Introduction
As one of the most common types of chronic disorder
in aged people, osteoporosis has a multifactorial etiology and has
been characterized by progressive bone substance loss,
microarchitecture impairments and an increased risk of fractures
(1,2). In addition to the systemic symptoms,
patients with osteoporosis also suffer from the dental diseases
periodontitis and dentition defect, which usually cause bone mass
insufficiency (3,4). Due to impaired alveolar bone
structure and metabolic disturbances, it is accordingly difficult
to treat these patients. A great deal of effort has been made to
alleviate this situation, yet few treatment technologies are
applied widely. In current clinical practice, most dentists prefer
to select drugs as facilitators (5). Osteoblasts usually originate from
mesenchymal stem cells and are of importance during the bone
formation process (6). Osteoblasts
often behave abnormally in bone metabolism disorders. Therefore,
numerous drugs for osteoporosis treatment are targeted at
regulating osteoblast function.
Osteoblasts express several types of calcium
channels. Of these channels, the L-type voltage-sensitive channel
is the one most clearly involved in functional osteoblast
regulation. A previous study has demonstrated that calcium channels
are associated with proliferation, apoptosis and differentiation in
osteoblasts (7). As a large number
of patients who suffer from bone metabolism disorders also require
hypertension treatment, the identification an appropriate
antihypertensive drug that is able to also treat bone disorders
would be of great significance. If a drug stimulates osteoblast
function while performing an antihypertensive effect, it is likely
to present a great benefit for elderly patients and doctors.
Benidipine (BD) is a dihydropyridine-type calcium
channel blocker and has been widely used for hypertension therapy.
It blocks the L-type and T-type calcium channels in different types
of cells, including osteoblasts (8). Due to the dual effects of BD on
hypertension and calcium channels, it is hypothesized to be a
suitable candidate for the treatment of patients with osteoporosis
and hypertension. Therefore, the aim of the present study was to
evaluate the effect of BD at different concentrations on
osteoblasts in vitro.
Materials and methods
Medicine preparation
A solution of BD (Kyowa Hakko Kirin Co., Ltd.,
Tokyo, Japan) was prepared by dissolving solid BD in
dimethylsulfoxide (DMSO) solvent. The stock solution was stored at
−20°C.
Cell culture
MC3T3-E1 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in α-MEM containing 100 U/ml
penicillin, 100 U/ml streptomycin and 10% fetal bovine serum (FBS)
in a humidified incubator at 37°C and 5% CO2. The cells
were subcultured every three days in the presence of 0.25%
trypsin.
MTT assay
MC3T3-E1 cells were seeded in 96-well plates (5,000
cells/well) and incubated overnight. BD solution at different
concentrations (final concentrations of
1×10−4–1×10−10 M) was then added. Cells
without BD treatment were used as a negative control and wells
without cells were set as blanks. One-, two-, and three-day further
incubations were performed, and then 20 μl MTT (5.0 mg/ml) was
added and the cells were incubated for another 4 h at 37°C.
Subsequently, the supernatant was removed, DMSO was added and the
optical density (OD) at 570 nm was measured on a microplate
spectrophotometer (Model 680 Microplate Reader; Bio-Rad, Hercules,
CA, USA). The proliferation rate of the cells was calculated
according to the following formula: (ODsample ‐
ODblank) / (ODcontrol ‐
ODblank).
Assay for alkaline phosphatase (ALP)
activity
MC3T3-E1 cells were seeded in 24-well plates
(2×104 cells/well) containing α-MEM medium and 10% FBS.
After 24 h, the culture medium was changed to α-MEM, 10% FBS and
osteogenic induction supplement containing 10 mmol/l disodium
β-glycerophosphate and 0.15 mmol/l ascorbic acid (Sigma, St. Louis,
MO, USA). A series of dilutions of BD (final concentrations,
1×10−6–1×10−9 M) were added to the culture
medium in the 24-well plates for 3, 5, 7, 10 and 14 days. MC3T3-E1
cells treated with only osteogenic induction supplement were used
as the control group. Following incubation, the MC3T3-E1 cells were
washed twice with ice-cold PBS and lysed by two cycles of freezing
and thawing. Aliquots of the supernatants were subjected to ALP
activity and protein content measurement using an ALP activity kit
and a bicinchonininc acid (BCA) protein assay kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). All the
results were normalized by protein content.
