Introduction
Multiple myeloma (MM) is a B-cell
lymphoproliferative disorder that remains an aggressive and
incurable disease. Despite the fact that novel targeted therapies
have significantly improved the clinical outcome of MM patients in
the frontline and recurrent settings, patients continue to
experience disease progression and relapse, which requires the
treatment to be changed (1).
Retreatment with previously employed agents may be of benefit.
Bortezomib, a first-in-class proteasome inhibitor,
has been shown to be effective in the treatment of relapsed or
refractory MM (RRMM). Bortezomib-based regimens have also
demonstrated enhanced activity, with high rates of complete
response (CR) and very good partial response (VGPR), in patients
with MM (2). In addition, a number
of studies have provided evidence that the bortezomib retreatment
of patients who have relapsed following bortezomib-containing
therapy is feasible and effective, resulting in substantial
clinical response rates (3–6).
However, the available studies to date have not specifically
addressed the optimal duration of bortezomib-based retreatment.
In a previous study, we administered a combination
of bortezomib, thalidomide and dexamethasone (VTD) to patients with
newly diagnosed MM (NDMM), and the overall response rate (ORR) was
observed to be 91% (7). The
present study concerns 20 of those patients who responded to the
VTD therapy and then presented with progressive or relapsed disease
and were retreated with bortezomib-based regimens. The results of
the retreatment and the occurrence of adverse events (AEs) were
evaluated.
Patients and methods
This study involved the retrospective analysis of 65
patients who received VTD treatment as an initial therapy for NDMM.
Of those patients, 20 who received bortezomib-based regimens as the
salvage therapy for RRMM at some point during their MM disease
course were included in the study group. The bortezomib-based
regimens included VTD (7), a
combination of bortezomib doxorubicin and dexamethasone (PAD),
bortezomib-pegylated liposomal doxorubicin-dexamethasone (PLD), VTD
plus allogeneic cytokine-induced killer cell therapy (8) and a combination of VTD with
cisplatin, doxorubicin, cyclophosphamide and etoposide (PACE). The
PAD regimen was composed of a three-week cycle of 1.3
mg/m2 bortezomib (Xian-Janssen Pharmaceutical Co., Ltd.,
Xian, Shanxi, China) on days 1, 4, 8, and 11, with 20 mg
dexamethasone (Shandong Lukang Pharmaceutical Group Co., Ltd.
Jining, Shangdong, China) on days 1–4 and 8–11 and 4.5
mg/m2 doxorubicin (Jiangsu Hansoh Pharmaceutical Co.,
Ltd., Lianyungang, Jiangsu, China) on days 1–4. The PLD regimen was
composed of 20 mg/m2 pegylated liposomal doxorubicin
(Shanghai Fudan-zhangjiang Bio-Pharmaceutical Co., Ltd., Shanghai,
China) on day 1, with bortezomib and dexamethasone at the same dose
and schedule as for the PAD regimen. The PACE regimen was composed
of 10 mg/m2 cisplatin (Jiangsu Hansoh Pharmaceutical
Co., Ltd.), 4.5 mg/m2 doxorubicin, 200 mg/day
cyclophosphamide (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang,
Jiangsu, China) and 40 mg/m2 etoposide (Jiangsu Hengrui
Medicine Co., Ltd.), all on days 1–4. It was recommended that
patients were treated with two cycles of bortezomib following a
confirmed CR or VGPR in the initial therapy (4). The main reasons for the short cycles
of the bortezomib-based therapies were to avoid AEs and to overcome
social factors such as the prohibitive cost of the treatment. All
patients provided written informed consent. The study was conducted
in accordance with the Declaration of Helsinki and with approval
from the Hospital Review Board of Wuxi People’s Hospital, Wuxi,
China.
The disease response following the initial therapy
and salvage therapy was evaluated according to the International
Myeloma Working Group criteria (9). Briefly, a CR was defined by a
negative immunofixation test result for serum and urine, <5%
plasma cells in the bone marrow and the disappearance of any
soft-tissue plasmacytoma, if present at the baseline; a VGPR was
defined as a reduction of ≥90% serum M-protein and urine M-protein
levels <100 mg/24 h; and a partial response (PR) was defined as
a ≥50% reduction of serum M-protein and reduction of 24-h M-protein
by ≥90% or to <200 mg, and a ≥50% reduction in the size of the
soft-tissue plasmacytomas. The ORR is the sum of the CR, VGPR and
PR values.
