Introduction
The use of an inhaled corticosteroid (ICS) and
long-acting β2-agonist (LABA) combination inhaler is recommended by
the Global Initiative for Asthma and most other asthma treatment
guidelines as the first choice to control chronic asthma for
patients in whom control with ICS monotherapy is difficult
(1–4). Budesonide/formoterol (BUD/FM)
inhalation aerosol is an ICS and LABA combination, which is
administered twice daily via one hydrofluoroalkane-pressurized
metered-dose inhaler and has been approved for use in many
countries for the long-term maintenance treatment of persistent
asthma (2–4). With regard to safety, while the
short-term safety in ICS (5–7) and
ICS/LABA (8–10) has been evaluated, the long-term
safety has yet to be fully investigated (11,12),
particularly in elderly patients with bronchial asthma. In the
present retrospective study, an evaluation of the 1-, 6- and
12-month safety of BUD/FM inhalation for elderly asthmatic patients
was performed, and the changes in serum potassium levels and pulse
rate were observed.
Patients and methods
Patients
Clinicopathological data for all the patients with
bronchial asthma were obtained by retrospective review from the
database at the University of Tsukuba, Mito Medical Center, Mito
Kyodo General Hospital (Mito, Japan). The consecutive patients who
were diagnosed with bronchial asthma and treated with BUD/FM
inhalation aerosol (two inhalations of 160/4.5 mg, twice daily;
Symbicort Turbuhaler; AstraZeneca, Osaka, Japan) between February
2010 and January 2012 at the hospital, were entered in this
study.
In order to evaluate the 1-year safety of BUD/FM
inhalation, a medical chart review of the patients up to January
2013 was performed. Demographic data, including age, gender and
comorbid diseases were retrieved from the patient medical records.
This retrospective study conformed to the Ethical Guidelines for
Clinical Studies issued by the Ministry of Health, Labor and
Welfare of Japan.
Study population
The patient population was divided into three age
groups: The <65 years group, the 65–74 years group and the ≥75
years group. The demographic data and safety of BUD/FM inhalation
therapy were compared among the three age groups. Adverse effects
were counted as they occurred during the study period. Blood
samples were obtained from the patients in order to measure the
serum potassium levels, and pulse rates were measured at
pretreatment and 1, 6 and 12 months following the initiation of the
BUD/FM inhalation therapy.
Statistical analysis
The serum potassium levels and pulse rate prior to
treatment and at 1, 6 and 12 months after treatment were compared
using Wilcoxon rank sum test. To compare the different groups of
patients, the chi-square test was also used. All statistical
analysis were performed using SPSS software, version 10.1 for
Windows (SPSS Inc., Chicago, IL, USA) and P<0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
Table I shows
patient characteristics. A total of 350 patients with bronchial
asthma were treated with BUD/FM inhalation during the study period.
There were 189 males and the median age was 60 years (range: 17–93
years). In total, 207 (59.2%) patients were aged <65 years, 68
(19.4%) patients were 65–74 years old and 75 (21.4%) patients were
≥75 years old (Table I). Among the
350 patients, 160 (45.7%) patients had comorbid diseases, including
cardiovascular diseases in 74 patients (54 patients had
hypertension, 12 had chronic heart failure, six had arrhythmia and
five had ischemic heart disease). Fifty-six patients (74.7%) aged
≥75 years had one or more comorbid diseases, and 64.7% and 24.1% of
patients aged 65–74 years and <65 years had one or more comorbid
diseases, respectively. There was a significant difference in the
incidence of one or more comorbid diseases between patients aged
≥65 years and those aged <65 years (P=0.001; chi-square test).
However, there was no difference in the incidence of comorbid
diseases between patients aged ≥65–74 years and those aged <75
years (P=0.603; chi-square test).
 | Table ICharacteristics of 350 patients with
bronchial asthma. |
Table I
Characteristics of 350 patients with
bronchial asthma.
