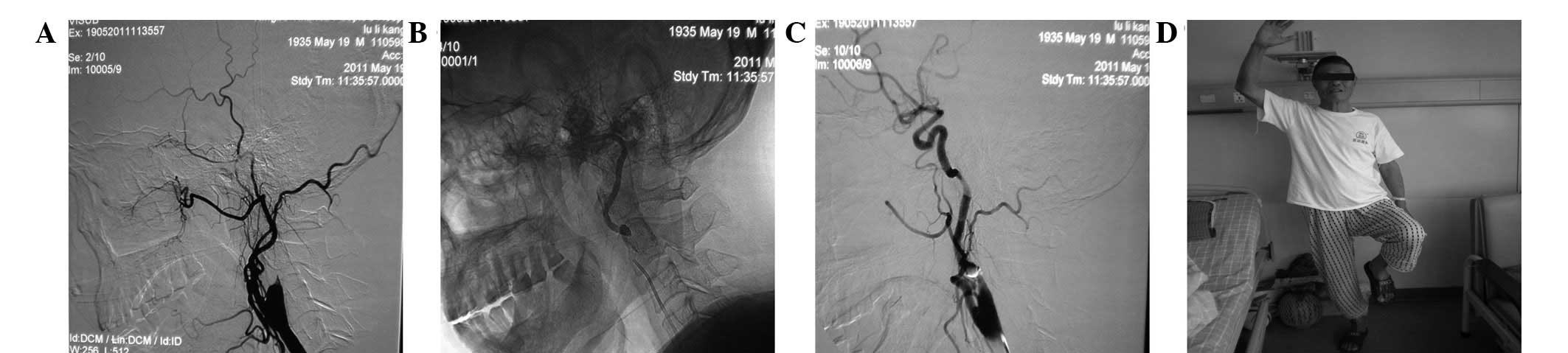
For patients with a thrombus in the middle cerebral
artery, the thrombosis treatments were as follows: i) for complete
vascular occlusion cases, retrieval with a guidewire (J-type
rotation) or repetitive interpenetration with a guidewire or
catheter were conducted, followed by thrombolysis. ii) 400,000
units of urokinase were intra-arterially perfused within 0.5 h,
followed by angiography. Urokinase (150,000 units) was then
arterially perfused and angiographic re-examination was conducted
every 10 min until the total urokinase amount reached 1,000,000 U.
Following thrombolysis, recanalization was performed on the
remaining thrombus and the stent-assisted revascularization was
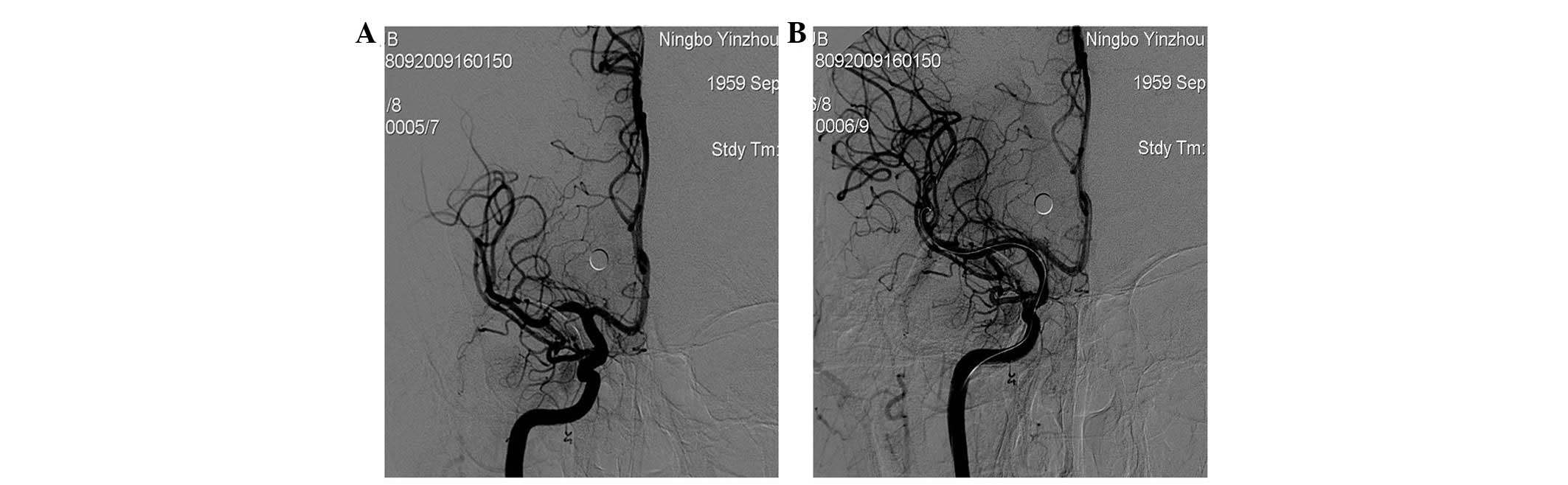
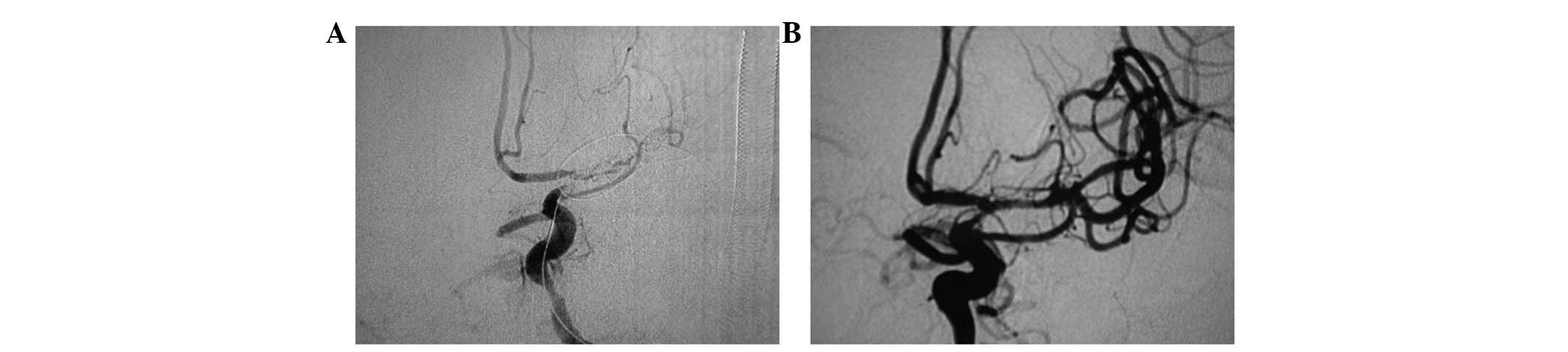
conducted for vascular stenosis ≥50% (the first stent, 1 case,
Fig. 3; the second stent, 2 cases,
Fig. 4).
During treatment, the maximum urokinase dosage was
1,150,000 U. Following thrombolysis, the arterial sheath was
retained for 1 h. The intraoperative and postoperative cardiogram,
blood pressure and blood oxygen saturation were continuously
monitored. Cranial CT scans were performed immediately after
surgery and 24 h later to determine the extent of intracranial
hemorrhaging. At 24 h after thrombolysis, Bayaspirin (300 mg/day)
was orally administered. For patients with stent-assisted
revascularization, Bayaspirin (300 mg/day) and Clopidogrel (75
mg/day) were used together for 3 months, followed by only Baspirin
(100 mg/day) for a prolonged period of time.
Evaluation of treatment efficacy
The treatment efficacy was evaluated according to
the indices as follows: i) Thrombolysis in Myocardial Infarction
(TIMI) grades of recanalization in imaging manifestation: grade 0,
no blood perfusion of the distal occluded artery; grade 1, partial
passage of contrast agent, partial filling of the distal stenotic
artery; grade 2, complete filling of the distal stenotic artery,
slow development and elimination of contrast agent; and grade 3,
rapid filling and elimination in the distal stenotic artery, same
with normal artery. In this study, TIMI grades 2 and 3 were defined
as recanalization. ii) Evaluation standard from National Institutes
of Health stroke scale (NIHSS; perioperative), iii) bleeding
complications and iv) modified Rankin scale (mRS) score
(postoperative 3 months; Table
I).
 | Table IClinical data of the subjects. |
Table I
Clinical data of the subjects.