Assay for mineralized matrix
formation
Cells were seeded in 24-well plates
(2×104 cells/well) and cultured overnight at 37°C in a
5% CO2 humidified incubator. The medium was then changed
to medium containing osteogenic induction supplement and BD
(1×10−6–1×10−9 M) for 21 days. The formation
of mineralized matrix nodules was determined by alizarin red S
(ARS) staining. Briefly, the cells were fixed in 95% ethanol for 30
min at room temperature. The fixed cells were washed with PBS and
stained with 1% ARS (pH 4.2) for 30 min at room temperature.
Quantitative analysis was performed by elution with 10% (w/v)
cetylpyridium chloride for 10 min at room temperature, and the OD
was measured at 570 nm.
RNA isolation and semiquantitative
reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted following three-day
incubation using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). Complementary DNA (cDNA) was produced using a
transcriptase PCR kit (ReverTra Dash; Toyobo Biochemicals, Osaka,
Japan). Aliquots of total cDNA were amplified, using PCR equipment
(PC701 thermal cycler; Astec, Fukuoka, Japan). The amplification
reaction products were resolved on 1.5% agarose/TAE gels by
electrophoresis at 100 mV, and were visualized by ethidium-bromide
staining. The primers used are presented in Table I.
 | Table IPrimer sequences used for RT-PCR. |
Table I
Primer sequences used for RT-PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| Runx2 |
TTCTCCAACCCACGAATGCAC |
CAGGTACGTGTGGTAGTGAGT |
| BMP2 |
TGGCCCATTTAGAGGAGAACC |
AGGCATGATAGCCCGGAGG |
| OCN |
GAACAGACTCCGGCGCTA |
AGGGAGGATCAAGTCCCG |
| GAPDH |
GACTTCAACAGCAACTCCCAC | TCCACCACCCTGT
TGCTGTA |
Western blot analysis
MC3T3-E1 cells were washed with cold PBS and lysed
in cold Tris-HCl (50 mM, pH 7.4), 10 mM EDTA, 4.3 M urea and 1%
Triton X-100. Proteins were subjected to SDS-PAGE using 10%
separation gel and transferred to a nitrocellulose membrane. The
membrane was blocked for 2 h at room temperature with 5% bovine
serum albumin in TBST solution (10 mM Tris-HCl, pH 8.0; 150 mM
NaCl; 0.05% Tween-20). Subsequently, the blots were incubated with
the corresponding primary antibodies (rabbit anti-Runx2, rabbit
anti-BMP2 and rabbit anti-OCN) (Biosynthesis Biotechnology Co.,
Ltd., Beijing, China) in the TBST solution overnight at 4°C,
followed by 2 h incubation with secondary goat anti-rabbit IgG
antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA)
conjugated with horseradish peroxidase, and visualized with an
enhanced luminol-based chemiluminescent (ECL) kit (Thermo Fisher
Scientific Inc., Waltham, MA, USA). The OD of the bands was
quantified using LAS-1000 luminescent image analyzer software
(Fujifilm, Berlin, Germany).