| Variable | Data |
|---|
| Age (years) | 60 (17–93)a |
| ≤65 | 207 (59.2%) |
| 65–74 | 68 (19.4%) |
| ≥75 | 75 (21.4%) |
| Gender |
| Male | 189 |
| Female | 161 |
| Comorbid
diseases |
| Present | 160 (45.7%) |
| Cardiovascular
diseases | 74 |
| Hypertension | 54 |
| Chronic heart
failure | 12 |
| Arrhythmia | 6 |
| Ischemic heart
disease | 5 |
| Others | 4 |
| Respiratory
diseases | 43 |
| COPD | 26 |
| Pneumonia | 5 |
| Others | 8 |
| Metabolic
diseases | 43 |
| Diabetes | 29 |
| Thyroid disease | 5 |
| Others | 9 |
| Renal and urologic
diseases | 14 |
| Autoimmune
diseases | 9 |
| Psychiatric
diseases | 8 |
| Malignant
diseases | 7 |
| Other diseases | 14 |
| Other
controllers |
| Leukotriene
antagonists | 41 |
| Xanthines | 19 |
| Oral steroids | 2 |
Treatment of bronchial asthma
An effective control of bronchial asthma was
obtained, so BUD/FM inhalation therapy in 141 (40.3%) of the 350
patients was terminated or changed to ICS inhalation within 3
months. Similarly, in 95 (27.1%) patients, the inhalation therapy
was terminated within 3–12 months. Therefore, the inhalation
therapy was continued for >12 months in 114 (32.6%)
patients.
Safety of BUD/FM inhalation
Adverse events
Four (1.1%) patients exhibited hoarseness, and three
of them were aged ≥75 years. Two (0.6%) patients developed a tremor
and both were <75 years of age. Two (0.6%) patients had oral
candidiasis and arrhythmia, and each of them was ≥75 years of age.
All adverse events were transient and disappeared shortly after the
termination of the inhalation therapy.
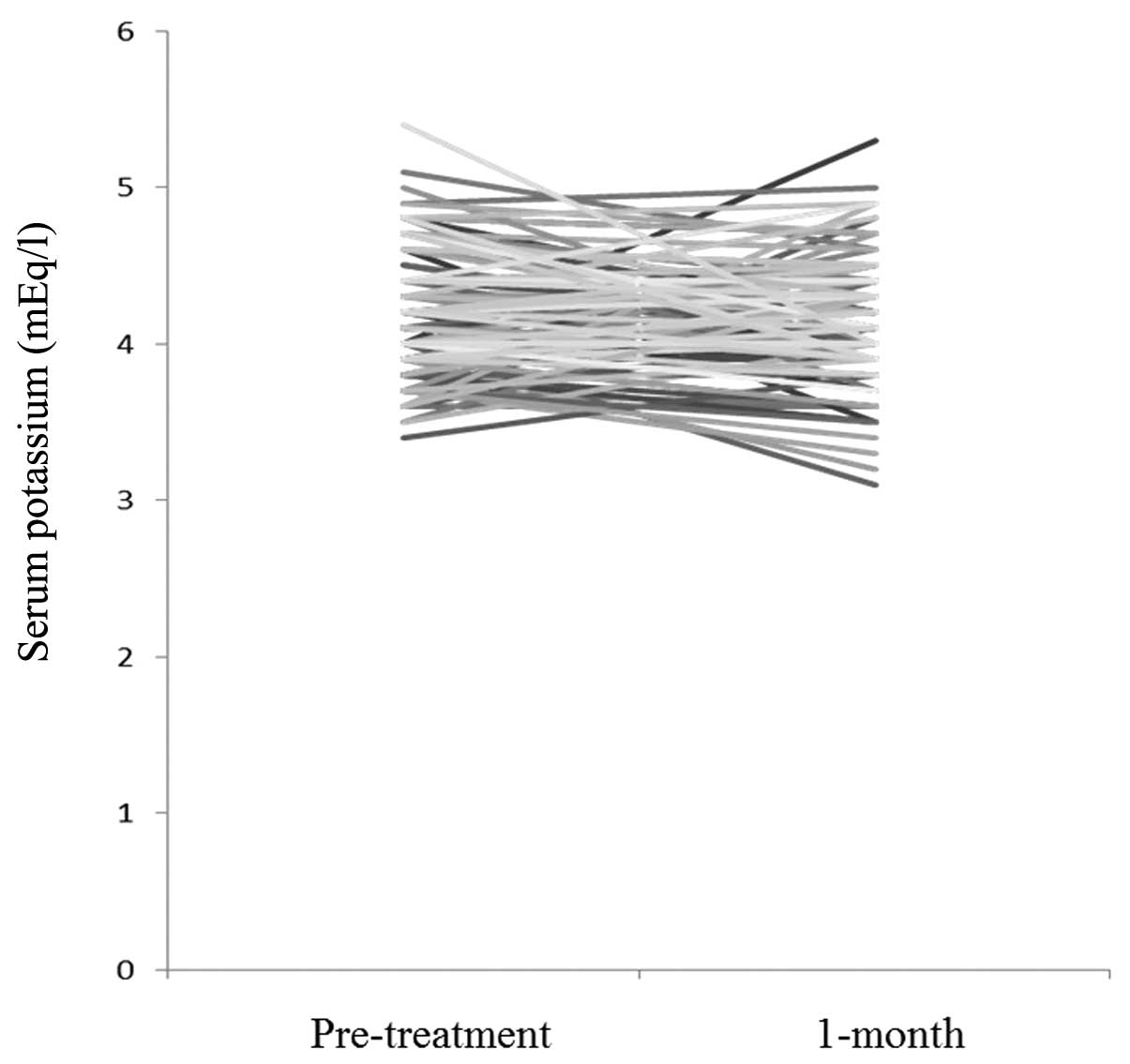
Serum potassium
Fig. 1 shows the
changes in serum potassium levels between pretreatment and 1 month
after the initiation of BUD/FM therapy. There was no statistical
difference between them (P=0.567). As shown in Table II, serum potassium levels at 6 and
12 months were not different from the levels before treatment
(P=0.941 and P=0.822, respectively). In the 65–74 years group and
the ≥75 years group, serum potassium levels at 1, 6 and 12 months
was not different from the levels before treatment. The changes in
serum potassium levels in patients with cardiovascular diseases,
and those ≥75 years with comorbid diseases were also examined.