| Group | Gender | Age (years) | Onset time (min) | Infarction site
score | Preoperative NIHSS
score | Postoperative NIHSS
score | NIHSS improvement ≥4
in 24 h | NIHSS score
(postoperative 3 months) | Mechanical
assistance | Thrombolysis time
(min) | Urokinase dosage
(IU) | Bleeding
complication | Postoperative TIMI
grade 2-3 | mRS score
(postoperative 3 months) |
|---|
| 1 | M | 78 | 290 | L-ICA | 6 | 3 | No | 0 | Yes | 50 | 115 | No | Yes | Yes |
| 1 | M | 52 | 260 | Basilar A | 25 | 8 | Yes | 0 | No | 60 | 40 | No | Yes | Yes |
| 1 | M | 59 | 195 | R-ICA | 15 | 8 | Yes | 2 | Yes (stent) | 70 | 100 | Yes | Yes | Yes |
| 1 | F | 66 | 200 | R-M1 | 21 | 6 | Yes | 2 | No | 55 | 40 | No | Yes | Yes |
| 1 | M | 61 | 210 | R-M2 | 9 | 4 | Yes | 1 | No | 50 | 40 | No | Yes | Yes |
| 1 | F | 78 | 390 | L-M2 | 12 | 9 | No | 4 | No | 60 | 75 | Yes | Yes | Yes |
| 1 | M | 54 | 280 | R-M1 | 12 | 7 | Yes | 2 | No | 90 | 80 | Yes | Yes | Yes |
| 1 | M | 34 | 90 | R-ICA | 7 | 2 | Yes | 1 | No | 75 | 50 | No | Yes | Yes |
| 1 | M | 51 | 240 | R-M1 | 8 | 2 | Yes | 0 | Yes | 80 | 60 | No | Yes | Yes |
| 1 | F | 41 | 260 | R-M1 | 3 | 1 | No | 0 | Yes (stent) | 70 | 60 | No | Yes | Yes |
| 1 | M | 59 | 195 | R-ICA | 8 | 2 | Yes | 0 | Yes (stent) | 90 | 100 | Yes | Yes | Yes |
| 1 | M | 57 | 200 | L-M1 | 11 | 8 | No | 2 | No | 60 | 60 | No | Yes | Yes |
| 1 | M | 41 | 210 | D-A2 | 8 | 6 | No | 2 | No | 55 | 75 | No | Yes | Yes |
| 1 | F | 63 | 240 | L-M2 | 9 | 6 | No | 1 | No | 60 | 80 | No | Yes | Yes |
| 1 | M | 36 | 280 | L-M1 | 12 | 9 | No | 3 | No | 85 | 80 | Yes | Yes | Yes |
| 1 | M | 61 | 90 | R-A2 | 3 | 1 | No | 0 | No | 55 | 50 | No | Yes | Yes |
| 1 | M | 51 | 240 | R-M1 | 8 | 2 | Yes | 0 | Yes (stent) | 50 | 60 | No | Yes | Yes |
| 1 | M | 33 | 360 | Right vertebral
A | 9 | 5 | Yes | 3 | Yes | 60 | 80 | No | Yes | Yes |
| 1 | M | 59 | 195 | Left vertebral
A | 15 | 8 | Yes | 2 | Yes | 60 | 100 | Yes | Yes | Yes |
| 1 | M | 57 | 200 | Right vertebral
A | 21 | 6 | Yes | 2 | No | 80 | 55 | No | Yes | Yes |
| 1 | F | 62 | 210 | R-M2 | 9 | 4 | Yes | 1 | No | 50 | 40 | Yes | Yes | Yes |
| 1 | F | 64 | 390 | Basilar A | 12 | 9 | No | 4 | No | 60 | 75 | Yes | Yes | Yes |
| 1 | F | 54 | 280 | R-M1 | 12 | 7 | Yes | 2 | No | 75 | 80 | Yes | Yes | Yes |
| 2 | M | 57 | 420 | L-ICA | 7 | 5 | No | 3 | Yes | 120 | 45 | Yes | No | Yes |
| 2 | M | 63 | 450 | L-ICA | 21 | 23 | No | Died | Yes | 60 | 80 | Died | No | No |
| 2 | M | 62 | 400 | Right vertebral
A | 10 | 9 | No | 5 | Yes | 60 | 80 | No | No | Yes |
| 2 | M | 78 | 390 | R-M1 | 30 | 20 | Yes | Died | Yes | 60 | 80 | Died | No | No |
| 2 | F | 73 | 380 | L-M1 | 15 | 18 | No | 8 | Yes | 50 | 80 | Yes | No | No |
Statistical analysis
Data were expressed as mean ± SD. Statistical
analysis was performed using SPSS 17.0 statistical software (SPSS,
Chicago, IL, USA). A Student’s t-test was used for measurement
data. For small-sized samples, the F-statistic was calculated
according to the Levene test (P=0.1). Then t-test and Cochran and
Cox t-tests were performed for homogeneity (P>0.1) and
heterogeneity of variance (P<0.1), respectively. A Chi-square
test was conducted for counted data. P<0.05 was considered to
indicate a statistically significant result.
