Introduction
Chemotherapy combined with radiation and surgery is
an important component of intensive therapies for high-risk
neuroblastoma. Combination therapy with multiple antitumor agents
induces tumor cell death. The primary mechanisms of cell death are
apoptosis, necrosis and autophagy. In particular, apoptosis and
necrosis have significantly different effects on the subsequent
immunological response (1,2). Apoptotic cells are commonly engulfed
by phagocytes, such as macrophages or dendritic cells (DCs), which
induce a sequential immune response (1,2).
Whether the engulfed apoptotic cells induce subsequent
tolerogenicity or immunogenicity depends on the function of the
phagocytes involved. Furthermore, it has been revealed that the
mechanisms of cell death induced by chemotherapeutic antitumor
agents have an effect on the immunogenicity of dying tumor cells
(2–4). Obeid et al (5) and Martins et al (6) reported that anthracyclins induce
immunogenic tumor cell death in a murine colon cancer model. In
this model, anthracyclins induced the translocation of calreticulin
(CRT) to the cell surface, and CRT was exposed by dying tumor cells
phagocytosed by DCs, resulting in the presentation of tumor
antigens and the induction of immunogenicity in tumor cells
(5,6). This evidence has provided insights
into the immunological benefits and drawbacks of conventional
chemotherapeutic antitumor agents. Moreover, a clear understanding
of the cellular basis of immunogenicity induced by dead tumor cells
treated with chemotherapeutic agents is likely to provide novel
strategies for the development of therapeutic vaccines for advanced
cancer (4,7). To date, only a few studies have
investigated the immunogenic effect of anthracyclins on
neuroblastoma cells (8).
Despite the availability of intensive, multimodal
treatments, the long-term survival of patients with high-risk
neuroblastoma remains unsatisfactory (9–11).
The majority of high-risk neuroblastomas respond to initial therapy
but subsequently relapse, and it has been suggested that tumor
cells may acquire drug resistance following multi-agent
chemotherapy. However, a passive, antibody-based immunotherapeutic
approach increased the two-year event-free survival rate,
indicating that immunological mechanisms are capable of promoting
the eradication of high-risk neuroblastoma cells (10,12).
Investigation into the potential immunological benefits of
chemotherapeutic agents may improve conventional chemotherapeutic
regimens and help to establish novel immunological therapies for
high-risk neuroblastoma (13).
In this study, doxorubicin was administered to a
murine neuroblastoma cell line ex vivo to induce tumor cell
death, and the immunogenicity of the dead tumor cells was examined.
In addition, the mechanism underlying the immune reaction following
phagocytosis of the dead neuroblastoma cells was investigated.
Materials and methods
Murine tumor cell line
The murine neuro-2a neuroblastoma cell line
(H2-Ka, CCL-131), which was developed in A/J mice, was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Cells were maintained in Minimum Essential
Medium (MEM) with 10% fetal bovine serum (ATCC) and 1%
penicillin-streptomycin (10,000 U/ml; Gibco; Invitrogen Life
Technologies, Carlsbad, CA, USA) at 37°C in 5% CO2.
Animals
Female A/J mice (H2-Ka; Japan SLC, Inc.,
Hamamatsu, Japan), aged 6–10 weeks, were maintained under standard
conditions. The Ethics Committee of The animal experiment, Saitama
Medical University, Saitama, Japan, approved the animal
procedures.
Induction of tumor cell death and cell
survival assay
Cell death induced by doxorubicin (Sigma-Aldrich,
St. Louis, MO, USA) and cisplatin (CDDP; Maruko®,
Yakult, Tokyo, Japan) was examined using the cell viability reagent
water-soluble tetrazolium salt 8 (WST-8; Wako Chemicals, Osaka,
Japan), according to the manufacturer’s instructions. Briefly,
neuro-2a cells (0.40×105 cells/90 μl/well) were plated
in 96-well plates and incubated overnight. Subsequently, 10 μl MEM
with 10% fetal bobine serum and 1% penicillin-streptmycin combined
with either doxorubicin (final concentration,
6.1×10−3-100 μM) or CDDP (final concentration,
1.0×10−7-1.0×10−1 mg/ml) was added to each
well, and the mixture was incubated for either 24 h (doxorubicin)
or 72 h (CDDP). WST-8 (10 μl/well) was added, and, after 2 h
incubation, absorbance at 450 nm was measured with a microplate
reader (Thermo Fisher Scientific, Yokohama, Japan). Experiments
were repeated at least three times to confirm that the results were
reproducible.
Isolation of cluster of differentiation
(CD)11b+ spleen cells from A/J mice
CD11b+ spleen cells were prepared as
antigen-presenting cells (APCs). Whole spleen cell suspensions were
prepared by passing spleen tissue minced with scissors through a
70-μm cell strainer (BD Biosciences, San Diego, CA, USA).
Erythrocytes were lysed in an erythrocyte lysis solution (BD Pharm
Lyse™; BD Biosciences) and washed with RPMI-1640 medium containing
10% fetal calf serum (FCS). The cells were then re-suspended in
MACS® running buffer (Miltenyi Biotec GmbH, Bergisch
Gladbach, Germany), incubated with CD11b+ magnetic beads
(Miltenyi Biotec GmbH) for 15 min at 4°C, and positively sorted
using an autoMACS® Pro Separator (Miltenyi Biotec GmbH).
