Introduction
Ischemic heart disease (IHD), which leads to
myocardial infarction (MI), is a major clinical problem. Numerous
patients continue to present with angina and myocardial ischemia
(1). A pathological study has
shown that ischemic apoptosis plays a critical role in acute MI
(2) and apoptosis and necrosis
have been reported to be associated with this clinical condition
(3). In cardiac myocytes in
vitro, the inhibition of NADPH oxidase reduces apoptosis
(4). In previous years, studies
have found that a number of traditional plants and their extracts
have antioxidative effects on IHD (5,6).
IHD is the principal etiology for the development of
congestive heart failure. Sustained ischemia causes several types
of damage to cardiac tissues, including cardiomyocyte apoptosis
(7). Heart failure following MI is
a common clinical problem and has a poor prognosis. It has been
demonstrated that free radicals are involved in the pathogenesis of
heart failure, subsequent to MI. Changes in myocardial antioxidant
levels, as well as oxidative stress, have been observed in the
surviving myocardium of rats subjected to MI (8,9). The
early stages of MI are accompanied by a significant increase in
oxidative stress and lipid peroxidation, with significant
reductions in glutathione (GSH), catalase (CATA) and superoxide
dismutase (SOD) levels. The mechanism of action of Bcl-2 in the
prevention of apoptosis has also been hypothesized to be mediated
by an antioxidant pathway. Reactive oxygen species (ROS) have been
implicated in myocardial hypertrophy, apoptosis, contractile
dysfunction and fibrosis (3).
Traditional Chinese medicine has been shown to have
specific prospective therapeutic effects on IHD. Guanxin Shutong
capsule (GXSTC) is a widely used Chinese medicinal formula, which
is clinically administered for palpitations, restlessness, short
breath, fatigue, dizziness and chest pain, promoting blood
circulation, removing blood stasis (9) and protecting against cardiovascular
diseases (10,11). GXSTC contains five traditional
Chinese drugs: Choerospondiatis, Salvia miltiorrhiza, lilac,
borneol and tabasheer. Studies have indicated that lilac possesses
antioxidative properties (10) and
choerospondiatis and Salvia miltiorrhiza exhibit
anti-ischemic therapeutic effects (12), as well as the ability to scavenge
active oxygen free radicals (13).
Experimental and clinical studies have indicated
that GXSTC has various cardiovascular pharmacological effects.
However, there is little information available concerning the
antioxidative mechanisms of GXSTC. Therefore, the purpose of the
present study was to evaluate whether orally administered CXSTC
protects the heart against oxidative stress and apoptosis in rats
with MI.
Materials and methods
Surgical preparation of the animals
Experimental procedures and protocols on animals
were approved by the Ethics Committee of Xi’an Jiaotong University
for Animal Research (Xi’an, China). Male Wistar rats, weighing
290–310 g (purchased from school of medicine, Xi’an Jiaotong
University, Xi’an, China), were anesthetized by the intraperitoneal
injection of 35 mg/kg pentobarbital sodium. Following intratracheal
intubation, a left thoracotomy was performed under
volume-controlled mechanical ventilation. The heart was raised from
the thorax and a ligature with a 6-0 Prolene suture was placed
around the proximal left anterior descending coronary artery. The
chest was then closed. The same surgical procedures were performed
in sham-operated rats, with the exception that the suture around
the coronary artery was not tied. Samples were collected from the
marginal region around the infarction as samples of infarcted
sites.
GXSTC consists of five traditional Chinese drugs:
57.1% choerospondiatis, 28.6% Salvia miltiorrhiza, 7.1%
lilac, 3.6% borneol and 3.6% tabasheer. GXSTC was provided by
Buchang Pharmaceuticals (batch no. 20120125; Xi’an, China).
Following removal of the capsules, the Guanxin Shutong powder was
dissolved in 40 mg/ml aseptic sodium carboxymethylcellulose
(CMC-Na; 0.5%). Within 24 h after modeling, the solution was
administered intragastrically each day at 0.2 g/kg GXSTC for the
high dosage group (GXSTCH), 0.1 g/kg GXSTC for the low dosage group
(GXSTCL) and 10 ml/kg CMC-Na for the sham surgery and MI + vehicle
groups (8 rats for each group). After 6 weeks of treatment, 24-h
urine samples and blood were harvested. Following decapitation, the
hearts were removed, snap-frozen in liquid nitrogen and stored at
−80°C until required for processing for protein or mRNA
extraction.
