Introduction
Acute myocardial infarction (AMI) is an issue
worldwide, which severely jeopardises human health. The majority of
patients who suffer from AMI succumb to its complications, such as
heart failure and arrhythmias. The mechanisms involved in repairing
the infarcted zone remain unknown. Cell therapy of AMI involves
transplanting various types of stem cells that have the potential
to differentiate in the infarcted zone, where they proliferate and
differentiate into cardiomyocytes, thus improving heart function
(1,2).
Embryonic stem cells (ESCs) have the ability to
respond to environmental demands. Previous studies have shown that
retinoic acid induces the differentiation of ESCs into
cardiomyocytes. Inductors also include dimethyl sulphoxide (DMSO),
cAMP, neuregulin and deoxycytidine (3–6).
Rajasingh et al found that leukaemia inhibitory factor (LIF)
and bone morphogenetic protein 2 (BMP-2) were able to
synergistically enhance the cardiomyocyte differentiation of
transplanted stem cells, whereas DMSO or retinoic acid were not
(7). In addition to using
inductors, investigators have precipitated the differentiation of
ESCs into cardiomyocytes naturally. Kehat et al observed
that cardiomyocytes differentiated from ESCs have the
characteristics of myocardial electrophysiology and surface
proteins. The authors transplanted these cardiomyocytes into the
infarcted zone of a pig and found that these cells integrated with
the receptor cardiomyocytes, and the heart began to beat again
(8).
Human embryonic germ cells (hEGCs) are stem cells
cultured from primordial germ cells, which reside in human
embryonic genital ridges in vivo. They are similar to human
embryonic stem cells (hESCs), which form the inner cell mass, in
biological characteristics. Our laboratory has established a system
for obtaining hEGCs through tissue culturing. The study of hESC
transplantation for the treatment of AMI started worldwide >10
years ago; however, the use of hEGCs as a transplantable cell
source for treating AMI has not, to the best of our knowledge, been
reported.
In the present study, ascorbic acid was used to
induce hEGCs to differentiate into cardiomyocytes in vitro
with the aim of identifying a simple and high-performance empirical
method for promoting hEGC differentiation. In addition, hEGCs were
injected into the infarcted zone in rat models of AMI in order to
observe and identify the survival and differentiation of hEGCs,
thereby providing experimental support for myocardiac tissue
engineering research and the treatment of AMI by stem cell
transplantation.
Materials and methods
Main reagents
The reagents used in this study were as follows:
Dulbecco’s modified Eagle’s Medium (DMEM; Gibco-BRL, Carlsbad, CA,
USA; high glucose); fetal calf serum (FCS; Hyclone, Beijing,
China); basic fibroblast growth factor (bFGF), LIF, ascorbic acid,
and Cx43 and cardiac troponin T (cTnT) mouse anti-human monoclonal
antibodies (R&D Systems, Shanghai, China); FITC-labeled rabbit
anti-mouse secondary antibody (Wuhan Boster Biological Technology,
Ltd., Wuhan, China); MAB1281 mouse anti-human nuclei monoclonal
antibody (Chemichon, Temecula, CA, USA); rabbit anti-GATA-4 (Wuhan
Boster Biological Technology, Ltd.); and EnVision™ Detection kit
[GK50075; comprising ChemMate™ DAKO EnVision™/HRP,
Rabbit/Mouse(ENV); ChemMate™ Substrate Buffer and ChemMate™
3,3′-diaminobenzidine (DAB) + Chromogen; Genentech, Inc., San
Francisco, CA, USA]; Hoechst33342Cell Staining solution (Biohao,
Beijing, China).
Source of embryos
Human embryos aborted at 5–10 weeks, were collected
from The First and Second Hospitals Affiliated to Soochow
University (Suzhou, China) and the Maternal and Child Health Center
(Suzhou, China), with the permission from pregnant women and the
Morality Committee. All the embryos were intact and not severely
contaminated.
Cell culture and proliferation of
hEGCs
The gonadal ridges of 5–10-week-old embryos were
cultured as explant tissue in vitro, in high glucose DMEM
supplemented with 15% FCS; LIF and bFGF were also added. The hEGCs
and cell cultures were gained from the surface of the homologous
human embryonic fibroblast, which was used as the feeder. After 8
days of primary culture, the hEGCs were digested and subcultured to
passage 4.
Formation of embryoid bodies (EBs) by
suspension culture
The subconfluent cells were selected for digestion
into a single cell suspension, then plated onto a gelatin
(0.1%)-coated 100-mm Petri dish at 37ºC in humid air with 5%
CO2 for 1 h to remove fibroblasts. Briefly,
1×105 cells in 1 ml cultivation medium containing 20%
FCS were suspended in bacteriological plates for 5 days. The medium
was replaced every other day.