CD11b+ cells were re-suspended in RPMI-1640 medium.
Generation of bone marrow-derived DCs
(BM-DCs)
BM cells were harvested from A/J mice by flushing
the femurs and tibias with RPMI-1640 medium (Gibco; Invitrogen Life
Technologies). The BM material was passed through a 70-μm cell
strainer (BD Biosciences) and the cells were centrifuged (440 × g
at room temperature) and re-suspended in erythrocyte lysis
solution. The surviving cells were washed with RPMI-1640 medium
containing 10% FCS, prior to being re-suspended in RPMI-1640 medium
supplemented with 10% FCS, 50 μM 2-ME (Sigma-Aldrich), 1% MEM
non-essential amino acid solution and 1% penicillin-streptmycin
solution (Gibco; Invitrogen Life Technologies). Following the
addition of recombinant mouse granulocyte-macrophage
colony-stimulating factor (GM-CSF; 20 ng/ml; R&D Systems, Inc.,
Minneapolis, MN, USA), the cells were plated and incubated at 37°C
in 5% CO2. Fresh medium containing 20 ng/ml GM-CSF was
added after three days of incubation, and adherent cells were
harvested by trypsinization after seven days. The expression of
CD11c and major histocompatibility complex (MHC) class II antigens
was assessed by fluorescence-activated cell sorting (FACS) using
phycoerythrin (PE)-conjugated anti-mouse CD11c antibodies
(Invitrogen Life Technologies) and fluorescein
isothiocyanate-conjugated anti-MHC class II antibodies (Miltenyi
Biotec GmbH).
In vitro CD8α+ T-cell
proliferation assay and interferon (IFN)-γ detection by ELISA
To evaluate the immunogenicity of
doxorubicin-treated, dead neuro-2a cells, doxorubicin-treated
neuro-2a cells were co-cultured with CD8α+ cells and
proliferation of the CD8α+ cells was evaluated. The
inguinal and mesenteric lymph nodes (LNs) and spleens were removed
from A/J mice. The LNs were ground between two glass slides, washed
with RPMI-1640, passed through a 70-μm cell strainer and
re-suspended in MACS running buffer. The cells were then incubated
with CD8α magnetic beads (Miltenyi Biotec GmbH) for 15 min at 4°C,
and positively sorted using the autoMACS® Pro Separator
(Miltenyi Biotec GmbH). CD8α+ cells were labeled with 10
μM carboxyfluorescein succinimidyl ester (CFSE; Enzo Life Sciences
Inc., Plymouth Meeting, PA, USA) and re-suspended in RPMI-1640
supplemented with 10% FCS, 50 μM 2-ME, 1% MEM-non-essential amino
acid solution, 1% penicillin-streptmycin and MEN vitamin solution
(Gibco; Invitrogen Life Technologies). Neuro-2a cells
(2.0×105) that had been treated with doxorubicin (final
concentration, 5 μM) for 24 h, as well as CFSE-labeled
CD8α+ cells (2.0×105) and CD11b+
spleen cells (0.5×105), were plated in 24-well
flat-bottomed plates coated with hamster anti-mouse CD3/CD28
antibodies (BD Pharmingen, San Diego, CA, USA) in 1 ml RPMI
supplemented with 10% FCS, 50 μM 2-ME, 1% MEM-non-essential amino
acid solution, 1% penicillin-streptmycin and MEN vitamin solution.
As an adjuvant, purified, single-stranded CpG-oligodeoxynucleotide
(ODN)-1826 (5′-TCCATGACGTTCCTGACGTT; Nippon Gene Co., Ltd., Toyama,
Japan) was added to each well (10 μg/well). The cells were
incubated for three days at 37°C in 5% CO2, and then
harvested. CFSE dilution of the CD8α+ cells was
evaluated using the FACScan system (BD Biosciences). Concentrations
of IFN-γ in the culture supernatant were measured using a mouse
IFN-γ ELISA kit (BD Biosciences), according to the manufacturer’s
instructions. IFN-γ concentrations were compared as an index of the
rate of CD8α+ lymphocyte proliferation.
Comparison of the antigen-presenting
capacity of CD11b+ spleen cells versus BM-DCs
To evaluate the capacity of antigen presentation by
CD11b+ spleen cells and BM-DCs, either CD11b+
spleen cells (0.5×105) or BM-DCs (0.5×105)
were co-cultured with doxorubicin-treated neuro-2a cells
(2.0×105) and CFSE-labeled CD8α+ cells
(2.0×105) in 24-well plates coated with hamster
anti-mouse CD3/CD28 antibodies without CpG-ODN. Following three
days of co-culture, CD8α+ cell proliferation was
confirmed using FACS. Concentrations of IFN-γ in the culture
supernatant were measured using a mouse IFN-γ ELISA kit (BD
Biosciences).
Evaluation of the ability of doxorubicin
treatment to induce immunogenic cell death in neuroblastoma
cells
To show that doxorubicin has the ability to induce
immunogenic cell death in neuroblastoma cells, doxorubicin or
CDDP-treated neuro-2a cells were co-cultured with BM-DCs and
CD8α+ lymphocytes, and IFN-γ concentrations in the
culture supernatants were compared. CDDP was used as a control
agent as it is unable to induce immunogenic cell death in tumor
cells (14). Neuro-2a cells
(2.0×105) treated with either doxorubicin (5 μM for 24
h) or CDDP (25 μg/ml for 72 h) were co-cultured with CFSE-labeled
CD8α+ cells (2.0×105) in 24-well plates
coated with hamster anti-mouse CD3/CD28 antibodies (BD Pharmingen).