Determination of the infarct size
The heart tissue was washed with PBS three times.
Sections were sliced and then incubated for 10 min in
nitrotetrazolium blue chloride for pathological examination.
Photographs were captured and the infarct size was measured with
Image-Pro Plus software (MediaCybernetics, Inc., Rockville, MD,
USA). Ischemic and left ventricular areas were determined in five
slices of each heart tissue sample. The ischemic risk area ratio
was defined as a percentage and calculated as follows: Total
ischemic area/total left ventricular area × 100.
Antioxidant assays
To determine the total SOD and CATA activities, as
well as the malondialdehyde (MDA) and GSH levels, blood was sampled
from the abdominal aorta and serum was obtained following
centrifugation at 3,000 × g for 10 min. The MDA and GSH levels, and
SOD and CATA activities, were measured spectrophotometrically using
diagnostic kits (Jiancheng Bioengineering Institute, Nanjing,
China), according to the manufacturer’s instructions.
Determination of serum creatine
kinase-isoenzyme (CK-MB), serum lactate dehydrogenase (LDH) and
serum glutamate oxaloacetic transaminase (SGOT)
Blood was sampled from the abdominal aorta and serum
was obtained by centrifugation at 3,000 × g for 10 min. CK-MB, LDH
and SGOT levels were determined spectrophotometrically at 660, 450
and 510 nm, respectively, using diagnostic kits (Jiancheng
Bioengineering Institute), according to the manufacturer’s
instructions.
Determination of heart SOD, GOT, nitric
oxide (NO) and NO synthase (NOS)
Heart tissue was homogenized in ice-cold 0.9% saline
solution and centrifuged at 600 × g for 10 min. The supernatant was
used to determine SOD, GOT, NO and NOS levels, which were measured
spectrophotometrically using diagnostic kits (Jiancheng
Bioengineering Institute) according to the manufacturer’s
instructions.
mRNA expression of NADPH oxidase
subunits
The mRNA expression levels of p22phox, p47phox,
gp91phox and p67phox in the renal cortical tissues were
quantitatively analyzed by reverse transcription polymerase chain
reaction (RT-PCR), as described previously (14,15).
Primer sequences for the analysis of p22phox, p47phox, p67phox,
gp91phox and GAPDH mRNA are shown in Table I. GAPDH was measured as an
invariant housekeeping gene for an internal control. Amplified cDNA
bands were detected by Goldview staining (RuiTaibio, Beijing,
China) and the quantities of mRNA were evaluated using the Gene
Genius imaging system (Syngene, Cambridge, UK).
 | Table IPrimers used for p22phox, p47phox,
p67phox, gp91phox and GAPDH. |
Table I
Primers used for p22phox, p47phox,
p67phox, gp91phox and GAPDH.
| mRNA | Primer | Sequence, 5′-3′ |
|---|
| p22phox | Sense |
ATGGAGCGGTGTGGACAGAAG |
| Antisense |
CGGACAGCAGTAAGTGGAGGAC |
| p47phox | Sense |
CCATCATCCTTCAGACCTATCG |
| Antisense |
AACCACCAGCCACTCTCG |
| p67phox | Sense |
CGTGTGTTGTTTGGCTTTGTG |
| Antisense |
CTGAGGCTGCGACTGAGG |
| gp91phox | Sense |
TAGCATCCATATCCGCATTG |
| Antisense |
CTAACATCACCACCTCATAGC |
| GAPDH | Sense |
AGTGGCAAAGTGGAGATT |
| Antisense |
GTGGAGTCATACTGGAACA |
Determination of Bcl-2, Bax, caspase-3,
gp91phox and p47phox protein expression
Rabbit monoclonal antibodies against Bcl-2, Bax,
p47phox (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
gp91phox and caspase-3 (Epitomics, Burlingame, CA, USA), were used
to determine the protein expression levels of gp91phox, p47phox,
Bcl-2 and Bax by western blot analysis, as described previously
(16,17).