There was no statistical differences between serum potassium levels
at these intervals (Table
II).
 | Table IIChange in serum potassium levels prior
to and following BUD/FM inhalation. |
Table II
Change in serum potassium levels prior
to and following BUD/FM inhalation.
| Patients | Pretreatment K level
(median, range; mEq/l) | Post-treatment K
level (median, range; mEq/l) | P-value |
|---|
| All patients |
| 1-month
interval | 4.1, 3.4–5.4 | 4.2, 3.1–5.3 | 0.567 |
| 6-month
interval | 4.1, 3.4–5.0 | 4.1, 3.5–5.1 | 0.941 |
| 12-month
interval | 4.2, 3.4–5.4 | 4.2, 3.5–5.2 | 0.822 |
| Patients aged ≥65
years |
| 1-month
interval | 4.2, 3.5–5.4 | 4.2, 3.6–4.9 | 0.286 |
| 6-month
interval | 4.2, 3.5–5.0 | 4.1, 3.6–5.1 | 0.457 |
| 12-month
interval | 4.2, 3.4–5.4 | 4.3, 3.5–5.2 | 0.989 |
| Patients aged ≥75
years |
| 1-month
interval | 4.2, 3.6–5.4 | 4.2, 3.6–4.9 | 0.691 |
| 6-month
interval | 4.1, 3.6–5.0 | 4.1, 3.6–4.8 | 0.909 |
| 12-month
interval | 4.1, 3.4–5.4 | 4.3, 3.5–5.0 | 0.472 |
| Patients with
cardiovascular diseases |
| 1-month
interval | 4.1, 3.4–4.9 | 4.3, 3.2–5.0 | 0.680 |
| 6-month
interval | 4.1, 3.5–5.0 | 4.3, 3.6–5.1 | 0.345 |
| 12-month
interval | 4.1, 3.0–5.0 | 4.2, 3.6–5.0 | 0.731 |