Following three days of co-culture, CD8α+ cell
proliferation was confirmed using FACS, and IFN-γ concentrations in
the culture supernatants were measured using a mouse IFN-γ ELISA
kit (BD Biosciences).
Results
Induction of neuro-2a cell death by
doxorubicin and CDDP
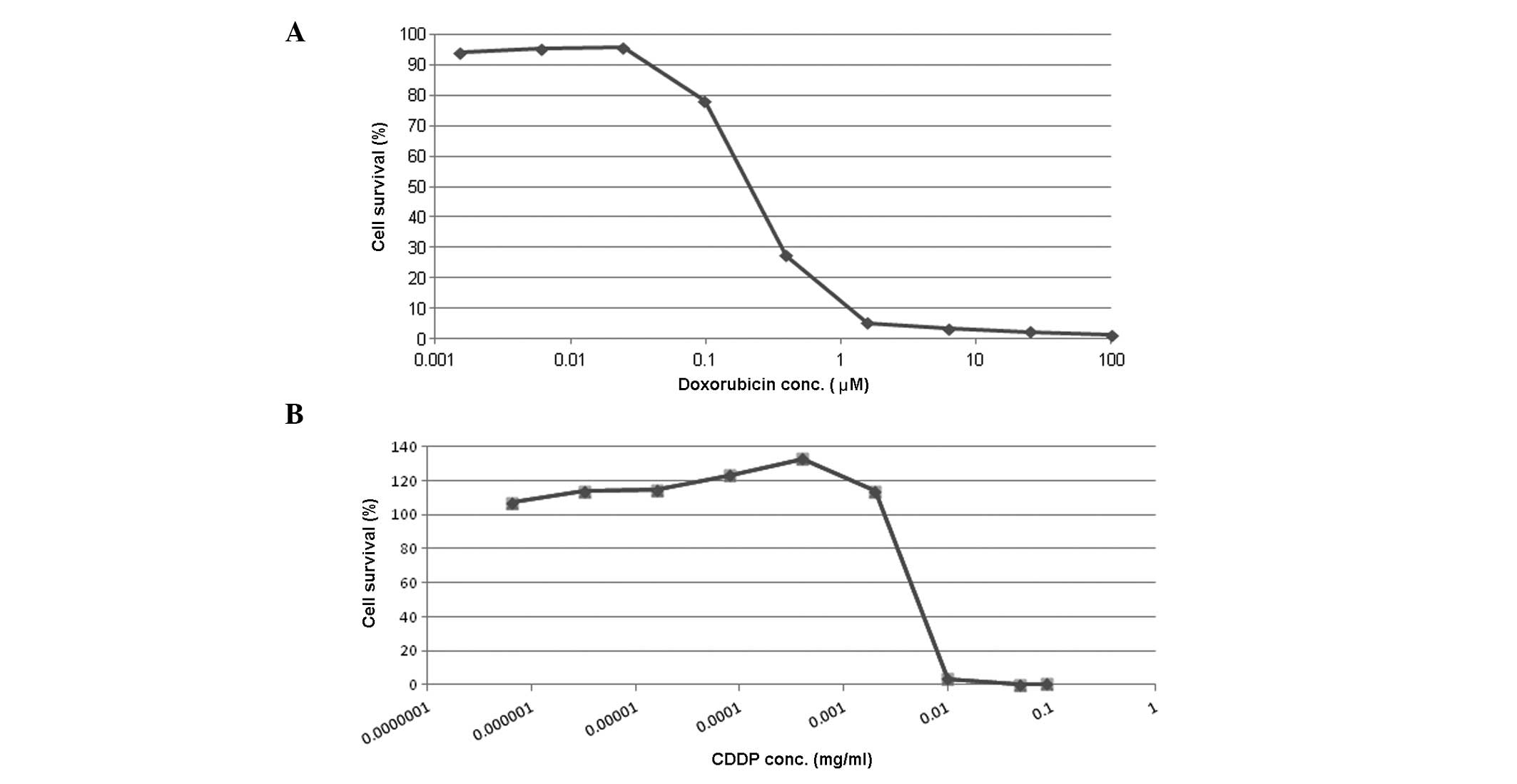
The effect of doxorubicin and CDDP treatment on
neuro-2a cell survival was dependent on the concentration of the
two agents (Fig. 1). Following a
24-h incubation period, doxorubicin at concentrations of <0.1 μM
caused minimal cell death, while at concentrations of >1.6 μM
doxorubicin caused the majority of cells (>98%) to die (Fig. 1A). CDDP also induced neuro-2a cell
death. Following a 72-h incubation period, >0.01 mg/ml CDDP
caused death in >98% of cells (Fig.
1B).