Results
The mechanically assisted thrombolysis was
successfully conducted on 23 patients, with a recanalization rate
of 82.1% (23/28) and an average recanalization time of 65.22 min.
The patient age, disease onset time, urokinase dosage and
preoperative NIHSS score in the recanalization group (23 cases) and
non-recanalization group (5 cases) are shown in Table II. The results show that there were
significant differences in disease onset time and age between the
two groups, with no statistical difference between the urokinase
dosage and preoperative NIHSS scores.
The indices in patients with bleeding complications
(14 cases) and without bleeding complications (9 cases) are shown
in Table III. There were
significant differences in disease onset time and urokinase dosage
between patients with and without bleeding complications. This
indicated that the probability of bleeding increased as the disease
onset time and urokinase dosage increased. The differences between
age, preoperative NIHSS score and recanalization time between the
two groups were not statistically significant.
 | Table IIIIndices in patients with and without
bleeding complications. |
Table III
Indices in patients with and without
bleeding complications.
| Indices | Bleeding
(n=14) | No bleeding
(n=9) | P-value |
|---|
| Age (years) | 53.29±12.74 | 58.33±11.19 | 0.340 |
| Disease onset time
(min) | 220.71±70.22 | 292.27±88.72 | 0.034 |
| Urokinase dosage
(U) | 61.79±20.43 | 81.11±18.84 | 0.033 |
| Preoperative NIHSS
score | 10.57±6.80 | 11.89±2.31 | 0.584 |
| Recanalization time
(min) | 61.43±10.64 | 71.11±14.74 | 0.081 |
In addition, the effect of mechanically assisted
thrombolysis on bleeding complications was investigated. The
results showed that there were 3 cases with bleeding complications
and 5 cases without bleeding complications for mechanically
assisted thrombolysis, and 6 cases with bleeding complications and
9 cases without bleeding complications for thrombolysis. This
indicated that mechanically assisted thrombolysis had no clear
effect on bleeding complications.
Discussion
In this study, the average recanalization time was
65.22 min, and the total recanalization rate was 82.1%, which is
relatively higher than that reported in previous studies (11,12,14,17–19).
In addition, the mechanically assisted thrombolysis does not
increase bleeding complications. This indicates that the
recanalization rate and recanalization time of mechanically
assisted thrombolysis are more favorable than those of the
traditional intravenous and intra-arterial thrombolysis methods.
Due to different criteria for patient selection, these data cannot
be directly compared with other reported results.
Barreto et al have studied the association
between thrombus burden (none, mild, moderate and severe, according
to thrombus length) and clinical prognosis in 135 patients with
acute cerebral infarction. The results showed that for patients
with a higher grade of thrombus burden and preoperative NIHSS
score, the probability of using mechanically assisted thrombolysis
was higher. Following treatment, the recanalization rate was not
significantly different from that in patients with a low thrombus
burden (20). In this study, the
influencing factors on the recanalization rate in mechanically
assisted thrombolysis were associated not with the NIHSS
preoperative score, but with patient age and disease onset time.
The disease onset time is associated with thrombus condition and
size, with a clear effect on recanalization rate. The 3 cases with
atrial fibrillation were elderly patients. The cerebral infarction
may be caused by embolism, but not thrombosis formation, with a
poor response to urokinase. Therefore, age may be indirectly
associated with the recanalization rate, though with a statistical
significance.