Differentiation of hEGCs into
cardiomyocytes
After suspension for 5 days, EBs were separately
plated onto a gelatin (0.1%)-coated 24-well plate and various
concentrations (0.01, 0.05, 0.1 and 0.2 mg/ml) of ascorbic acid
were added. The medium was replaced with fresh medium every other
day.
Immunocytochemistry
Cells were collected at 1, 2, 3 and 4 weeks of
induction and fixed in 4% paraformaldehyde, quenched with 3%
hydrogen peroxide and non-specific background was blocked with a
serum-free blocking solution. Primary antibodies were diluted in
dilution buffer and incubated with the cells for 30 min at room
temperature. Cells were washed in phosphate-buffered saline and a
polymer-linked peroxidase-labeled secondary antibody was added for
30 min at room temperature. Cells were then stained with DAB and
haematoxylin, fixed with neutral gum and photographed.
Cardiomyocytes were identified immunocytochemically using
anti-GATA-4 and anti-cTnT antibodies. Controls underwent staining
without the primary antibody.
Statistical analysis
Conversion rate of cardiac-like cells: three visual
fields of the cell creep slices induced by the inductors were
recorded. The total cell count and the count of cells positive for
GATA-4 were recorded under a light microscope with a magnification
of ×200 (Olympus, Shanghai, China). The conversion rate of hEGC
differentiation into cardiac-like cells in the three fields was
calculated. Results are expressed as the means ± standard deviation
and statistically evaluated by Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Immunofluorescence
Cells were collected following induction for 2
weeks, and the expression levels of myocardial connectin Cx43
protein and cardiac troponin (cTnT) were detected by
immunofluorescence. Cells were blocked by treatment with cold
methanol in PBS for 30 min. After rinsing three times in PBS for 5
min each, rat anti-human (1:50) antibody diluted solution was added
overnight at 4ºC. Cells were rinsed three times in PBS for 5 min
each. Subsequently, the cells were incubated with FITC-labeled
rabbit anti-mouse secondary antibody at a dilution of 1:50 in PBS
for 1 h at room temperature. Cells were rinsed three times in PBS
for 5 min each. Cells were then covered with 50% buffered glycerol
and observed under a fluorescence microscope (Olympus).
Experimental animals and grouping
Forty Sprague-Dawley rats, of clean grade and either
gender (weight, 200–250 g) were provided by the Experimental Animal
Center of Soochow University (Suzhou, China). These rats were
randomly divided into two groups: The experimental group (n=28) and
the control group (n=12). In the experimental group, hEGCs were
injected into the edge of the infarcted zone of the rat models with
AMI. Four points were selected and 12 μl cell suspension was
injected into each point. In the control group, the same process
was followed but only PBS was injected into the selected points to
serve as the negative control.
Preparation of the cells for
transplantation
One hour prior to transplantation, the hEGC colony
subcultured to the fourth passage was extracted with aseptic
inoculating needles, and digested with 0.25% trypsinase and 0.02%
EDTA. When cytoplasm recovery occurred and the cell colonies were
scattered, blood serum medium was added to stop the trypsinization,
and then a pipette was used to blow air across the cells and to
strike the bottom of the bottle gently and repeatedly until the
cells formed a steady cell suspension. The cells were centrifuged
for 5 min at 1,000 × g, washed with sterile PBS and then blown on
and struck until the cells were well distributed for cell counting.
The cells were taken up with a microinjector at a concentration of
1×106 ml (50 μl).
Establishment of the rat model of
myocardial infarction
The surgery group: The AMI model was established by
ligation of the left anterior descending branch of the coronary
artery. Rats were anesthetized with an intraperitoneal injection of
4% chloral hydrate and, following disinfection of the surgical
area, tracheal intubation was performed and mechanical ventilation
was initiated. With monitoring by electrocardiography (CONTEC), the
thoracic cavity was opened from the left sternal border in the
third or fourth intercostal muscles and the cardiac pericardium was
opened until the heart was totally exposed. The left anterior
descending branch of the coronary artery was ligated with 5.0
surgical silk sutures. Needle-shaped electrodes were attached under
the four limbs. Lead II electrocardiograms (ECGs) were recorded.
The myocardial infarction model was established until persistent ST
elevation on the ECG indicative of myocardial infarction was
observed. After the surgery, 400,000 units penicillin was injected
into the rats in case of infection. The rats recovered at 30ºC.
Strict aseptic conditions were maintained during the whole process;
therefore, dermal sutures were unnecessary.
The sham surgery group: Between the lower margin of
the left atrial appendage and pulmonary conus, namely the left
anterior descending branch of the coronary artery, a non-invasive
suture needle was used to perforate the lower part of the blood
vessel without ligature. Other procedures were similar to those in
the surgery group.