Statistical analysis
All data are expressed as mean ± SD. Statistical
analysis was performed by Student’s t-test or one-way ANOVA,
followed by a Tukey’s multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of GXSTC on infarct size
The ischemic risk area ratio was 33.93±6.56% in the
MI + vehicle group. Treatment with GXSTC at doses of 0.1 and 0.2
g/kg body weight resulted in dose-dependent reductions in infarct
size, with ischemic risk area ratios of 23.45±4.67 and 17.82±5.89%,
respectively. Significant differences were observed in the ratios
between the MI + vehicle and GXSTCL and GXSTCH groups (Table II).
 | Table IIEffect of GXSTC on the myocardial
infarct size following ligature of the left anterior descending
coronary artery. |
Table II
Effect of GXSTC on the myocardial
infarct size following ligature of the left anterior descending
coronary artery.
| Group | Infarct size,
cm2 | Left ventricular
areas, cm2 | Infarct-to-left
ventricular areas, % |
|---|
| MI + vehicle | 1.33±0.29 | 2.59±0.19 | 33.93±6.56 |
| Sham | 0 | 3.13±0.21 | 0 |
| GXSTCL | 0.87±0.09 | 2.84±0.13 | 23.45±4.67a |
| GXSTCH | 0.59±0.06 | 2.72±0.26 | 17.82±5.89b |
Antioxidant assays
The serum SOD, GSH and CATA levels of the MI +
vehicle group were significantly decreased (1,560.90±130.02,
149.80±18.95 and 15.18±1.16 U/ml, respectively), while the serum
MDA level was significantly increased (9.8 ± 0.93 nmol/ml) compared
with the corresponding levels in the sham-operated rats (Fig. 1). The GXSTCL and GXSTCH groups
showed significantly reduced serum levels of MDA (6.86±0.58 and
4.59±0.15 nmol/ml, respectively). By contrast, the levels of SOD,
GSH and CATA were increased (to 2,110.8.56±190.94 and
2,300.92±180.49 U/ml; 180.58±18.77 and 298.37±16.54 mg/l; and
28.34±2.19 and 32.35±2.56 U/ml, respectively) (P<0.05; Fig. 1).
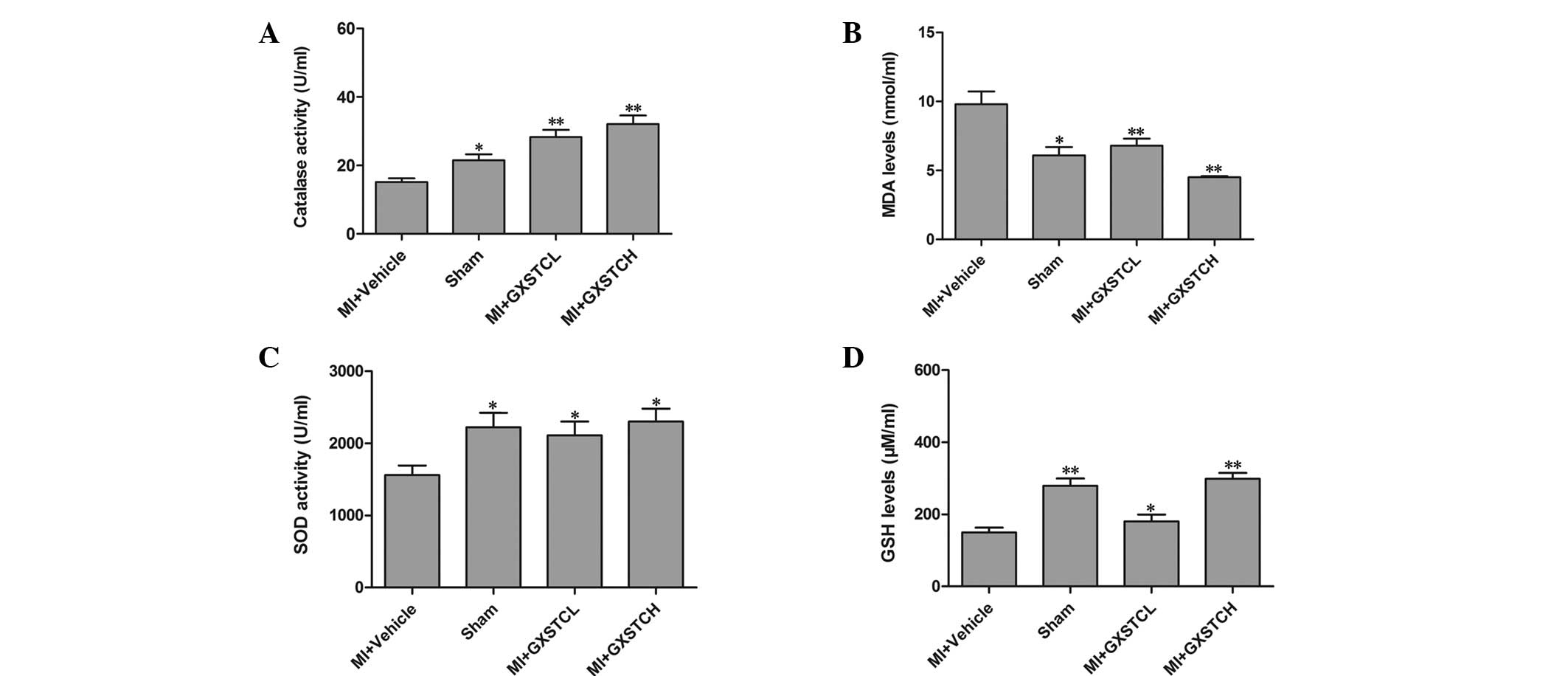 | Figure 1Effect of GXSTC on the serum levels of
(A) CATA, (B) MDA, (C) SOD and (D) GSH. Sham (sham-operated
control; n=12), MI + vehicle (orally administered vehicle; n=12),
MI + GXSTCH (orally administered 0.2 g/kg GXSTC; n=12) and MI +
GXSTCL (orally administered 0.1 g/kg GXSTC; n=12).