Safety was assessed throughout the study, and AEs
were graded according to the National Cancer Institute Common
Toxicity Criteria (version 3.0) (10) and reported up to 30 days after the
last dose of bortezomib.
Statistical analyses were performed using SPSS
statistical software, version 13.0 (SPSS, Inc., Chicago, IL, USA).
Time-to-event analyses were conducted using Kaplan-Meier
methodology. The duration of response (DOR) was assessed only for
patients achieving at least a PR and was calculated from the date
of the first response to the date of progression. The time to
progression (TTP) was from the date of the first administration of
bortezomib to the date of progression.
Results
Patient characteristics
The baseline demographic and clinical
characteristics of the 20 patients who received bortezomib-based
retreatment are summarized in Table
I. The median age of the patients at diagnosis was 63 years
(range, 39–72 years). There were more men (n=14, 70%) than women
and more patients identified with IgA myeloma (n=7, 35%) than with
other myeloma types in this study. The karyotype analysis of the
bone marrow of all the patients yielded normal results (data not
shown).
 | Table IBaseline demographic and clinical
patient characteristics. |
Table I
Baseline demographic and clinical
patient characteristics.
| Characteristics | Patients (n=20) |
|---|
| Median age at
diagnosis, years (range) | 63 (39–72) |
| Males, n (%) | 14 (70) |
| Myeloma type, n |
| IgG | 9 |
| IgA | 7 |
| IgM | 1 |
| Light chain | 2 |
| IgD | 1 |
| Median time from
diagnosis to bortezomib-based retreatment, months (range) | 20.5 (5–30.5) |
| Therapies prior to
bortezomib-based retreatment |
| Median number of
prior lines of therapy including bortezomib, n (range) | 4 (2–11) |
| Received in prior
regimen (other than bortezomib), n (%) |
| Thalidomide and
dexamethasone | 6 (30) |
| Melphalan and
prednisone | 6 (30) |
| Vincristine,
doxorubicin and dexamethasone (VAD) | 14 (70) |
| α-interferons | 2 (10) |
| Thalidomide | 20 (100) |
| Bone marrow
transplant | 1 (5) |
| Cyclophosphamide,
vincristine, melphalan and prednisone (COMP) | 6 (30) |
| Other | 2 (10) |
Initial VTD treatment and response
VTD treatment was used as the initial therapy in the
20 patients with MM. The study group received a median of two
cycles (range, 2–4 cycles) of bortezomib treatment. Bortezomib (1.3
mg/m2) was administered on days 1, 4, 8 and 11 of each
21-day cycle. All patients achieved a PR or better with the initial
VTD therapy; 10 patients (50%) achieved a CR, 8 (40%) achieved a
VGPR and 2 (10%) achieved a PR. The median DOR was 18.2 months
(range, 3.6–29.5 months) and the median TTP was 20 months (range,
6–32 months). Bortezomib was re-administered in the consecutive
relapses.
Interim anti-MM therapy
Between the initial VTD treatment and the
bortezomib-based retreatment, the majority of the patients received
MM-specific interim therapy. The interim therapies, as single
agents or in combination, are summarized in Table I. A number of patients received
more than one MM-specific therapy. For all the patients, the median
time between the last dose of the initial bortezomib treatment and
the first dose of an alternative antineoplastic therapy was 15
months (range, 4–25 months). The median time between the initial
treatment and the retreatment with bortezomib (regardless of the
interim therapies) was 20.5 months (range, 5–30.5 months).