Transplantation of hEGCs following
myocardial infarction
The prepared hEGC suspension was directly injected
into the infarcted zone in the heart of each rat. Under direct
vision, after the ligature was removed from the anterior descending
coronary artery, hEGCs were injected into the edge of the infarcted
zone of the heart. Four points were selected and 12 μl cell
suspension with a concentration of 1×106 ml was injected
into each. In the control group, the same process was followed, but
only PBS was injected into the selected points to serve as the
negative control. The pectus was closed and mechanical ventilation
was maintained until spontaneous respiration occurred.
Immunohistochemistry
One day and 1, 2 and 4 weeks following the
transplantation, seven rats in the experimental group and three in
the control group were selected. The rats were anaesthetised with
an intraperitoneal injection of 4% hydral and their hearts were
removed and washed in PBS. The left and right atria of the heart
together with the right ventricle were eliminated, the left
ventricle was fixed with 4% paraformaldehyde and embedded in
paraffin for serial sectioning. Primary antibodies against MAB1281
(1:100) and GATA-4 (1:100), as well as universal secondary
antibodies were adopted in a 2-step immunohistochemical method
(5). Cells were observed under a
microscope (Olympus). All processes were conducted strictly
according to the manufacturer’s instructions.
Results
Characteristics of cells following
induction
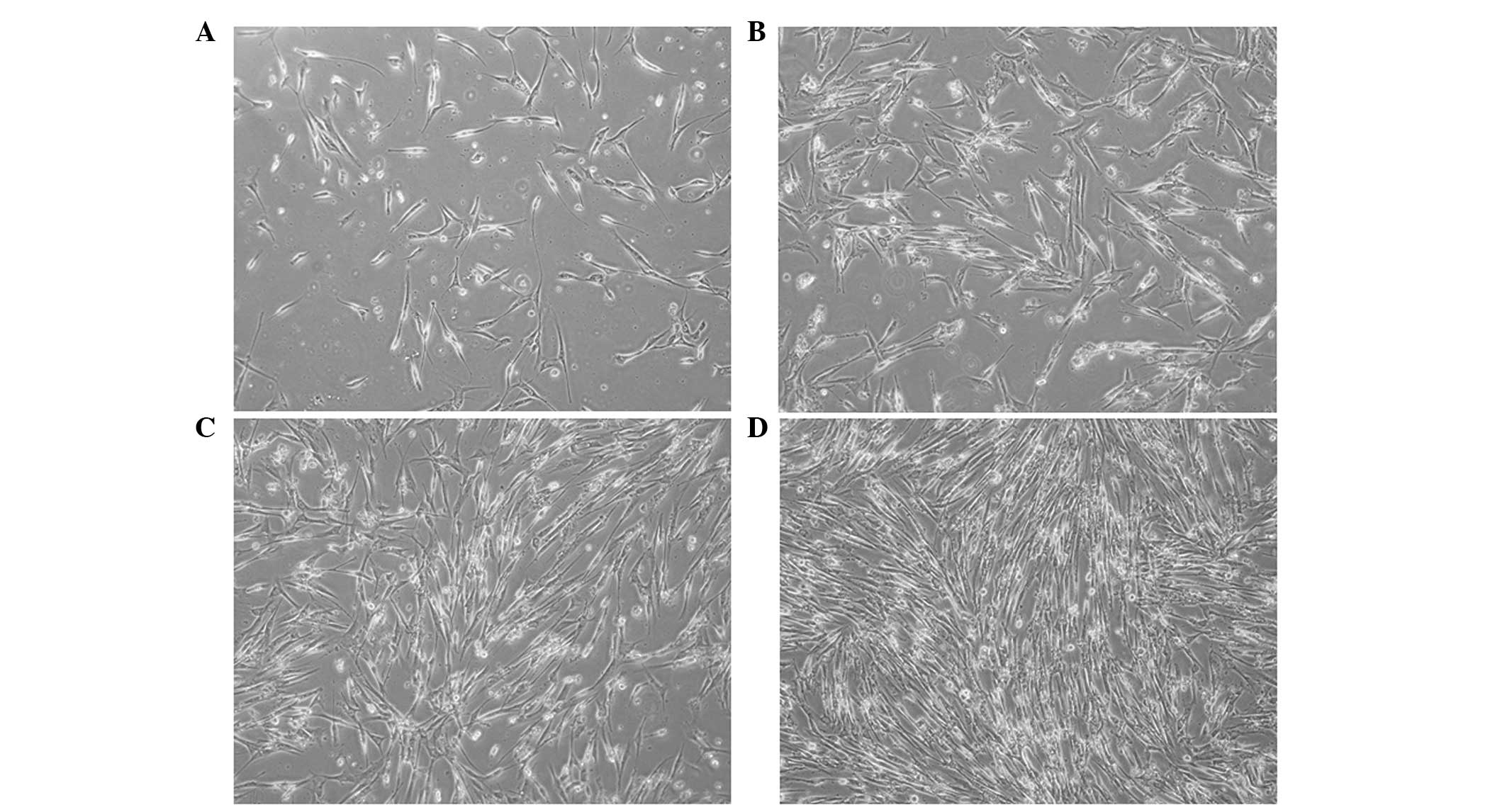
Spindle-shaped and irregular cells increased in
number at the periphery of EBs after 1 week (Fig. 1A). Prominences in flanking cells
were connected after 2 weeks (Fig.