*P<0.05 and **P<0.01, vs. MI + vehicle.
GXSTC, Guanxin Shutong capsule; CATA, catalase; MDA,
malondialdehyde; SOD, superoxide dismutase; GSH, glutathione; MI,
myocardial infarction. |
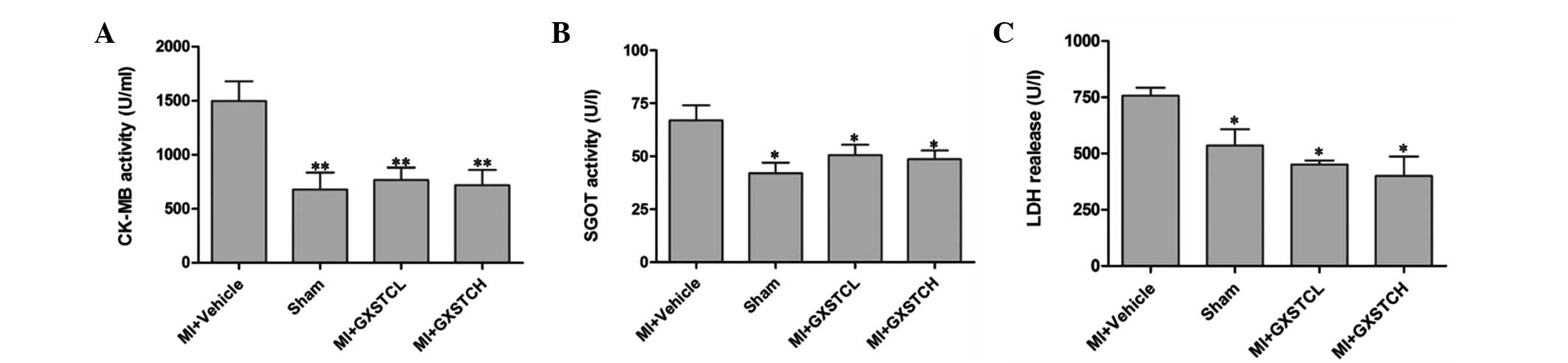
Effect of GXSTC on the serum levels of
CK-MB, LDH and GOT
The serum levels of CK-MB, LDH and GOT in the MI +
vehicle group were increased significantly (1,499.43±180.30,
756.97±35.64 and 66.99±7.06 U/ml, respectively) compared with those
in the sham group (Fig. 2). GXSTC
at doses of 0.1 and 0.2 g/kg body weight significantly reduced the
serum level of CK-MB to 767.85±113.0 and 719.51±140.11 U/ml,
respectively, and of LDH to 451.20±18.18 and 400.48±86.24 U/ml,
respectively (P<0.05; Fig. 2).
In addition, GXSTC at both doses significantly decreased SGOT
activity.
Effect of GXSTC on the levels of SOD,
GOT, NO and NOS in the heart
The SOD, NO and NOS levels in the hearts of the MI +
vehicle group were significantly decreased whereas the levels of
GOT were significantly increased compared with those in the
sham-operated rats. In the CXSTCH group, the levels of SOD, NOS and
NO (192.81 ± 5.91 and 0.58 ± 0.03 U/mg protein and 11.55 ± 1.05
μg/mg protein, respectively) were significantly increased compared
with those in the MI + vehicle group (90.74 ± 2.08 and 0.27 ± 0.01
U/mg protein and 5.84 ± 1.31 μg/mg protein, respectively), whereas
the levels of GOT were significantly decreased (from 668.99 ± 70.06
to 480.85 ±47.11 U/mg protein) (P<0.05, Fig. 3). In addition, the GXSTCL group
also showed significantly decreased levels of GOT and increased
levels of SOD, NO and NOS in the heart (Fig. 3).