In this study, due to mechanical assistance factors,
once the vessel was unblocked, the intra-arterial urokinase
perfusion could be stopped to minimize bleeding complications.
Therefore the urokinase dosage is not associated with the
recanalization rate, indicating the importance of mechanically
assisted thrombolysis. Therefore, the therapy that acts the most
rapidly is significant in obtaining a favorable recanalization
rate. Strengthening of public health education on stroke for timely
medical treatment and perfection of pre-hospital and in-hospital
fast rescue system are the key for successful thrombolytic
therapy.
Numerous factors are associated with bleeding
complications, including patient age, disease onset time,
preoperative NIHSS score, thrombolytic drug dosage, recanalization
time, platelet index, blood glucose level and collateral
circulation to the infarcted cortex (4,21–26).
In this study, only the effects of patient age, disease onset time,
preoperative NIHSS score, urokinase dosage and recanalization time
on bleeding complication were investigated, due to the small sample
size. The results show that there was a significant difference in
disease onset time and urokinase dosage between patients with and
without bleeding complications. The probability of bleeding
increases as the disease onset time and urokinase dosage increase
(27). In contrast to previous
findings, there is no statistical difference in disease onset age,
preoperative NIHSS score and recanalization time between the two
groups. This is similar to the findings of by Barreto et
al(20). In addition, there
was no clear effect of mechanically assisted thrombolysis on
bleeding complications. The reasons may be that the endovascular
surgery increases the vascular endothelial injury, but this
side-effect is offset by the decrease of urokinase dosage and
thrombolytic time. Therefore the mechanically assisted
intra-arterial thrombolysis is safe. Notably, although the bleeding
rate in this study is high, the majority of bleedings are ‘imaging
bleeding’, but not symptomatic bleeding. This is similar to a
previous finding which showed that bleeding patients with
recanalization may obtain a favorable prognosis (28). The bleedings may be a leakage of a
small volume caused by disruption of the blood-brain barrier.
Stent-assisted revascularization has become an
important method in the treatment of acute cerebral infarction
(29–34). In the current study, a stent was
used in 4 cases for mechanical recanalization, easing vascular
stenosis and preventing vascular re-occlusion. In particular, a
Solitaire FR stent (EV3, Irvine, CA, USA) was used in one case with
a favorable result. However, this still requires further
investigation due to the small sample size. In addition, the use of
first stent-assisted revascularization is controversial, due to a
high postoperative bleeding rate. With the popularization of
digital subtraction angiography (DSA) with CT scanning, the
intracranial bleeding following thrombolysis may be observed
without moving the patient. For patients with combined severe
vascular stenosis (without intracranial bleeding), the application
of first stent-assisted revascularization may be a good choice for
avoiding postoperative vascular re-occlusion.
There are certain deficiencies in this study.
Firstly, due to limitations of the mechanical thrombus disruption
and retrieval device, saline injection and repetitive
interpenetration with a microwire and microcatheter were mainly
used in this study, with the occasional use of a stent. With the
progress of interventional devices (The Penumbra system; Penumbra,
Alameda, CA, USA), the recanalization rate in simple mechanical
thrombus disruption and retrieval will be >50% in the near
future and thrombolysis is likely to be a complementary therapy for
thrombus fragments in distal infarction sites. Secondly, the
selected patients in this study were treated within 6 h after
disease onset, with abnormal patients excluded by cranial CT
scanning. This ensures a short time from the emergency visit to
obtaining treatment (<45 min), and thus results in a favorable
therapeutic outcome. However, patients with early cerebral edema
were excluded from receiving thrombolytic therapy. In addition, the
definition of disease onset time is sometimes unclear. The cerebral
infarction or progressive aggravation is observed after a patient
wakes up. Therefore, applications of clinical-diffusion mismatch,
(NIHSS score ≥8, magnetic resonance diffusion abnormalities ≤25 ml)
(35), perfusion-diffusion
weighted imaging mismatch (36) or
CT perfusion scanning (37) in
screening and treating patients are more beneficial and require
further investigation.
The safety of mechanically assisted thrombolysis for
the treatment of acute cerebral infarction is equivalent to that of
simple intra-arterial thrombolysis, but the former has a higher
efficiency. This method may reduce the urokinase dosage and the
recanalization time, and also increase the recanalization rate.