1B). The connections between adjacent cells became apparent and
formed an intercalated-like structure in 3 weeks (Fig. 1C). The cells tended to be uniform
after 4 weeks of treatment with 0.05 or 0.1 mg/ml ascorbic acid
(Fig. 1D). The cell morphology did
not change significantly following treatment with 0.01 mg/ml
ascorbic acid; however, the majority of cells died under treatment
with 0.2 mg/ml ascorbic acid.
Optimal induction concentration
The effective concentration of ascorbic acid was
0.01–0.1 mg/ml and the inductive effect was dose-dependent.
Comparisons in the conversion rates of the experimental and control
groups revealed significant differences (P<0.01). Significant
differences were also observed between the conversion rates at all
time points (P<0.05) with the highest conversion rate at 3
weeks. Comparisons of the results for 0.1 mg/ml ascorbic acid with
those of 0.05 and 0.01 mg/ml revealed significant differences
(P<0.05). The results showed that the optimum concentration of
ascorbic acid was 0.1 mg/ml (Table
I).
 | Table IConversion rate of cardiac-like cells
of hEGCs treated with ascorbic acid (%). |
Table I
Conversion rate of cardiac-like cells
of hEGCs treated with ascorbic acid (%).
| Groups | Concentration of
ascorbic acid (mg/ml) | 1 week | 2 weeks | 3 weeks | 4 weeks |
|---|
| Control | 0.00 | 0 | 0 | 0 | 0 |
| Experimental | 0.01 | 26.7±4.17a | 37.5±0.65a | 54.5±1.73a | 38.0±3.27a |
| 0.05 | 41.3±2.34a | 67.0±3.11a | 80.6±3.35a | 62.2±6.01a |
| 0.10 | 46.0±2.01a | 71.3±1.36a | 85.2±1.79a | 77.4±1.49a |
| 0.20 | 0 | 0 | 0 | 0 |
Expression of GATA-4 and cTnT detected by
immunocytochemistry
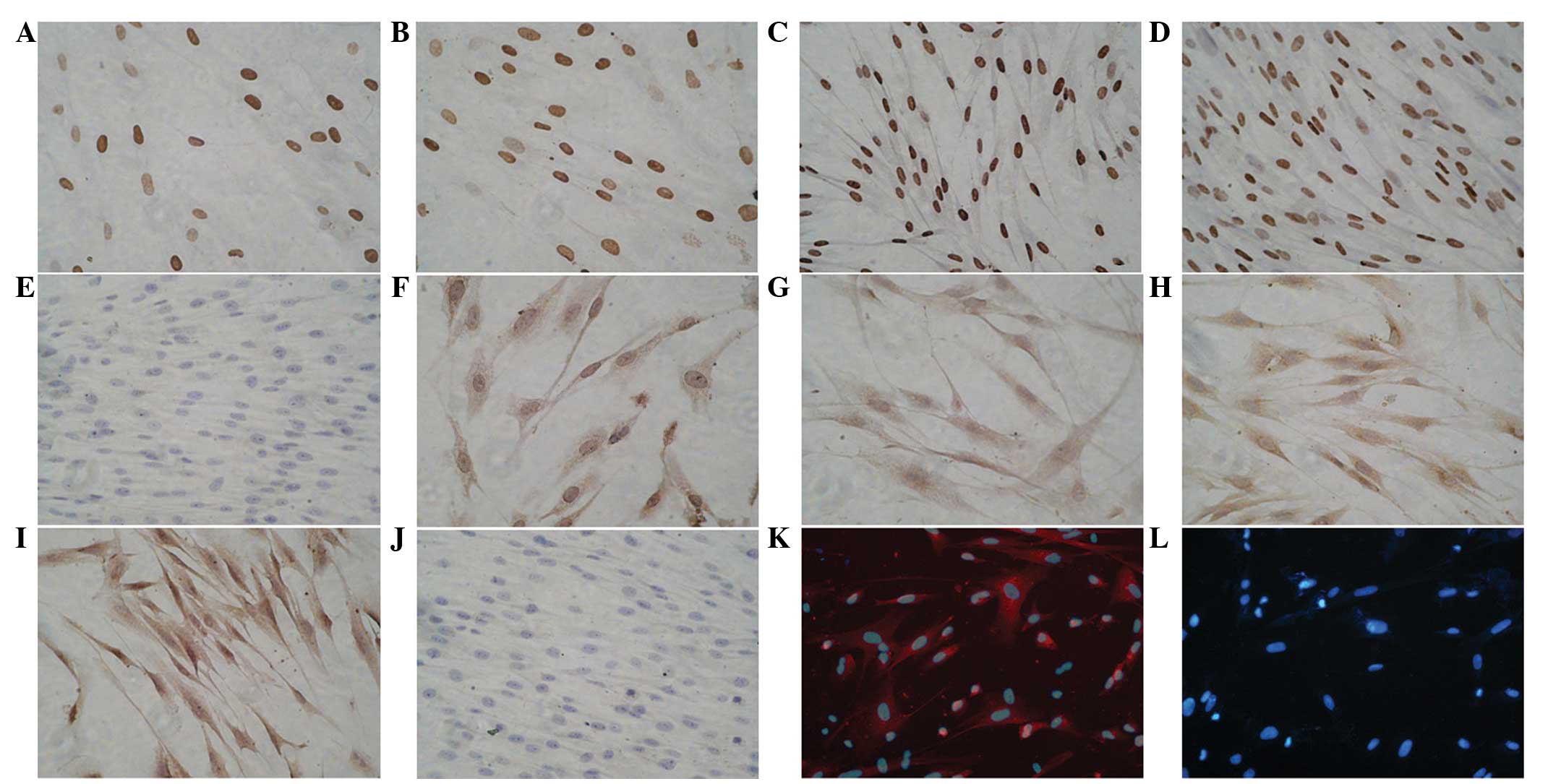
Following treatment with 0.1 mg/ml ascorbic acid,
the primordial myocardium-specific transcription factor, GATA-4,
was stained positively in the differentiated cells after 1 week of