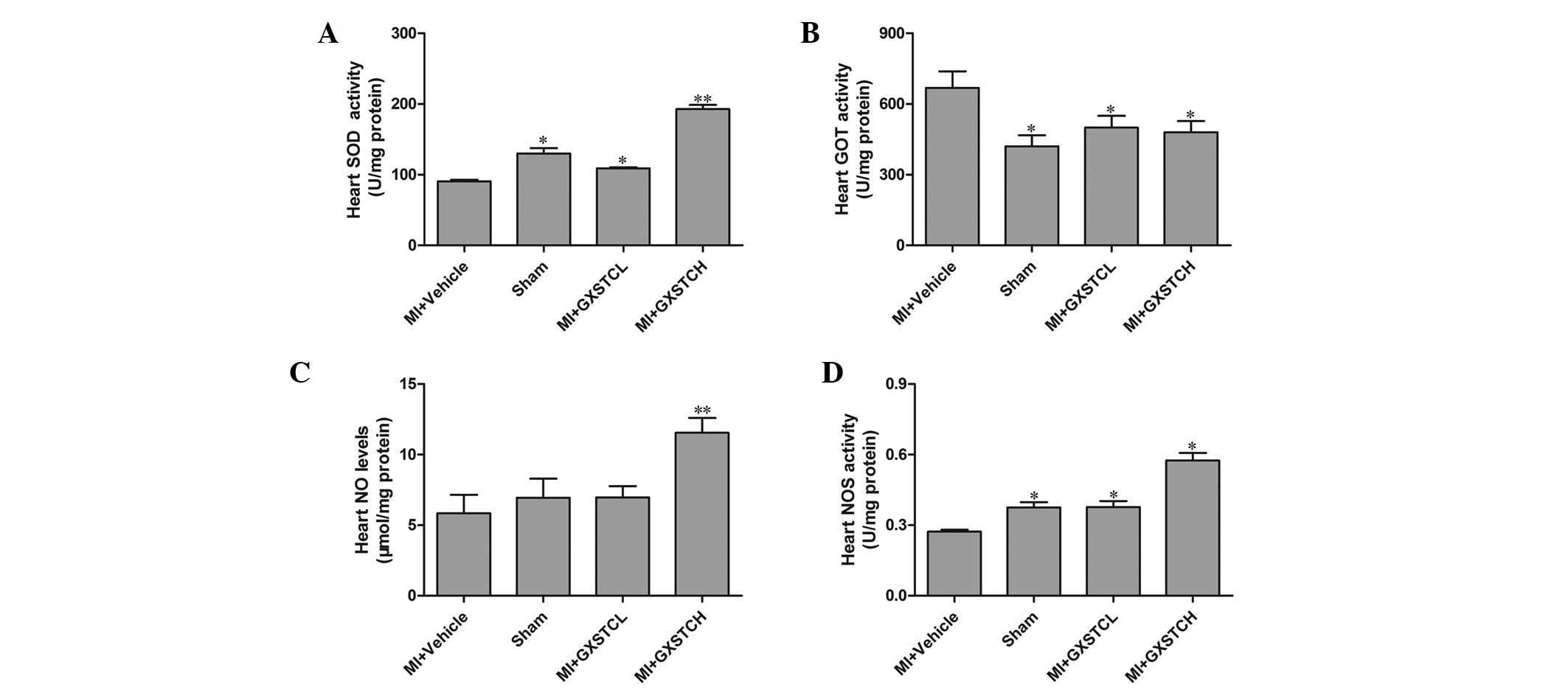 | Figure 3Effect of GXSTC on the levels of (A)
SOD, (B) GOT, (C) NO and (D) NOS in the heart. Sham (sham-operated
control; n=12), MI + vehicle (orally administered vehicle; n=12),
MI + GXSTCH (orally administered 0.2 g/kg GXSTC; n=12) and MI +
GXSTCL (orally administered 0.1 g/kg GXSTC; n=12).
*P<0.05 and **P<0.01, vs. MI + vehicle.