| Patients ≥65 years
with cardiovascular diseases |
| 1-month
interval | 4.2, 3.7–4.9 | 4.3, 3.5–4.9 | 0.967 |
| 6-month
interval | 4.3, 3.7–5.0 | 4.1, 3.6–5.1 | 0.795 |
| 12-month
interval | 4.3, 3.7–5.0 | 4.3, 3.7–5.0 | 0.948 |
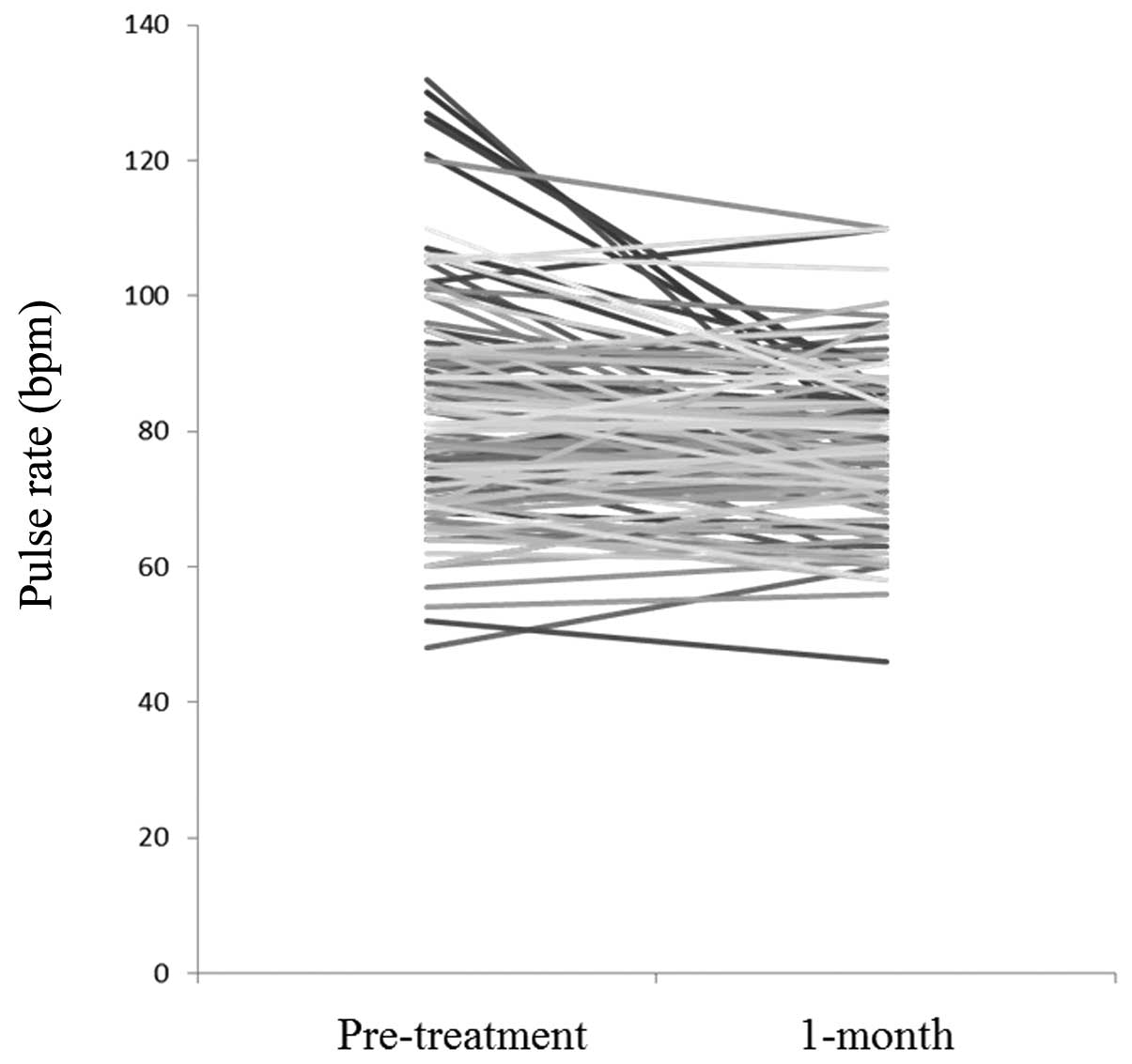
Pulse rate
Fig. 2 shows
changes in pulse rate between pretreatment and 1 month after the
initiation of BUD/FM therapy. The pulse rate 1 month after the
initiation of BUD/FM therapy was significantly lower than the pulse
rate prior to treatment (P=0.001). In the ≥75 years group, the
pulse rate was also decreased at the 6-month interval (P=0.046);
these results may be due to the achievement of control of bronchial
asthma. However, there was no statistically significant difference
in pulse rate between any other age groups or intervals (Table III). The change in pulse rate in
asthmatic patients with cardiovascular diseases and those ≥75 years
with comorbid diseases were also examined, and no statistical
differences between any age groups and any intervals were
identified (Table III).
 | Table IIIChange in pulse rates prior to and
following BUD/FM inhalation. |
Table III
Change in pulse rates prior to and
following BUD/FM inhalation.