induction; however, the positive cell number was low and brownish
yellow granules were observed (Fig.
2A). An increased number of positive cells were stained 2 weeks
following induction; the nucleus appeared oval-shape and stained
brown in color (Fig. 2B). The
expression of GATA-4 peaked after 3 weeks; the nucleus was long
fusiform in shape and presented dark brown (Fig. 2C). The positive cell number
decreased gradually and tended to be uniform after 4 weeks of
induction (Fig. 2D). No expression
of GATA-4 was observed in the normal tissue group (Fig. 2E).
Following treatment with 0.1 mg/ml ascorbic acid,
cTnT expression was not observed until 2 weeks after induction and
the expression was weak and the endochylema was stained brownish
yellow (Fig. 2F and G). cTnT
expression increased gradually over 3 and 4 weeks. cTnT was stained
brown around the nucleus after 3 weeks of induction (Fig. 2H and I). No expression of cTnT was
detected in the normal tissue group (Fig. 2J).
Immunofluorescence of differentiated
cardiomyocytes
Following treatment with 0.1 mg/ml ascorbic acid,
the immunofluorescence assay showed that after induction for 2
weeks, numerous Cx43-stained cells emitted strong red fluorescence.
The cells were polygonal and spindle-shaped (Fig. 2K). In the control group, the nuclei
were stained blue with Hoechst 33342 (Fig. 2L).
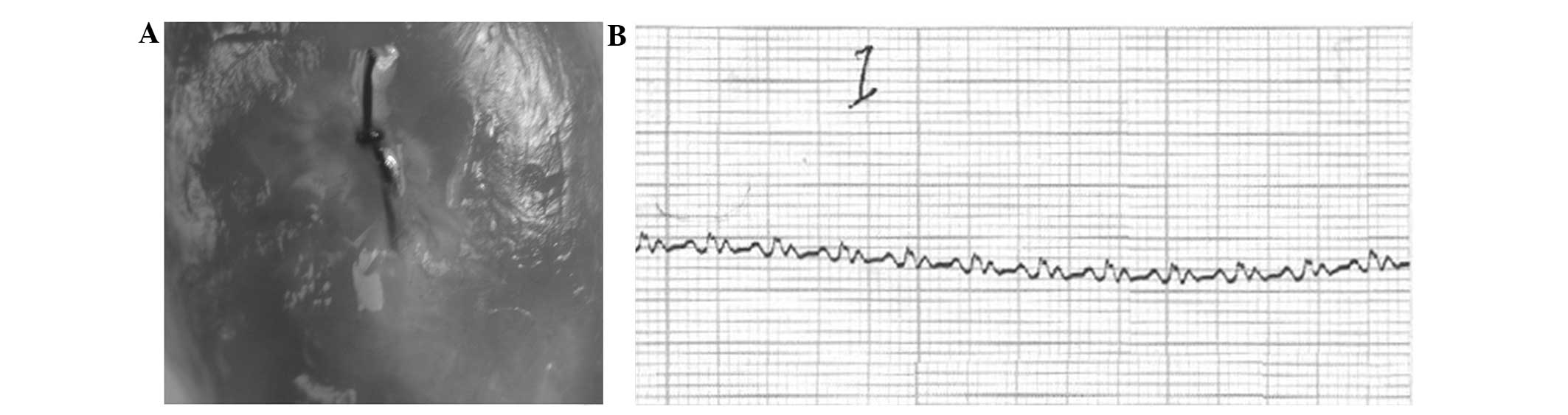
Rat model of AMI
After ligation of the left anterior descending
artery in the surgery group, the distal end cardiac muscles of
ligation were significantly pallid and their activity was weakened
or disappeared at a certain range (Fig. 3A). The ECG showed limb lead
ST-segment elevation to various degrees in the surgery group after
1–20 min of ligation and the pathological Q wave appeared (Fig. 3B). The sham surgery group were
normal.