CD8α+ lymphocyte proliferation
and IFN-γ production in co-culture with doxorubicin-treated
neuro-2a cells and CD11b+ spleen cells
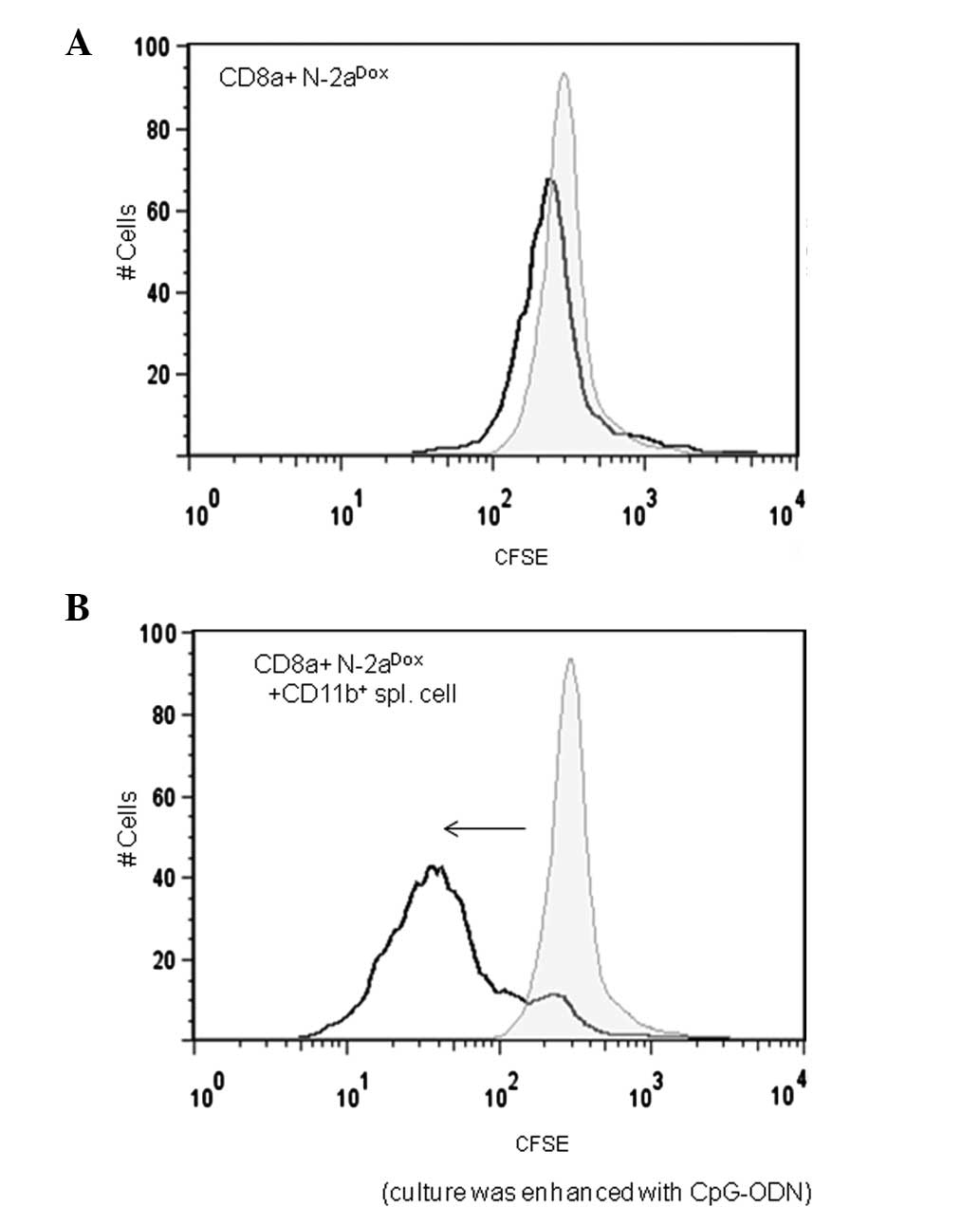
When CD8α+ cells were cultured with
neuro-2a cells alone, CD8α+ cell proliferation was not
observed (Fig. 2A). However, when
CD8α+ lymphocytes were co-cultured with
CD11b+ spleen cells and doxorubicin-treated neuro-2a
cells, FACS examination on the third day of the co-culture revealed
a dilution of the CFSE. This was indicated by a reduction in the
CFSE intensity of the CFSE-positive cell population relative to the
initial staining. These data indicate that CFSE-labeled
CD8α+ lymphocytes had proliferated and that the CFSE in
the cytoplasm was diluted (Fig.
2B). CpG-ODN was added to the culture as an adjuvant for the
lymphoproliferation reaction.