Statistical analysis
One-way analysis of variance and Tukey’s multiple
comparison tests were performed to detect any significant effects
that occurred as a result of the experimental variables. All
results are expressed as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effect of BD on the proliferation of
MC3T3-E1 cells
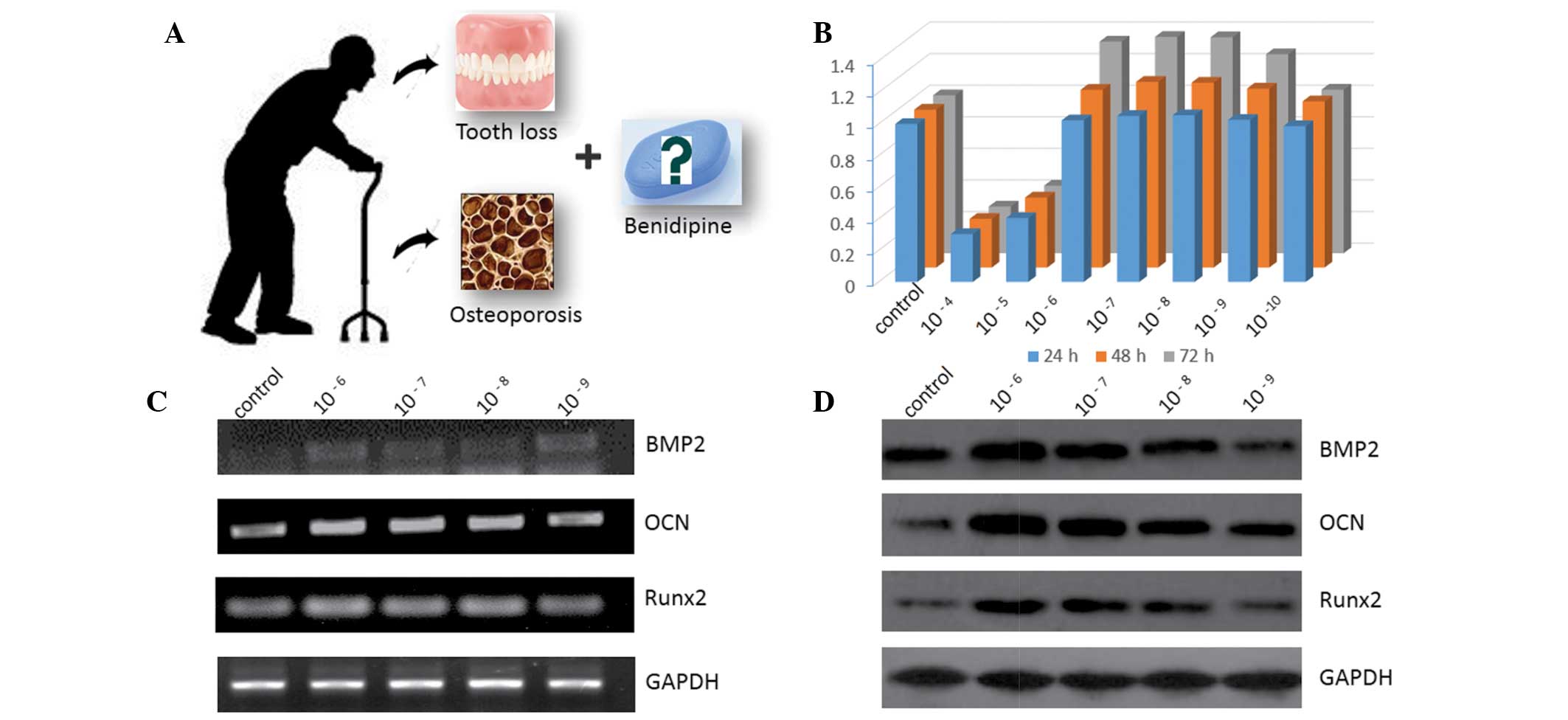
As demonstrated in Fig.
1B, the effect of BD on the proliferation of MC3T3-E1 cells was
time-dependent and the proliferation rate decreased with increasing
BD concentrations. Following one-, two- and three-day treatment, BD
promoted proliferation at concentrations of
1×10−6–1×10−9 M. The higher concentrations of
BD inhibited cell proliferation whereas no significant difference
from the control was observed when the lower concentrations of BD
were applied.
Effect of BD on gene and protein
expression
Following treatment with different concentrations of
BD for three days, BMP2, OCN and Runx2 mRNA levels were markedly
upregulated compared with those in the control group in a
concentration-dependent manner (Fig.
1C). As demonstrated in Fig.
1D, BMP2, OCN and Runx2 protein levels were enhanced following
BD treatment; the most prominent enhancements were observed in the
groups of cells treated with 1×10−6 and
1×10−7 M BD.