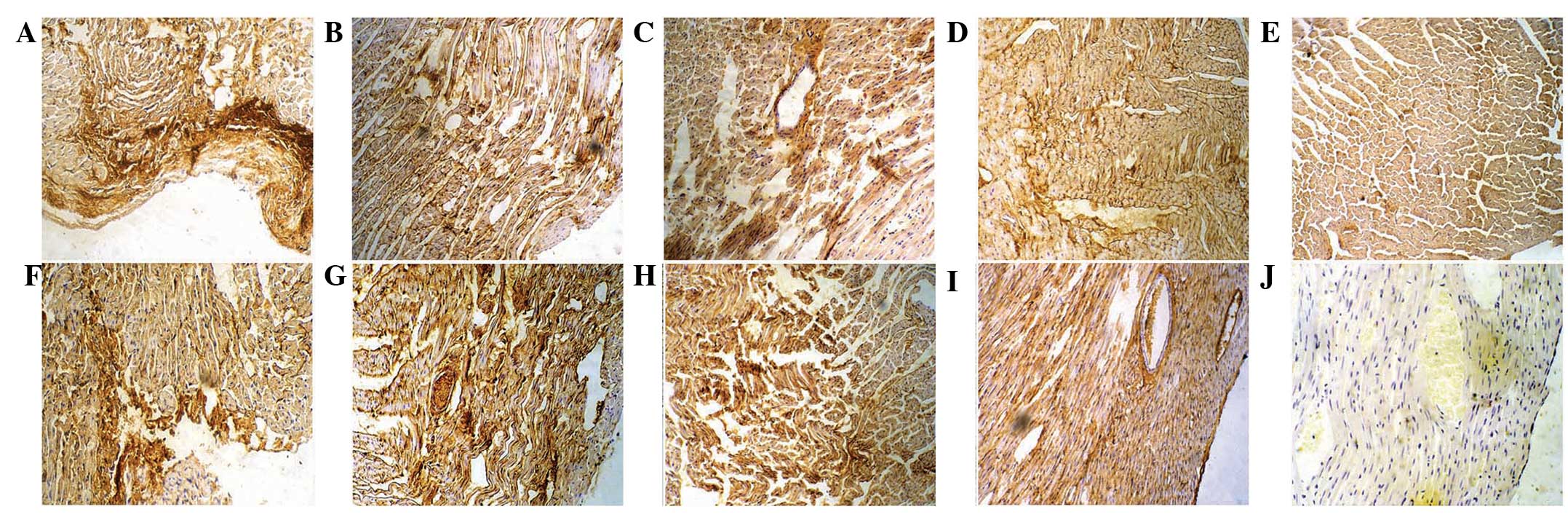
Results of MAB1281 and GATA-4
immunohistochemistry
One day after transplantation, MAB1281-positive
cells were distributed along the epicardium; the cells appeared
round with a deep brown nucleus and were arranged in a disorderly
manner (Fig. 4A). One week
following transplantation, cardiac muscles in the infarcted zone
showed necrosis and were disordered. There were MAB1281-positive
cells around the myocardium, and cells were spindle-shaped and thin
(Fig. 4B). Two weeks after
transplantation, MAB1281-positive cells were arranged in the
infarcted zone of the myocardium and were parallel to the cardiac
muscles. They were long and spindle-shaped with a brown ovoid
nucleus (Fig. 4C). Four weeks
after transplantation, the majority of the cells in the infarcted
zone were MAB1281 positive with a short column and brown nucleus,
and the cardiomyocytes were aligned (Fig. 4D). The control group was negative
for MAB1281 (Fig. 4E).