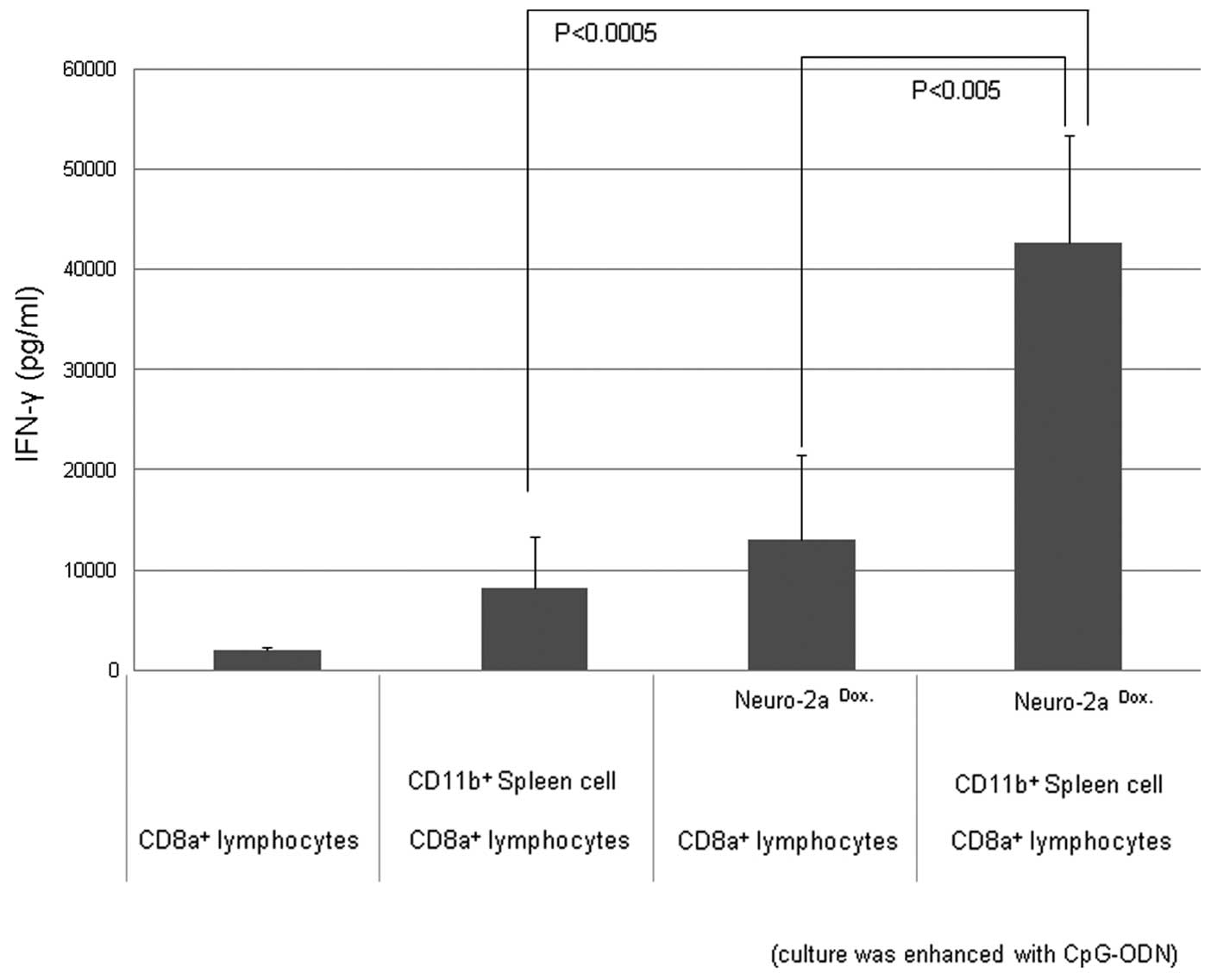
Production of IFN-γ following co-culture
of CD8α+ responder cells, CD11b+ APCs and
doxorubicin-treated tumor cells under CD3/CD28 stimulation
IFN-γ production was observed when CD8α+
lymphocytes were co-cultured with CD11b+ spleen cells
and doxorubicin-treated neuro-2a cells with stimulation by CD3 and
CD28 antibodies, and CpG-ODN as an adjuvant (Fig. 3).
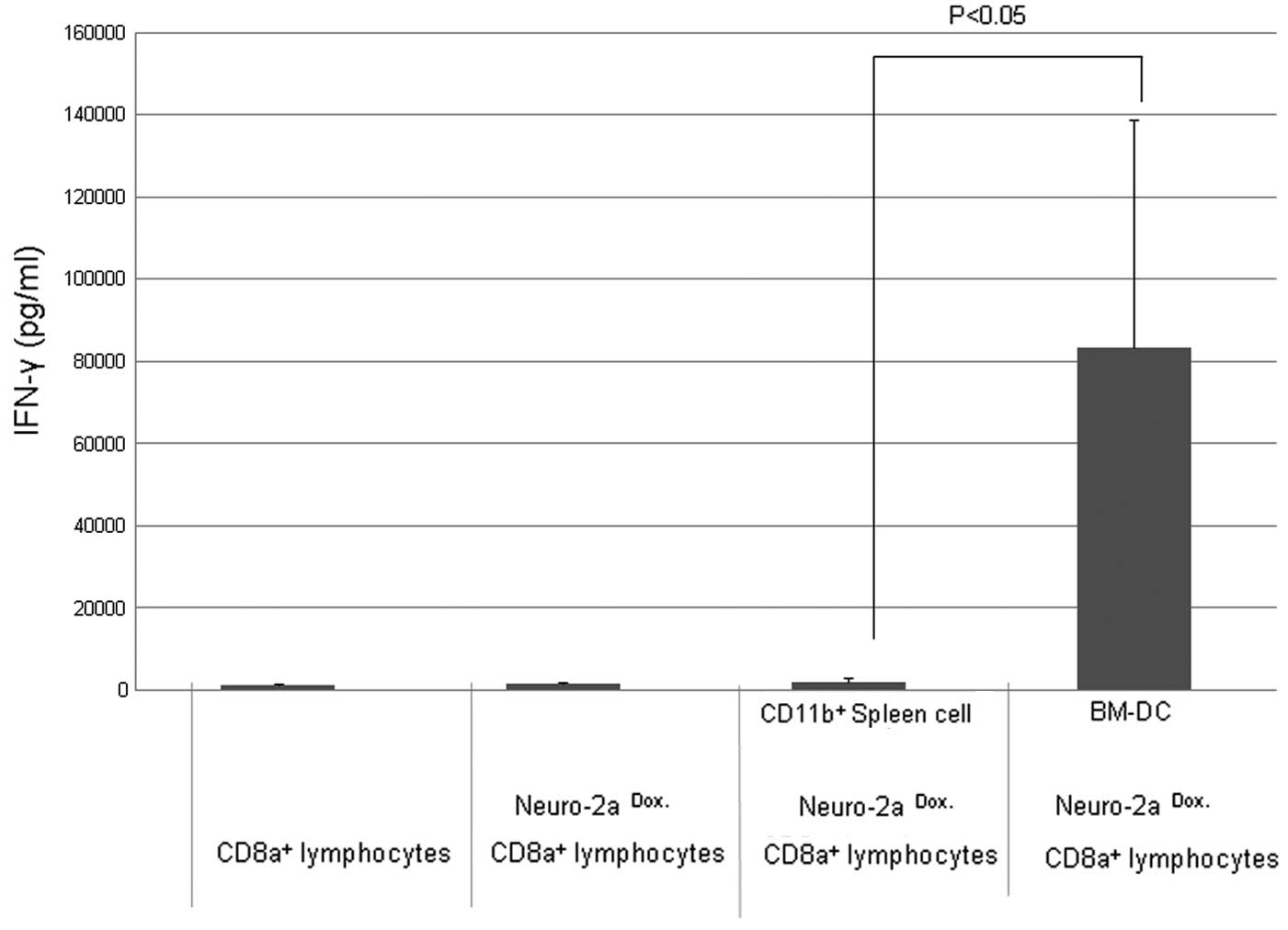
BM-DCs induce IFN-γ production more
effectively than CD11b+ spleen cells
When CD8α+ lymphocytes were cultured
using BM-DCs as the APCs, IFN-γ production was markedly increased
compared with cultures using CD11b+ spleen cells.
Enhancement by CpG-ODN was not necessary to confirm the promotion
of IFN-γ production in culture using BM-DCs (Fig. 4)
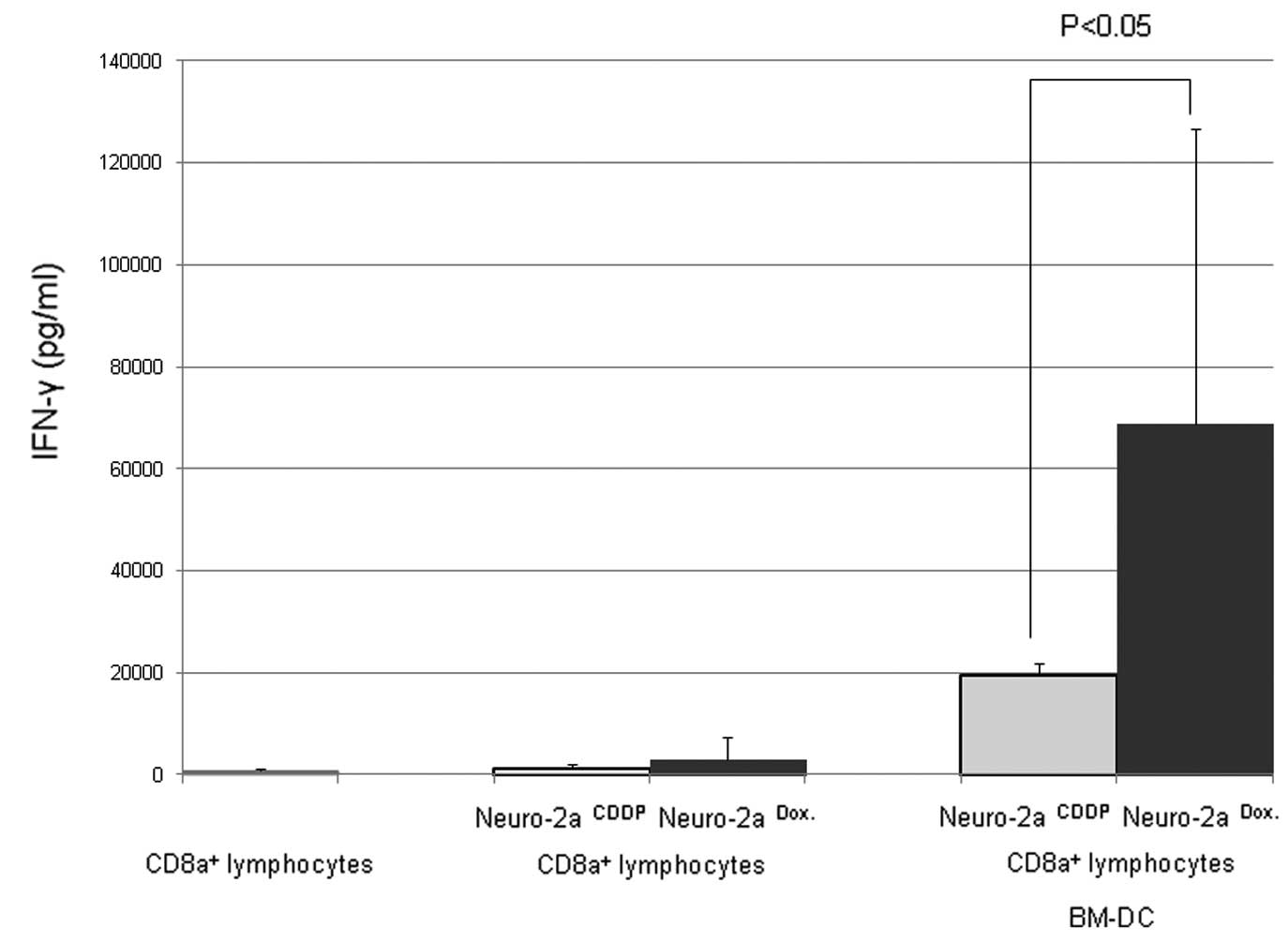
Doxorubicin effectively induces
immunogenic cell death of neuroblastoma cells compared with
CDDP
As shown in Fig. 5,
when doxorubicin-treated neuro-2a cells were co-cultured with
BM-DCs and CD8α+ lymphocytes, IFN-γ production was
increased compared with that when CDDP-treated neuro-2a cells were
used. Thus, doxorubicin was shown to be able to induce immunogenic
cell death in neuroblastoma cells. CDDP was used as a control agent
as it is unable to induce immunogenic cell death in tumor cells
(14). These results highlight the
potential advantages of doxorubicin as it not only causes tumor
cell death, but also induces antitumor lymphocyte reactions against
the neuroblastoma cells.
Discussion
Intensive multi-agent chemotherapy is the key to the
induction and consolidation of remission in advanced neuroblastoma.
However, cell-based immunotherapy is another promising approach.
With the aim of improving the prognosis of patients with advanced