Bortezomib-based retreatment
The patients received a median of two therapies
(range, 0–9 therapies) prior to the bortezomib-based retreatment
(Table I) and a median of two
cycles of bortezomib (range, 1–4 cycles) as the retreatment; 60%
received two cycles. For the retreatment, 40% of the patients
received the VTD regimen and 40% of the patients received the PAD
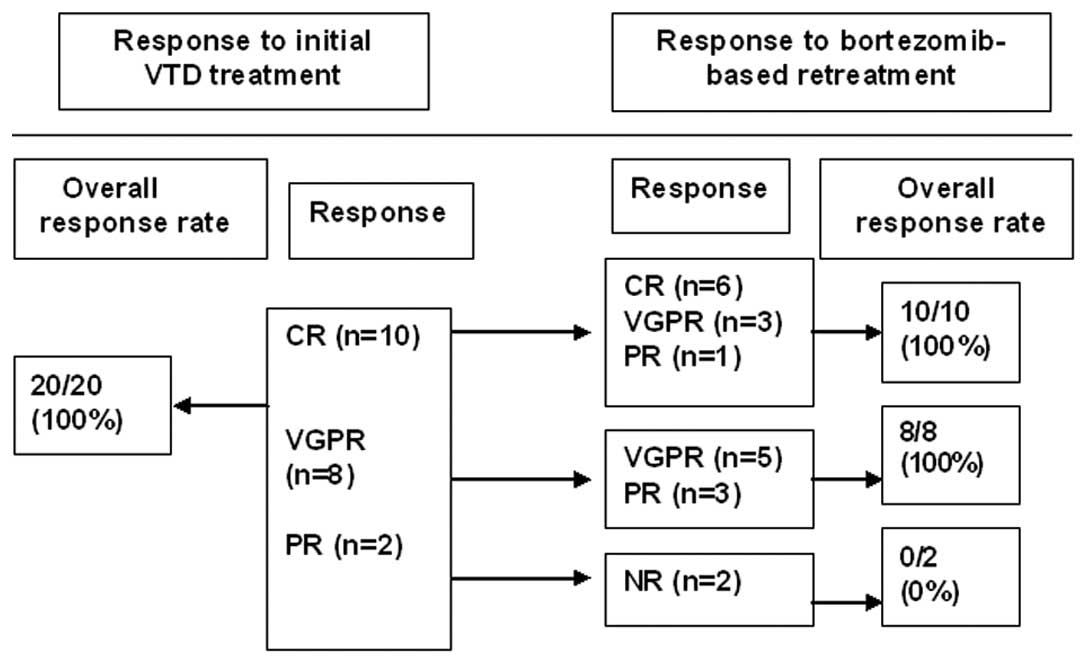
regimen. The ORR to the bortezomib-based retreatment was 90%. Six
(30%), eight (40%) and four (20%) patients achieved a CR, VGPR and
PR, respectively. The association between the response to the
initial VTD therapy and the response to the bortezomib-based
retreatment is shown in Fig. 1. Of
the 10 patients who achieved a CR during the initial VTD treatment,
six experienced a repeat CR during the retreatment. Five of eight
patients with an initial VGPR had a repeat VGPR, while the other
three patients had a PR with the bortezomib-based retreatment. Of
the two patients who achieved a PR during the initial VTD
treatment, neither of them responded to the bortezomib-based
retreatment. The median DOR to the bortezomib-based retreatment was
6.6 months (range, 0–11 months) and the median TTP was 8.7 months
(range, 1–12.5 months).
Patient 1 was treated four times with bortezomib,
with a 23.5-month break between the first and second, a 11.5-month
interval between the second and third, and a 12-month break between
the third and fourth therapy courses. The first, third and fourth
treatments resulted in CRs which lasted 21.5, 10 and 7 months,
respectively, and the second treatment resulted in a PR which
lasted 11 months. Patient 2 was treated three times with
bortezomib, with a 25-month break between the first and second and
a 21-month interval between the second and third therapy courses.
The initial treatment resulted in a CR which lasted 14.7 months,
the second treatment resulted in a VGPR which lasted 8 months and
the third treatment resulted in a PR which lasted 6 months.
Fig. 2 shows the M protein levels
(IgA normal levels, 69–382 mg/dl; IgM normal levels, 63–277 mg/dl)
in relation to the events during the treatment of patient 1 and
2.