Effect of BD on the differentiation of
MC3T3-E1 cells
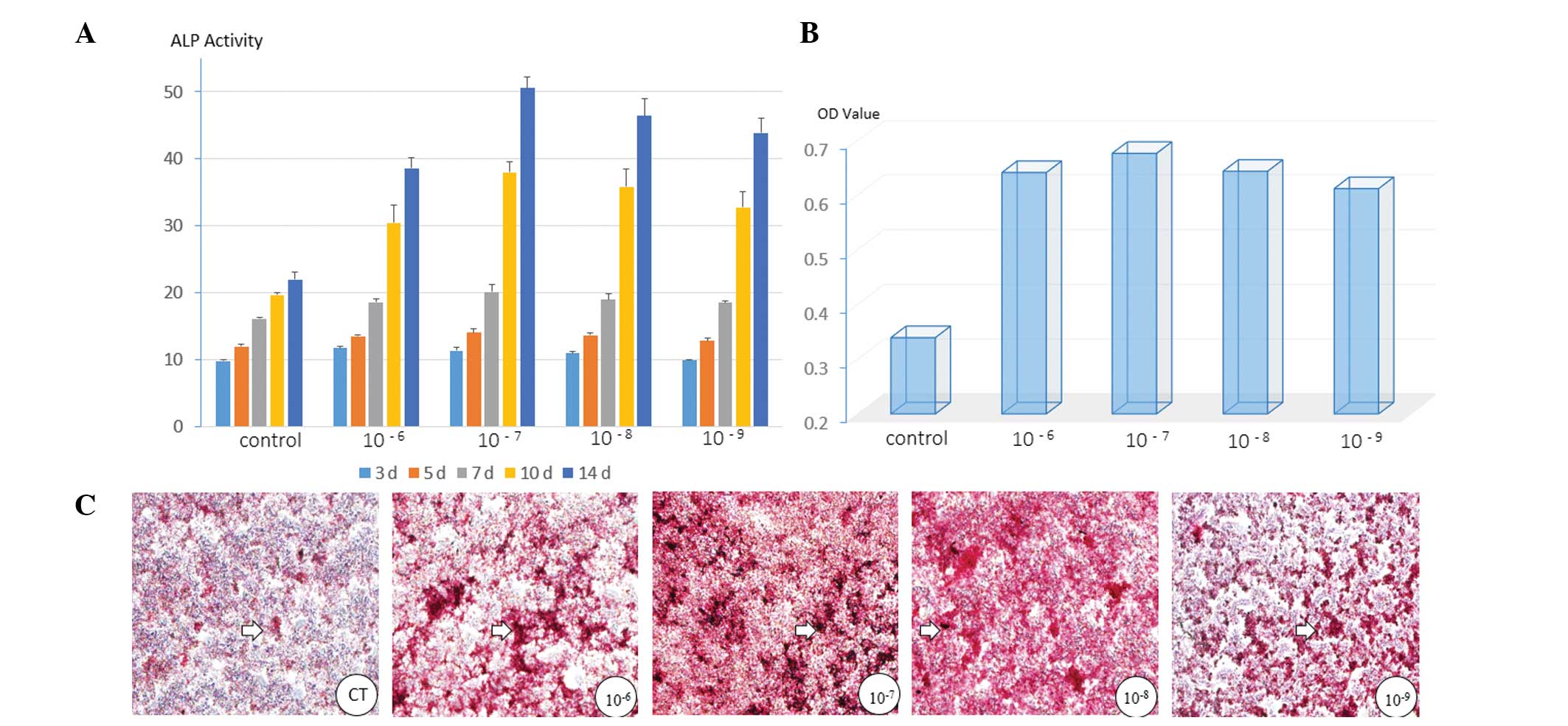
As presented in Fig.
2A, all four groups treated with BD exhibited elevated levels
of ALP activity compared with those in the control group. The
highest level was observed in the cells treated with
1×10−7 M BD. From day 3 to day 14, the level of ALP
activity in each of the groups of cells increased in a
time-dependent manner.
BD also promoted the formation of mineralized matrix
nodules in the MC3T3-E1 cells. The results of the quantitative
analysis of the ARS staining were in accordance with the
morphological observations. BD at 1×10−7 M also resulted
in the clearest promotive effect in the cells (Fig. 2B and C).