neuroblastoma, this study analyzed the interaction between these
two therapeutic strategies. It was hypothesized that immunogenic
cell death and the subsequent innate cellular immune reactions are
particularly important.
Engulfment of apoptotic tumor cells by innate immune
cells, such as macrophages or DCs, induces sequential immune
reactions. These innate cellular phagocytes are considered to have
a pivotal role in the mechanism of tumor immunity (2,14–17).
In particular, BM-derived immature DCs phagocytose, mature and
later contribute to immune reactions against cancer cells (2,13,18,19).
Alternatively, tumor-associated macrophages infiltrate the tumor,
engulfing dead cells and inducing a tolerogenic reaction to tumor
cells (15,16,20).
Moreover, the induction of apoptotic tumor cell death by
chemotherapeutic agents and the subsequent immunogenic reactions
are important factors in the immunological antitumor reaction.
Using murine colon cancer (CT26) and fibrosarcoma (MCA205) models,
Obeid et al (5) reported
that anthracyclins induce immunogenic tumor cell death via CRT
exposure on the cell surface. In these models, it has been
suggested that dying cancer cells with exposed CRT are phagocytosed
by DCs, prior to a sequential antitumor immune reaction being
induced (21).
Although controversial, a previous study reported
that circulating tumor cells (CTCs) contribute to the development
of metastases in neuroblastoma (22). It was proposed that CTCs, which are
undetectable using conventional radiological and microscopic
techniques, spread systematically and are regulated by a different
molecular mechanism to that of the primary tumor lesion (23). Therefore, in the present study, it
was hypothesized that the doxorubicin-induced immunogenic cell
death of neuro-2a cells was likely to induce an immune reaction