| Patients | Pretreatment pulse
rare (median, range; bpm) | Post-treatment pulse
rate (median, range; bpm) | P-value |
|---|
| All patients |
| 1-month
interval | 80, 48–132 | 77, 46–110 | 0.001 |
| 6-month
interval | 81, 48–120 | 80, 52–120 | 0.081 |
| 12-month
interval | 80, 48–120 | 77, 50–109 | 0.050 |
| Patients aged ≥65
years |
| 1-month
interval | 78, 54–110 | 77, 56–110 | 0.210 |
| 6-month
interval | 81, 54–106 | 76, 56–105 | 0.163 |
| 12-month
interval | 80, 54–106 | 76, 56–109 | 0.288 |
| Patients aged ≥75
years |
| 1-month
interval | 81, 60–110 | 78, 58–110 | 0.093 |
| 6-month
interval | 81, 60–106 | 76, 56–105 | 0.046 |
| 12-month
interval | 81, 60–106 | 77, 56–109 | 0.128 |
| Patients with
cardiovascular diseases |
| 1-month
interval | 80, 52–120 | 79, 46–110 | 0.140 |
| 6-month
interval | 80, 54–120 | 80, 56–120 | 0.915 |
| 12-month
interval | 78, 60–120 | 77, 60–92 | 0.762 |
| Patients aged ≥65
years with cardiovascular diseases |
| 1-month
interval | 81, 54–102 | 80, 56–97 | 0.325 |
| 6-month
interval | 81, 54–102 | 80, 56–98 | 0.726 |
| 12-month
interval | 80, 54–102 | 76, 58–92 | 0.807 |
Discussion
BUD, a potent and safe ICS with a high affinity for
glucocorticoid receptors, is approved in many countries for the
treatment of asthma (2–4). The addition of inhaled LABA provides
a more effective means of improving lung function and asthma
control than increasing the dose of ICS in patients whose asthma is
not adequately controlled. FM is unique amongst LABAs as it has a
fast onset of action and a long duration of effect (2,3). BUD
and FM, as individual components and in combination, possess
well-defined efficacy as well as pharmacological and safety
profiles when administered through a single metered-dose inhaler
(4,8–10).
In the present study, an evaluation of safety over 12 months of the
usual dose of BUD/FM was performed, and the serum potassium levels
and pulse rate were examined in elderly patients with bronchial
asthma.
There has been increasing interest in the treatment
of elderly patients with bronchial asthma (13). However, clinical information
regarding asthmatic patients aged ≥75 years has been scarcely
available as such patients are not usually included in clinical
trials and retrospective care analysis. Therefore, there is
scientific uncertainty regarding the risks and benefits of
treatment with ICS/LABA in asthmatic patients aged ≥75 years. To
assess the short-term efficacy and safety of the treatment in the
elderly, we previously reviewed our clinical data from consecutive
patients with bronchial asthma treated with BUD/FM (14). Even with the existence of comorbid
diseases in the elderly, BUD/FM was effective with no high
incidences of adverse events, such as hoarseness, tremor,
arrhythmia and oral candidiasis (14). The incidences observed were similar
to those reported in previous studies in young adults and
middle-aged patients (8–10).
Previous studies have investigated the 1-year safety
of the usual dosage of FM (5–7) and
BUD/FM inhalation for asthma patients (8–10).
In these studies, the mean age of the patients evaluated was ~30–40
years (8–10). Rosenhall et al evaluated the
1-year safety of BUD/FM (8), and
Maspero et al examined mometasone furoate/FM (9), and they concluded that both
treatments were safe and well tolerated in patients with persistent
asthma (8,9). However, in these studies, there was
no investigation into the changes in serum potassium levels and
pulse rate. With regard to the evaluation of these parameters,
Hinkle et al studied heart rate in pediatric subjects with
stable asthma (15), and
Malolepszy et al examined serum potassium levels and heart
rate in high-dose FM in patients with acute bronchial obstruction
(16). Recently, Saito and
Hasunuma studied the short-term safety of high dose BUD/FM in
asthmatic patients, whose mean age was 44.3 years (11). In their study, >10% of patients
showed palpitation, tachycardia and decreased serum potassium
levels in a 2.5-fold higher dose of BUD/FM than the usual dose of
therapy (11). In the present
study, the median age of the patients was 60 years, and 40.8% and
21.4% of them were ≥65 and ≥75 years of age, respectively. Aging is
associated with a high prevalence of comorbid diseases (13). Elderly patients are predisposed to
comorbid diseases, such as diabetes, cardiovascular and
cerebrovascular diseases. In the present study, the ≥65 years group
had a higher proportion of patients with comorbid diseases than
those <65 years of age. However, there were neither significant
reductions in serum potassium levels nor changes in pulse rate in
the three age groups, including those with comorbid diseases.
The results of the present study revealed novel
findings; however, there were a number of limitations in this
study. This was a small-sized retrospective study in a single
institution. Serum potassium levels and pulse rate were not
evaluated in every patient and a quality of life analysis was not
performed in this study. Therefore, we consider that conclusive
outcomes were not derived from this study. However, the methodology
used, which was based on an audit of the information from clinical
practice documented in the patient records, may provide some
clinical information that was not available from clinical trials.