References
|
1.
|
Yong H, Foody J, Linong J, et al: A
systematic literature review of risk factors for stroke in China.
Cardiol Rev. Sep 17–2012.(Epub ahead of print).
|
|
2.
|
Takagi T, Kato T, Sakai H and Nashimura Y:
Early neurologic improvement based on the National Institutes of
Health Stroke Scale score predicts favorable outcome within 30
minutes after undergoing intravenous recombinant tissue plasminogen
activator therapy. J Stroke Cerebrovasc Dis. 12:322–329. 2012.
|
|
3.
|
Arnold M, Schroth G, Nedeltchev K, et al:
Intra-arterial thrombolysis in 100 patients with acute stroke due
to middle cerebral artery occlusion. Stroke. 33:1828–1833. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pera J, Undas A, Topor-Madry R, et al:
Fibrin clot properties in acute stroke: what differs cerebral
hemorrhage from cerebral ischemia? Stroke. 43:1412–1414. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kuoppala M, Åkeson J, Svensson P, et al:
Risk factors for haemorrhage during local intra-arterial
thrombolysis for lower limb ischaemia. J Thromb Thrombolysis.
31:226–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Alexandrov AV, Demchuk AM, Felberg RA, et
al: High rate of recanalization and dramatic clinical recovery
during tPA infusion when continuously monitored with 2-MHz
transcranial Doppler monitoring. Stroke. 31:610–614. 2000.
View Article : Google Scholar
|
|
7.
|
Röther J, Schellinger PD, Gass A, et al:
Effect of intravenous thrombolysis on MRI parameters and functional
outcome in acute stroke <6 hours. Stroke. 33:2438–2445.
2002.
|
|
8.
|
Restrepo L, Bang OY, Ovbiagele B, et al:
Impact of hyperlipidemia and statins on ischemic stroke outcomes
after intra-arterial fibrinolysis and percutaneous mechanical
embolectomy. Cerebrovasc Dis. 28:384–390. 2009. View Article : Google Scholar
|
|
9.
|
Farkas J, Hinrichs C, Cariaga T, et al:
Intra-arterial thrombolysis using r-TPA: Initial angiographic
outcomes. ASNR Proceedings. 93:80–81. 2002.
|
|
10.
|
Xiao L, Tong JJ and Shen J: Endoluminal
treatment for venous vascular complications of malignant tumors.
Exp Ther Med. 4:323–328. 2012.PubMed/NCBI
|
|
11.
|
Linfante I and Akkawi NM: Advances in
treatment of acute ischemic stroke. Curr Neurol Neurosci Rep.
6:28–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nesbit GM, Luh G, Tien R and Barnwell SL:
New and future endovascular treatment strategies for acute ischemic
stroke. J Vasc Interv Radiol. 15:S103–S110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Xavier AR, Tiwari A, Purai N, et al:
Safety and efficacy of intracranial stenting for acute ischemic
stroke beyond 8 h of symptom onset. J Neurointerv Surg. 4:94–100.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Bergui M, Stura G, Daniele D, et al:
Mechanical thrombolysis in ischemic stroke attributable to basilar
artery occlusion as first-line treatment. Stroke. 37:145–150. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fourth National Cerebrovascular Diseases
Conference: Guidelines for the diagnosis and treatment of cerebral
infarction. Chinese Journal of Neurology. 29:376–381. 1996.
|
|
16.
|
Guideline of Cerebrovascular Disease
Prevention and Treatment in China. Journal of Apoplexy and Nervous
Diseases. 23:4–8. 2006.
|
|
17.
|
Smith WS, Sung G, Starkman S, et al:
Safety and efficacy of mechanical embolectomy in acute ischemic
stroke: results of the MERCI trial. Stroke. 36:1432–1438. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Qureshi AI, Siddiqui AM, Suri M, Fareed K,
et al: Aggressive Mechanical Clot Disruption and Low-dose
Intra-arterial Third-generation Thrombolytic Agent for Ischemic
Stroke: A Prospective Study. Neurosurgery. 51:1319–1329. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Leary MC, Saver JL, Gobin YP, et al:
Beyond tissue plasminogen activator: mechanical intervention in
acute stroke. Ann Emerg Med. 41:838–846. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Barreto AD, Albright KC, Hallevi H, et al:
Thrombus burden is associated with clinical outcome after
intra-arterial therapy for acute ischemic stroke. Stroke.