following phagocytosis by innate cellular phagocytes. To confirm
the mechanism of action, several co-culture experiments were
performed. When CD8α+ lymphocytes were stimulated by
CD3/CD28 antibodies and co-cultured with doxorubicin-treated
neuro-2a cells and CD11b+ cells, a marked increase in
the proliferation of CD8α+ lymphocytes and IFN-γ
production was observed. These results indicate that
CD11b+ cells phagocytose dead tumor cells and induce
activation of CD8α+ cytotoxic T-lymphocyte
proliferation. Moreover, phagocytosis of apoptotic cells by BM-DCs
markedly increased IFN-γ production. This mechanism may have
contributed to the antitumor effect of doxorubicin on mouse
neuroblastoma cells.
Data from the present study indicate that
doxorubicin is capable of inducing immunogenic neuroblastoma cell
death. Previously, chemotherapy agents have been regarded as
detrimental from an immunological point of view due to their
myelosuppressive effect. However, recent studies have suggested
that chemotherapy may increase tumor immunity (4,24).
Induction of immunogenic cell death represents a key mechanism for
augmentation of tumor immunity. Results from the present ex
vivo study indicate that induction of neuroblastoma cell death
by doxorubicin may enhance survival of tumor-bearing mice in
vivo and promote proliferation of CD8α+ lymphocytes
and IFN-γ production in vitro. Since cell death of neuro-2a
cells was induced ex vivo, the immunogenic effect of
doxorubicin occurs independently of the host immune system. This
demonstrates that doxorubicin has an advantageous immunological
antitumor effect on neuroblastoma cells via the induction of
immunogenic cell death. In this mechanism, phagocytosis by BM-DCs
is considered to contribute to the rejection of neuro-2a cells.