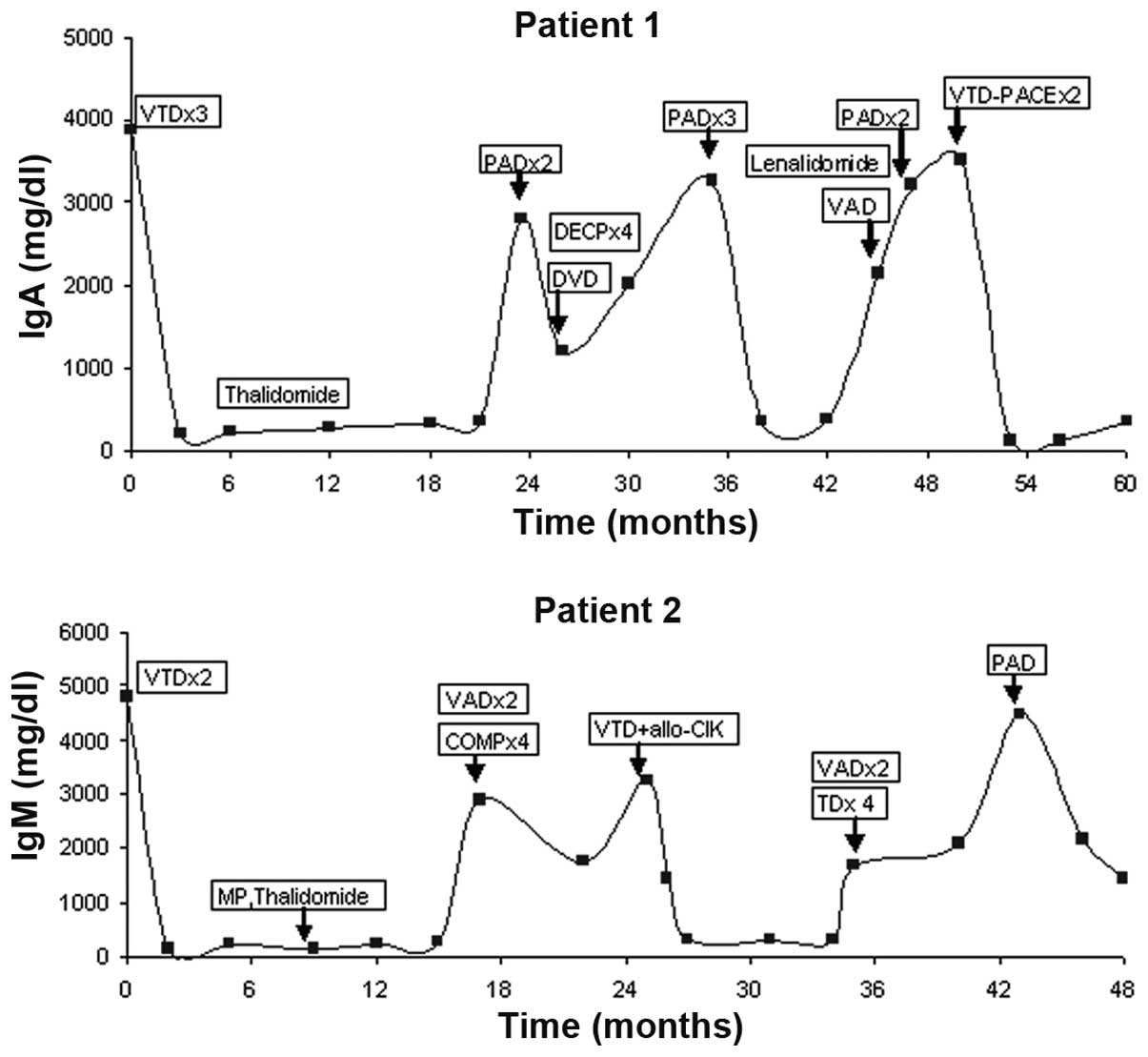 | Figure 2Diagram showing the M protein levels
in relation to the events during the treatment of two of the
patients. VTD, bortezomib, thalidomide and dexamethasone; PAD,
bortezomib, doxorubicin and dexamethasone; DVD, doxorubicin,
vincristine and dexamethasone; DECP, dexamethasone, etoposide,
cyclophosphamide and cisplatin; VAD, vincristine, adriamycin and
dexamethasone; PACE, cisplatin, doxorubicin, cyclophosphamide and
etoposide; MP, melphalan and prednisone; COMP, cyclophosphamide,
vincristine, melphalan and prednisone; allo-CIK, allogenic
cytokine-induced killer; TD, thalidomide and dexamethasone. |
Safety and tolerability
The AEs during the bortezomib-based retreatment are
shown in Table II. During the
retreatment, the most common AEs were thrombocytopenia and
neutropenia. The majority of the AEs were grade I or II.
Interruption of the therapy was required in three patients;
discontinuation was due to pulmonary infection, diarrhea and
neutropenia. Two patients passed away during the retreatment
period: One succumbed due to sepsis and one due to disease
progression. All mortalities were considered to not be associated
with bortezomib.
 | Table IIAdverse events during the
bortezomib-based retreatment. |
Table II
Adverse events during the
bortezomib-based retreatment.