39:3231–3235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kidwell CS, Saver JL, Carnado J, et al:
Predictors of hemorrhagic transformation in patients receiving
intra-arterial thrombolysis. Stroke. 33:717–724. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kase CS, Furlan AJ, Wechsler LR, et al:
Cerebral hemorrhage after intra-arterial thrombolysis for ischemic
stroke: the PROACT II trial. Neurology. 57:1603–1610. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kikuchi K, Kawahara KI, Miura N, et al:
Secondary prevention of stroke: Pleiotropic effects of optimal oral
pharmacotherapy. Exp Ther Med. 4:3–7. 2012.PubMed/NCBI
|
|
24.
|
Kim SK, Yim SV and Lee BC: Association
between cytochrome P450 promoter polymorphisms and ischemic stroke.
Exp Ther Med. 3:261–268. 2012.PubMed/NCBI
|
|
25.
|
Christoforidis GA, Mohammad Y, Kehagias D,
et al: Angiographic assessment of pial collaterals as a prognostic
indicator following intra-arterial thrombolysis for acute ischemic
stroke. AJNR Am J Neuroradiol. 26:1789–1797. 2005.PubMed/NCBI
|
|
26.
|
Christoforidis GA, Slivka A, Mohammad Y,
et al: Size matters: hemorrhage volume as an objective measure to
define significant intracranial hemorrhage associated with
thrombolysis. Stroke. 38:1799–1804. 2007.
|
|
27.
|
Christoforidis GA, Slivka AP, Karakasis C,
et al: Hemorrhage rates and outcomes when using up to 100 mg
intra-arterial t-PA for thrombolysis in acute ischemic stroke.
Interv Neuroradiol. 16:297–2305. 2010.PubMed/NCBI
|
|
28.
|
Carpenter CR, Keim SM, Milne WK, et al:
Thrombolytic therapy for acute ischemic stroke beyond three hours.
J Emerg Med. 40:82–92. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Castaño C, Dorado L, Guerrero C, et al:
Mechanical thrombectomy with the Solitaire AB device in large
artery occlusions of the anterior circulation: a pilot study.
Stroke. 41:1836–1840. 2010.
|
|
30.
|
Zaidat OO, Wolfe T, Hussain SI, et al:
Interventional acute ischemic stroke therapy with intracranial
self-expanding stent. Stroke. 39:2392–2395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Machi P, Costalat V, Lobotesis K, et al:
Solitaire FR thrombectomy system: immediate results in 56
consecutive acute ischemic stroke patients. J Neurointerv Surg.
4:62–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Gralla J, Brekenfeld C, Mordasini P, et
al: Mechanical thrombolysis and stenting in acute ischemic stroke.
Stroke. 43:280–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Levy EI, Mehta R, Gupta R, et al:
Self-expanding stents for recanalization of acute cerebrovascular
occlusions. AJNR Am J Neuroradiol. 28:816–822. 2007.PubMed/NCBI
|
|
34.
|
Castaño C, Serena J and Dávalos A: Use of
the new Solitaire (TM) AB device for mechanical thrombectomy when
Merci clot retriever has failed to remove the clot. A case report.
Interv Neuroradiol. 15:209–214. 2009.PubMed/NCBI
|
|
35.
|
Janjua N, El-Gengaihy A, Pile-Spellman J,
et al: Late endovascular revascularization in acute ischemic stroke
based on clinical-diffusion mismatch. AJNR Am J Neuroradiol.
30:1024–1027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Chalela JA, Kidwell CS, Nentwich LM, et
al: Magnetic resonance imaging and computed tomography in emergency
assessment of patients with suspected acute stroke: a prospective
comparison. Lancet. 369:293–298. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Natarajan SK, Snyder KV, Siddiqui AH, et
al: Safety and effectiveness of endovascular therapy after 8 hours
of acute ischemic stroke onset and wake-up strokes. Stroke.
40:3269–3274. 2009. View Article : Google Scholar : PubMed/NCBI
|