In children with advanced neuroblastoma, initial
induction of remission by intensifying chemoradiotherapy improves
the survival rate. However, a number of children exhibit relapses
following initial treatment, which is likely due to neuroblastoma
cells acquiring resistance to the antitumor mechanism (10–12).
Novel insights into the effects of conventional therapy may improve
current therapeutic regimens, and the development of innovative
therapies for advanced neuroblastoma remains particularly
important. Analysis of the immunological effect of conventional
chemotherapeutic agents and clinical trials of antitumor
immunological therapies are likely to contribute to further
increase the survival rate of patients with advanced neuroblastoma.
In conclusion, the investigation of the immunological efficacy of
conventional antitumor agents provides useful information for
improving intensive chemotherapy and for the development of
immunological approaches for neuroblastoma therapy.
Acknowledgements
This study was supported in part by a Saitama
Medical University Internal Grant (no. 212114), a Grant-in-Aid for
Scientific Research (C) (no. 22591984) and by the Kawano Masanori
Memorial Public Interest Incorporated Foundation for Promotion of
Pediatrics.
References
|
1
|
Liu G, Wu C, Wu Y and Zhao Y: Phagocytosis
of apoptotic cells and immune regulation. Scand J Immunol. 64:1–9.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jubinsky PT, Dickens DS and Short MK: New
roles for mononuclear phagocytes in cancer biology. J Pediatr
Hematol Oncol. 30:584–591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kepp O, Tesniere A, Schlemmer F, et al:
Immunogenic cell death modalities and their impact on cancer
treatment. Apoptosis. 14:364–375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen G and Emens LA: Chemoimmunotherapy:
reengineering tumor immunity. Cancer Immunol Immunother.
62:203–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Obeid M, Tesniere A, Ghiringhelli F, et
al: Calreticulin exposure dictates the immunogenicity of cancer
cell death. Nat Med. 13:54–61. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martins I, Kepp O, Galluzzi L, et al:
Surface-exposed calreticulin in the interaction between dying cells
and phagocytes. Ann NY Acad Sci. 1209:77–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zitvogel L, Apetoh L, Ghiringhelli F and
Kroemer G: Immunological aspects of cancer chemotherapy. Nat Rev
Immunol. 8:59–73. 2008. View
Article : Google Scholar
|
|
8
|
Tufi R, Panaretakis T, Bianchi K, et al:
Reduction of endoplasmic reticulum Ca2+ levels favors
plasma membrane surface exposure of calreticulin. Cell Death
Differ. 15:274–282. 2008.
|
|
9
|
Maris JM, Hogarty MD, Bagatell R and Cohn
SL: Neuroblastoma. Lancet. 369:2106–2120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maris JM: Recent advances in
neuroblastoma. N Engl J Med. 362:2202–2211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hara J: Development of treatment
strategies for advanced neuroblastoma. Int J Clin Oncol.
17:196–203. 2012. View Article : Google Scholar
|
|
12
|
Brodeur GM: Neuroblastoma: biological
insights into a clinical enigma. Nat Rev Cancer. 3:203–216. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verneris MR and Wagner JE: Recent
developments in cell-based immune therapy for neuroblastoma. J
Neuroimmune Pharmacol. 2:134–139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martins I, Kepp O, Schlemmer F, et al:
Restoration of the immunogenicity of cisplatin-induced cancer cell
death by endoplasmic reticulum stress. Oncogene. 30:1147–1158.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ullrich E, Menard C, Flament C, et al:
Dendritic cells and innate defense against tumor cells. Cytokine
Growth Factor Rev. 19:79–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: balance, tolerance, and diversity. Curr
Opin Immunol. 22:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sica A: Role of tumour-associated
macrophages in cancer-related inflammation. Exp Oncol. 32:153–158.
2010.PubMed/NCBI
|
|
18
|
Buhtoiarov IN, Sondel PM, Eickhoff JC and
Rakhmilevich AL: Macrophages are essential for antitumour effects
against weakly immunogenic murine tumours induced by class B
CpG-oligodeoxynucleotides. Immunology. 120:412–423. 2007.
View Article : Google Scholar
|
|
19
|
Apetoh L, Ghiringhelli F, Tesniere A, et
al: Toll-like receptor 4-dependent contribution of the immune
system to anticancer chemotherapy and radiotherapy. Nat Med.
13:1050–1059. 2007. View
Article : Google Scholar
|
|
20
|
Tesniere A, Apetoh L, Ghiringhelli F, et
al: Immunogenic cancer cell death: a key-lock paradigm. Curr Opin
Immunol. 20:504–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sica A and Bronte V: Altered macrophage
differentiation and immune dysfunction in tumor development. J Clin
Invest. 117:1155–1166. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuroda T, Morikawa N, Matsuoka K, et al:
Prognostic significance of circulating tumor cells and bone marrow
micrometastasis in advanced neuroblastoma. J Pediatr Surg.
43:2182–2185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuroda T: Cellular kinetics of
neuroblastoma and the role of surgery. Pediatr Surg Int.
27:913–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Emens LA: Chemoimmunotherapy. Cancer J.
16:295–303. 2010. View Article : Google Scholar
|