Discussion
Cell proliferation is a key attribute of the bone
repair process. In the present study, it was demonstrated that the
effects of BD on the proliferation of MC3T3-E1 cells varied
according to the concentration of BD. A low concentration of BD had
no effect on cell proliferation. At a BD concentration of
1×10−6 M, cell proliferation was promoted and this
effect was observed in the cells treated with concentrations down
to 1×10−9 M BD, whereas inhibition was observed when
cells were treated with the higher concentrations. Thus, the
concentrations 1×10−6–1×10−9 M BD were
selected for the remaining experiments. The result is inconsistent
with a previous study (9) and this
may be attributable to the different techniques applied. The
inhibitory effect is likely to be the result of cytotoxicity.
A large number of proteins that have been associated
with bone cells are specifically required for osteoblast
differentiation, such as Runx2, BMP2 and OCN (10). Runx2 is a master regulator of
osteogenic gene expression and osteoblast differentiation. It has
been reported that Runx2 knockout mice exhibit no bone tissues or
osteoblasts, indicating that osteoblast differentiation is
completely blocked in the absence of Runx2 (11,12).
In addition to being required for osteoblast differentiation, Runx2
is necessary for the proper function of mature osteoblasts,
including the synthesis of bone matrix (13).
OCN is the most specific gene for osteoblast
differentiation and mineralization. OCN is expressed during the
postproliferative period, reaches its maximum expression during
mineralization and accumulates in the mineralized bone (14). BMP2 is a member of the transforming
growth factor-β superfamily and has a key regulatory role as a
cell-cell signaling molecule during bone formation and repair.
BMP2, which is a potent osteogenic protein required for osteoblast
differentiation and bone formation, induces low levels of
expression of osteoblast marker genes such as OCN and ALP in
calvarial cells from Cbfa1−/− animals (12). Furthermore, it has been shown that
mice lacking BMP2 in the limb mesenchyme exhibit a clear defect in
bone mineral density shortly after birth, indicating that BMP2 has
a unique role in bone formation (15).
ALP, a cell membrane-associated enzyme, appears
early during osteoblast differentiation and is the most widely
recognized marker of osteoblastic differentiation (16). ALP activity correlates with matrix
formation in osteoblasts prior to the initiation of mineralization.
In the present study, BD enhanced ALP activity at five time points
in a time-dependent manner, but no significant
concentration-dependent manner was observed. While the appearance
of ALP activity is an early marker of differentiation, mineralized
nodule formation is considered as a late marker for maturation
(17,18). Consistent with the ALP activity
result, an increased mineralization level was observed in the
BD-treated cells. This was further confirmed by the quantitative
analysis.
In addition to the conventional antihypertensive
function of BD, several studies have suggested that BD increases
the ALP activity of osteoblastic cells and also stimulates mineral
matrix deposition (19,20). In addition, BD has been shown to
decrease receptor activator of nuclear factor κB ligand expression
in human osteoblasts, indicating the suppression of osteoclast
differentiation (21). The
systematic experiments conducted in the present study also
demonstrated that BD promoted the proliferation, osteogenic
differentiation and mineralization of MC3T3-E1 cells at the
cellular and molecular levels, when applied at concentrations of
1×10−6–1×10−9 M. These findings indicate that
BD may be a novel candidate for the combined treatment of
osteoporosis and hypertension. BD promoted osteogenesis most
markedly at concentrations of 1×10−7 and
1×10−8 M, which is in accordance with the serum drug
levels for antihypertensive therapy (22).
The results of the present study demonstrated that
BD promotes cell proliferation and osteogenic differentiation at
concentrations from 1×10−6 to 1×10−9 M by
upregulating Runx2, BMP2 and OCN gene expression levels. Therefore,
it was concluded that BD at the appropriate concentrations may have
a positive effect on osteoblast function in addition to its
conventional usage, and may be a suitable candidate for the
treatment of patients with osteoporosis and hypertension.
References
|
1
|
Warriner AH and Saag KG: Osteoporosis
diagnosis and medical treatment. Orthop Clin North Am. 44:125–135.
2013. View Article : Google Scholar
|
|
2
|
Mosekilde L, Vestergaard P and Rejnmark L:
The pathogenesis, treatment and prevention of osteoporosis in men.