One day following transplantation, GATA-4-positive
cells were dispersedly distributed under the epicardium and
appeared round with a deep brown nucleus and a small amount of
cytoplasm (Fig. 4F). One week
after transplantation, GATA-4-positive cells were observed in the
infarcted zone of the myocardium and along blood vessels; the cells
were thin and spindle-shaped with a brown nucleus (Fig. 4G). Two weeks after transplantation,
GATA-4-positive cells were arranged in the infarcted zone of the
myocardium and were parallel with the cardiac muscles; the cells
appeared long and spindle-shaped with a brown ovoid nucleus
(Fig. 4H). Four weeks after
transplantation, most cells of the infarcted zone were positive for
GATA-4, had a short column and brown nucleus, and the
cardiomyocytes were aligned in order (Fig. 4I). The control group was negative
for GATA-4 expression (Fig.
4J).
Discussion
Doetschman et al observed that, under certain
conditions, ESCs rapidly differentiate into cardiomyocytes with a
spontaneous rhythmic contraction; therefore, ESCs have become a
useful tool for studying gene expression and heart function
(9). It has been shown that
differentiated cells in vitro are similar to cardiomyocytes
in vivo in electrophysiological aspects and the cell cycle,
which is indicative of broad prospects for the future of
cardiomyocyte transplantation therapy (10). One of the major findings of the
present study was that ascorbic acid served as a valuable inductor
for the cardiac differentiation of hEGCs with high efficiency and
efficacy in vitro. hEGCs differentiated into cardiomyocytes
following treatment with ascorbic acid within a certain
concentration range (0.05–0.1 mg/ml). The majority of cells died
when the concentration reached 0.2 mg/ml due to the low pH and the
precipitation of crystals. Therefore, hEGCs were treated with 0.1
mg/ml ascorbic acid and cardiomyocyte-like cells were obtained,
which proliferated rapidly reaching a maximum at 10–12 days after
induction. The immunofluorescence assay detected expression of Cx43
and cTnT in the cardiomyocyte-like cells.
A large reduction in the number of cardiomyocytes
following myocardial infarction results in anatomical changes of
the heart and remodeling of the left ventricle, ultimately
resulting in heart failure. No type of reconstruction of the blood
supply to the coronary artery or further medical treatment is able
to prevent the loss of cardiomyocytes and left ventricular
remodeling. However, transplantation of stem cells enables the
regeneration of cardiomyocytes with normal function, thus
inhibiting the formation of scar tissues and improving the cardiac
function (11). A study has also
shown the ability of hESC-derived cardiomyocyte transplantation to
attenuate post-MI scar thinning and left ventricular dysfunction
(12). In the present study, the
mouse anti-human monoclonal antibody specific to nuclei (MAB1281),
with a strong species specificity, was adopted to act as the tracer
in immunohistochemistry. The present study showed that 1 day and 1,
2 and 4 weeks following transplantation the hEGCs gradually
migrated into the myocardium, mostly to the infarcted zone with
only a few migrating to the normal region in the heart. In the
control group, rats were injected with the same volume of PBS and
no expression of MAB1281 cells was detected.
Studies have shown that many tissue-specific
transcription factors are associated with heart development, such
as GATA-4, NKx2.5, Hey2, c-myb, Sox6 and Tbx (13–16),
among which GATA-4 is the most closely associated. GATA-4 has been
found to induce stem cell differentiation to cardiomyocytes,
playing an important role in heart development at the early stage
and it is also the earliest sign of cardiomyocyte progenitor cells.
ESCs expressing GATA-4 may only differentiate to cardiomyocytes
(17–20). In this study, hEGCs were
transplanted into the infarcted zone of rats with myocardial
infarction and 1 day and 1, 2 and 4 weeks later, positive
expression of GATA-4 was observed in the progenitor cells, mainly
in the nuclei. The number of GATA-4 positive cells increased with
time. There was no expression in the control group. The data
presented in the present study suggest that hEGCs survive after
transplantation, then differentiate into cardiomyocyte progenitor
cells. As the treatment was prolonged, the cells transplanted into
the infarcted zone were arranged in an orderly manner, and were
spindle-like, short and cylindrical in shape, which indicated that
the cells began to differentiate to cardiomyocytes with a normal
structure. Thus, the potential to treat myocardial infarction was
demonstrated.
References
|
1
|
Müller-Ehmsen J, Kedes LH, Schwinger RH
and Kloner RA: Cellular cardiomyoplasty - a novel approach to treat
heart disease. Congest Heart Fail. 8:220–227. 2002.