Additionally, reporting the treatment experiences in patients with
bronchial asthma, including those of elderly patients with some
comorbid diseases, may be of clinical significance.
In conclusion, the usual dosage of BUD/FM (two
inhalations of 160/4.5 mg, twice daily) showed no adverse effects
on the serum potassium levels and pulse rate in Japanese adults,
including the elderly with persistent asthma.
References
|
1
|
Global Initiative for Asthma (GINA).
Global Strategy for Asthma Management and Prevention NHLBI/WHO
Workshop Report. National Heart, Lung, and Blood Institute,
National Institutes of Health; 2010
|
|
2
|
Edwards SJ, von Maltzahn R, Naya IP and
Harrison T: Budesonide/formoterol for maintenance and reliever
therapy of asthma: a meta analysis of randomised controlled trials.
Int J Clin Pract. 64:619–627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Korenblat PE and Rosenwasser LJ:
Budesonide/formoterol pressurized metered-dose inhaler for patients
with persistent asthma. Allergy Asthma Proc. 31:190–202. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McCormack PL and Lyseng-Williamson KA:
Budesonide/formoterol: a review of its use as maintenance and
reliever inhalation therapy in asthma. Drugs. 67:2407–2431. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Donohue JF, Hanania NA, Fogarty C,
Campbell SC, Rinehart M and Denis-Mize K: Long-term safety of
nebulized formoterol: results of a twelve-month open-label clinical
trial. Ther Adv Respir Dis. 2:199–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Randell J, Saarinen A, Walamies M,
Vahteristo M, Silvasti M and Lähelmä S: Safety of formoterol after
cumulative dosing via Easyhaler and Aerolizer. Respir Med.
99:1485–1493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hamilos DL, D’Urzo A, Levy RJ, et al:
Long-term safety study of levalbuterol administered via
metered-dose inhaler in patients with asthma. Ann Allergy Asthma
Immunol. 99:540–548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenhall L, Elvstrand A, Tilling B, et
al: One-year safety and efficacy of budesonide/formoterol in a
single inhaler (Symbicort Turbuhaler) for the treatment of asthma.
Respir Med. 97:702–708. 2003.PubMed/NCBI
|
|
9
|
Maspero JF, Nolte H and Chérrez-Ojeda I;
P04139 Study Group. Long-term safety of mometasone
furoate/formoterol combination for treatment of patients with
persistent asthma. J Asthma. 47:1106–1115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peters SP, Prenner BM, Mezzanotte WS,
Martin P and O’Brien CD: Long-term safety and asthma control with
budesonide/formoterol versus budesonide pressurized metered-dose
inhaler in asthma patients. Allergy Asthma Proc. 29:499–516. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saito T and Hasunuma T: Safety and
tolerability of high-dose budesonide/formoterol via
Turbuhaler® in Japanese patients with asthma: a
randomized, double-blind, crossover, active comparator-controlled,
phase III study. Clin Drug Investig. 32:51–61. 2012.PubMed/NCBI
|
|
12
|
Noonan M, Leflein J, Corren J and
Staudinger H: Long-term safety of mometasone furoate administered
via a dry powder inhaler in children: Results of an open-label
study comparing mometasone furoate with beclomethasone dipropionate
in children with persistent asthma. BMC Pediatr. 9:432009.
View Article : Google Scholar
|
|
13
|
Reed CE: Asthma in the elderly: diagnosis
and management. J Allergy Clin Immunol. 126:681–687. 2010.
View Article : Google Scholar
|
|
14
|
Kagohashi K, Kurishima K, Satoh H, et al:
Safety and efficacy of budesonide/formoterol in a single inhaler
(Symbicort Turbuhaler) for the treatment of asthma. J New Remedies
and Clinics. 59:2087–2092. 2010.(In Japanese).
|
|
15
|
Hinkle J, Hinson J, Kerwin E, et al: A
cumulative dose, safety and tolerability study of arformoterol in
pediatric subjects with stable asthma. Pediatr Pulmonol.
46:761–769. 2011. View Article : Google Scholar
|
|
16
|
Malolepszy J, Böszörményi Nagy G, Selroos
O, Larsso P and Brander R: Safety of formoterol Turbuhaler at
cumulative dose of 90 microg in patients with acute bronchial
obstruction. Eur Respir J. 18:928–934. 2001. View Article : Google Scholar : PubMed/NCBI
|