| Adverse event | Total patients | Severity (grade) |
|---|
|
|---|
| I | II | III | IV |
|---|
| Diarrhea | 2 | 1 | | 1 | 0 |
| Thrombocytopenia | 10 | 4 | 4 | 2 | 0 |
| Neutropenia | 8 | 2 | 4 | 2 | 0 |
| Weakness | 2 | 2 | 0 | 0 | 0 |
| Pulmonary
infection | 1 | 0 | 0 | 1 | 0 |
| Peripheral
neuropathy | 2 | 1 | 1 | 0 | 0 |
| Herpes zoster | 2 | 1 | 1 | 0 | 0 |
Discussion
Over the past 10 years, the introduction of novel
agents such as thalidomide, bortezomib and lenalidomide has
markedly changed the treatment of patients with NDMM or RRMM
(11). Although the single agent
activity of these compounds has been reported in MM, their major
impact in the management of the disease is observed through
combination regimens. Preclinical studies have demonstrated that
bortezomib enhances the antiproliferative and proapoptotic activity
of cytotoxic agents such as melphalan and doxorubicin against
myeloma cells (12). Preclinical
observations have also identified synergistic anti-myeloma activity
of bortezomib when combined with an immunomodulatory drug (13). The results of a number of clinical
trials have shown that bortezomib-based regimens are an effective
treatment against NDMM and RRMM (14). Briefly, bortezomib-based regimens
show potential for increasing the depth (CR/VGPR) and durability of
responses, overwhelming possible resistance and improving
survival.
In patients with RRMM, there is an urgent
requirement to optimize treatment regimens to extend the duration
of survival. The duration of the first remission and the timing of
the relapse are key determinants for the treatment strategy at the
relapse. The National Comprehensive Cancer Network clinical
practice guidelines state that patients with MM who are not
refractory to the initial therapy (relapse >6 months after
completion of the previous therapy) may be retreated with the same
regimen (15). Bortezomib is a
weak substrate for multi-drug resistance efflux pumps and has the
potential to avoid resistance. In a phase III VISTA trial, it was
shown that patients relapsing following bortezomib-based therapy
were not intrinsically more resistant to subsequent therapies
compared with those relapsing following traditional chemotherapy
with melphalan and prednisone, and they were successfully treated
with subsequent bortezomib-based therapies (16,17).
The effectiveness of the retreatment with bortezomib may be a
result of certain patients experiencing a clinical response to the
initial treatment and therefore remaining sensitive to the
bortezomib-based retreatment. It is also possible that the addition
of other agents to the bortezomib-based retreatment may have
contributed to the responses observed.
The present retrospective case series represents the
first review of bortezomib-based retreatment exclusively in the
Chinese clinical practice setting, in patients who had responded to
VTD treatment for the initial therapy of NDMM. The results
presented demonstrate the efficacy of short-course bortezomib-based
retreatment, with an ORR of 90%. The response to the
bortezomib-based retreatment was most notable in the patients who
had exhibited a good response to the initial VTD therapy.
Crucially, in one of the heavily pretreated relapsed MM patients, a
triple response (PR, VGCR and CR) following the bortezomib-based
retreatment was achieved, and in another patient a double response
(VGPR and PR) was achieved. These findings suggest that the
treatment-free interval of >6 months following the initial
bortezomib therapy was associated with a superior response rate and
may be predictive of the efficacy of bortezomib-based retreatment.
Although the number of patients studied is small, the data indicate
a trend towards a greater sensitivity to short-course
bortezomib-based retreatment than during initial treatment in
patients who had responded to VTD treatment for the initial therapy
of NDMM, even in heavily pre-treated patients.
The toxicities reported in the present study were
manageable and generally predictable. It is possible to manage the
hematological toxicity of the treatment using dose modifications
and/or growth factor support. Severe myelosuppression was uncommon.
Grade III or IV neutropenia, related febrile neutropenia and sepsis
were rare. The thrombocytopenia observed was transient and not
associated with serious bleeding complications. The
treatment-induced peripheral neuropathy (PN) was lower during the
bortezomib-based retreatment. The short duration of the therapy and
the dose modifications in the study may have minimized the
cumulative PN arising from the bortezomib-based retreatment. These
findings suggest that retreatment with short-course
bortezomib-based combination regimens may be a well-tolerated and
effective therapeutic option for patients who respond to the
initial VTD therapy and that it is possible to re-use bortezomib in
subsequent lines of therapy without resistance.
The responses to the bortezomib-based retreatment in
the present study were rapid, with 60% of responders achieving
their best response by the end of cycle two. However, the
bortezomib-based retreatment was associated with a shorter DOR and
TTP than the initial VTD treatment was, which is commonly observed
with subsequent lines of therapy (3–5,16)
due to the progressive disease course. The higher CR rates in the
VISTA and phase II studies may be due to the prolonged courses of
bortezomib-based retreatment improving the quality of the response
in a proportion of the patients (3–5,16).