|
|
2
|
Lecina M, Ting S, Choo A, Reuveny S and Oh
S: Scalable platform for human embryonic stem cell differentiation
to cardiomyocytes in suspended microcarrier cultures. Tissue Eng
Part C Methods. 16:1609–1619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wobus AM, Kaomei G, Shan J, Wellner MC,
Rohwedel J, Ji GJ, Fleischmann B, Katus HA, Hescheler J and Franz
WM: Retinoic acid accelerates embryonic stem cell-derived cardiac
differentiation and enhances development of ventricular
cardiomyocytes. J Mol Cell Cardiol. 29:1525–1539. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun M, Yan X, Bian Y, Caggiano AO and
Morgan JP: Improving murine embryonic stem cell differentiation
into cardiomyocytes with neuregulin-1: differential expression of
microRNA. Am J Physiol Cell Physiol. 301:C21–C30. 2011. View Article : Google Scholar
|
|
5
|
Chen Y, Shao JZ, Xiang LX, Guo J, Zhou QJ,
Yao X, Dai LC and Lu YL: Cyclic adenosine 3′,5′-monophosphate
induces differentiation of mouse embryonic stem cells into
cardiomyocytes. Cell Biol Int. 30:301–307. 2006.
|
|
6
|
Xu C, Police S, Rao N and Carpenter MK:
Characterization and enrichment of cardiomyocytes derived from
human embryonic stem cells. Circ Res. 91:501–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rajasingh J, Bord E, Hamada H, Lambers E,
Qin G, Losordo DW and Kishore R: STAT3-dependent mouse embryonic
stem cell differentiation into cardiomyocytes: analysis of
molecular signaling and therapeutic efficacy of cardiomyocyte
precommitted mES transplantation in a mouse model of myocardial
infarction. Circ Res. 101:910–918. 2007. View Article : Google Scholar
|
|
8
|
Kehat I, Khimovich L, Caspi O, Gepstein A,
Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J and Gepstein
L: Electromechanical integration of cardiomyocytes derived from
human embryonic stem cells. Nat Biotechnol. 22:1282–1289. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doetschman T, Eistetter H, Katz M, Schmidt
W and Kemler R: The in vitro development of blastocyst-derived
embryonic stem cell lines: formation of visceral yolk sac, blood
islands myocardium. J Embryol Exp Morphol. 87:27–45.
1985.PubMed/NCBI
|
|
10
|
Westfall MV, Pasyk KA, Yule DI, Samuelson
LC and Metzger JM: Ultrastructue and cell-cell coupling of cardiac
myocytes differentiating in embryonic stem cell cultures. Cell
Motil Cytoskeleton. 36:43–54. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sargent CY, Berguig GY and McDevitt TC:
Cardiomyogenic differentiation of embryoid bodies is promoted by
rotary orbital suspension culture. Tissue Eng Part A. 15:331–342.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leor J, Gerecht S, Cohen S, Miller L,
Holbova R, Ziskind A, Shachar M, Feinberg MS, Guetta E and
Itskovitz-Eldor J: Human embryonic stem cell transplantation to
repair the infarcted myocardium. Heart. 93:1278–1284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liberatore CM, Searcy-Schrick RD and
Yutzey KE: Ventricular expression of tbx5 inhibits normal heart
chamber development. Dev Biol. 223:169–180. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Turbendian HK, Gordillo M, Tsai SY, Lu J,
Kang G, Liu TC, Tang A, Liu S, Fishman GI and Evans T: GATA factors
efficiently direct cardiac fate from embryonic stem cells.
Development. 140:1639–1644. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen-Barak O, Yi ZH, Hagiwara N, Monzen
K, Komuro I and Brilliant MH: Sox6 regulation of cardiac myocyte
development. Nucleic Acids Res. 31:5941–5948. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishida M, El-Mounayri O, Kattman S,
Zandstra P, Sakamoto H, Ogawa M, Keller G and Husain M: Regulated
expression and role of c-Myb in the cardiovascular-directed
differentiation of mouse embryonic stem cells. Circ Res.
110:253–264. 2012. View Article : Google Scholar
|
|
17
|
Shamblott MJ, Axelman J, Wang S, Bugg EM,
Littlefield JW, Donovan PJ, Blumenthal PD, Huggins GR and Gearhart
JD: Derivation of pluripotent stem cells from cultured human
primodial germ cells. Proc Natl Acad Sci USA. 95:13726–13731. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Snyder M, Huang XY and Zhang JJ: Stat3
directly controls the expression of Tbx5, Nkx2.5, and GATA4 and is
essential for cardiomyocyte differentiation of P19CL6 cells. J Biol
Chem. 285:23639–23646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stennard FA, Costa MW, Elliott DA, Rankin
S, Haast SJ, Lai D, McDonald LP, Niederreither K, Dolle P, Bruneau
BG, Zorn AM and Harvey RP: Cardiac T-box factor Tbx20 directly
interacts with Nkx2.5, GATA4, and GATA5 in regulation of gene
expression in the developing heart. Dev Biol. 262:206–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Small EM and Krieg PA: Transgenic analysis
of the atrialnatriuretic factor (ANF) promoter: Nkx2.5 and GATA-4
binding sites are required for atrial specific expression of ANF.
Dev Biol. 261:116–131. 2003. View Article : Google Scholar : PubMed/NCBI
|