Drugs. 73:15–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Bertulucci LA, Pereira FM, de Oliveira
AE, Brito LM and Lopes FF: Periodontal disease in women in
post-menopause and its relationship with osteoporosis. Rev Bras
Ginecol Obstet. 34:563–567. 2012.(In Portuguese).
|
|
4
|
Alania KN, Iverieli MB, Abashidze NO,
Gogishvili KhB and Chigladze TT: Oral cavity features in patients
suffering from osteogenesis imperfecta. Georgian Med News.
193:34–41. 2011.(In Russian).
|
|
5
|
Lippuner K: The future of osteoporosis
treatment - a research update. Swiss Med Wkly.
142:w136242012.PubMed/NCBI
|
|
6
|
Teti A: Bone development: overview of bone
cells and signaling. Curr Osteoporos Rep. 9:264–273. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blair HC, Schlesinger PH, Huang CL and
Zaidi M: Calcium signalling and calcium transport in bone disease.
Subcell Biochem. 45:539–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao K, Nagashima K and Miki H:
Pharmacological, pharmacokinetic, and clinical properties of
benidipine hydrochloride, a novel, long-acting calcium channel
blocker. J Pharmacol Sci. 100:243–261. 2006. View Article : Google Scholar
|
|
9
|
Kosaka N and Uchii M: Effect of benidipine
hydrochloride, a dihydropyridine-type calcium antagonist, on the
function of mouse osteoblastic cells. Calcif Tissue Int.
62:554–556. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ducy P, Zhang R, Geoffroy V, Ridall AL and
Karsenty G: Osf2/Cbfa1: a transcriptional activator of osteoblast
differentiation. Cell. 89:747–754. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakashima K, Zhou X, Kunkel G, et al: The
novel zinc finger-containing transcription factor osterix is
required for osteoblast differentiation and bone formation. Cell.
108:17–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Komori T, Yagi H, Nomura S, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ducy P, Starbuck M, Priemel M, et al: A
Cbfa1-dependent genetic pathway controls bone formation beyond
embryonic development. Genes Dev. 13:1025–1036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neve A, Corrado A and Cantatore FP:
Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol.
228:1149–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuji K, Bandyopadhyay A, Harfe BD, et al:
BMP2 activity, although dispensable for bone formation, is required
for the initiation of fracture healing. Nat Genet. 38:1424–1429.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beertsen W and Van den Bos T: Alkaline
phosphatase induces the deposition of calcified layers in relation
to dentin: an in vitro study to mimic the formation of afibrillar
acellular cementum. J Dent Res. 70:176–181. 1991. View Article : Google Scholar
|
|
17
|
Liu D, Zhang J, Wang G, Liu X, Wang S and
Yang M: The dual-effects of LaCl3 on the proliferation,
osteogenic differentiation, and mineralization of MC3T3-E1 cells.
Biol Trace Elem Res. 150:433–440. 2012.
|
|
18
|
Liu D, Zhang J, Li Y, Wang S and Yang M:
The effects of Ce on the proliferation, osteogenic differentiation
and mineralization function of MC3T3-E1 cells in vitro. Biol Trace
Elem Res. 149:291–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishiya Y, Kosaka N, Uchii M and Sugimoto
S: A potent 1,4-dihydropyridine L-type calcium channel blocker,
benidipine, promotes osteoblast differentiation. Calcif Tissue Int.
70:30–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishiya Y and Sugimoto S: Effects of
various antihypertensive drugs on the function of osteoblast. Biol
Pharm Bull. 24:628–633. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimizu H, Nakagami H, Yasumasa N, Mariana
OK, Kyutoku M, Koriyama H, Nakagami F, Shimamura M, Rakugi H and
Morishita R: Links between hypertension and osteoporosis:
benidipine ameliorates osteoporosis in ovariectomized hypertensive
rats through promotion of osteoblast proliferation and inhibition
of osteoclast differentiation. Curr Cardiovasc Risk Rep. 6:274–280.
2012. View Article : Google Scholar
|
|
22
|
Yun HY, Yun MH, Kang W and Kwon KI:
Pharmacokinetics and pharmacodynamics of benidipine using a slow
receptor-binding model. J Clin Pharm Ther. 30:541–547. 2005.
View Article : Google Scholar : PubMed/NCBI
|