However, prolonged therapy may be associated with continued
toxicity in certain patients, as well as the inconvenience of
having to attend frequent hospital appointments for a long period
of time. Therefore, the findings of the present study may indicate
highly active, tolerable and convenient treatment options with a
reduced treatment burden for certain patients, particularly for
elderly patients.
Clearly, the higher response rate is a significant
observation. However, this study has limitations due to the small
sample size and retrospective design; a longer follow-up and
prospective clinical trials are required to validate these initial
observations. This study is likely to contribute to the
identification of the optimal sequence of treatments for individual
patients while balancing efficacy and toxicity.
References
|
1
|
Kumar SK, Rajkumar SV, Dispenzieri A, et
al: Improved survival in multiple myeloma and the impact of novel
therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moreau P, Richardson PG, Cavo M, Orlowski
RZ, San Miguel JF, Palumbo A and Harousseau JL: Proteasome
inhibitors in multiple myeloma: 10 years later. Blood. 120:947–959.
2012.PubMed/NCBI
|
|
3
|
Hrusovsky I, Emmerich B, von Rohr A, et
al: Bortezomib retreatment in relapsed multiple myeloma - results
from a retrospective multicentre survey in Germany and Switzerland.
Oncology. 79:247–254. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sood R, Carloss H, Kerr R, et al:
Retreatment with bortezomib alone or in combination for patients
with multiple myeloma following an initial response to bortezomib.
Am J Hematol. 84:657–660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolf J, Richardson PG, Schuster M, LeBlanc
A, Walters IB and Battleman DS: Utility of bortezomib retreatment
in relapsed or refractory multiple myeloma patients: a multicenter
case series. Clin Adv Hematol Oncol. 6:755–760. 2008.PubMed/NCBI
|
|
6
|
Conner TM, Doan QD, Walters IB, LeBlanc AL
and Beveridge RA: An observational, retrospective analysis of
retreatment with bortezomib for multiple myeloma. Clin Lymphoma
Myeloma. 8:140–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen Y, Zhou X, Wang Z, et al: Coagulation
profiles and thromboembolic events of bortezomib plus thalidomide
and dexamethasone therapy in newly diagnosed multiple myeloma. Leuk
Res. 35:147–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou X, Zhu J, Sun H, Shao L, Xu M and Guo
H: Family haploidentical donor-derived cytokine-induced killer cell
biotherapy combined with bortezomib in two patients with relapsed
multiple myeloma in a non-allogeneic transplant setting. Leuk
Lymphoma. 54:209–211. 2013. View Article : Google Scholar
|
|
9
|
Durie BG, Harousseau JL, Miguel JS, et al;
International Myeloma Working Group. International uniform response
criteria for multiple myeloma. Leukemia. 20:1467–1473. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Cancer Institute Cancer Therapy
Evaluation Program. Common Toxicity Criteria version 3.0.
http://ctep.cancer.gov/forms/ctc2nom-4-30-99-final3.pdfuri.
Accessed September 2008
|
|
11
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar
|
|
12
|
Ma MH, Yang HH, Parker K, et al: The
proteasome inhibitor PS-341 markedly enhances sensitivity of
multiple myeloma tumor cells to chemotherapeutic agents. Clin
Cancer Res. 9:1136–1144. 2003.PubMed/NCBI
|
|
13
|
Richardson PG, Mitsiades C, Ghobrial I and
Anderson K: Beyond single-agent bortezomib: combination regimens in
relapsed multiple myeloma. Curr Opin Oncol. 18:598–608. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kapoor P, Ramakrishnan V and Rajkumar SV:
Bortezomib combination therapy in multiple myeloma. Semin Hematol.
49:228–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anderson KC, Alsina M, Bensinger W, et al:
NCCN clinical practice guidelines in oncology: multiple myeloma. J
Natl Compr Canc Netw. 7:908–942. 2009.PubMed/NCBI
|
|
16
|
Mateos MV, Richardson PG, Schlag R, et al:
Bortezomib plus melphalan and prednisone compared with melphalan
and prednisone in previously untreated multiple myeloma: updated
follow-up and impact of subsequent therapy in the phase III VISTA
trial. J Clin Oncol. 28:2259–2266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mohty B, El-Cheikh J, Yakoub-Agha I,
Avet-Loiseau H, Moreau P and Mohty M: Treatment strategies in
relapsed and refractory multiple myeloma: a focus on drug
sequencing and ‘retreatment’ approaches in the era of novel agents.
Leukemia. 26:73–85. 2012.
|