GXSTC, Guanxin Shutong capsule; SOD, superoxide dismutase; GOT,
glutamate oxaloacetic transaminase; NO, nitric oxide; NOS, nitric
oxide synthase; MI, myocardial infarction. |
Effect of GXSTC on the mRNA expression of
NADPH oxidase subunits
MI model rats showed significantly higher p22phox,
p47phox, p67phox and gp91phox mRNA expression levels compared with
sham-operated rats. Treatment with GXSTC (0.1 and 0.2 g/kg/day)
significantly decreased p22phox, p47phox, p67phox and gp91phox mRNA
expression (Fig. 4).
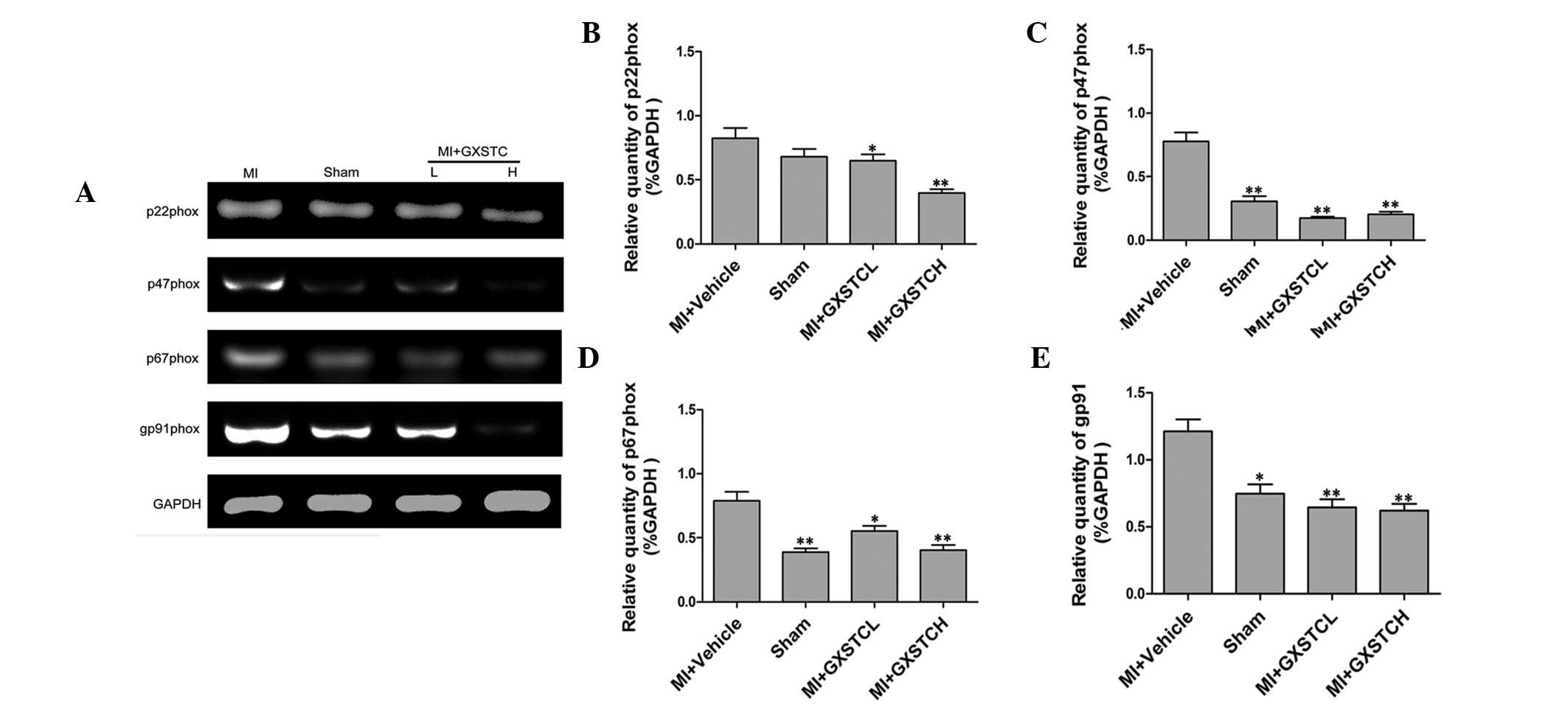 | Figure 4Effect of GXSTC on p22phox, p47phox,
p67phox and gp91phox mRNA expression in the heart. (A) Bands
correspond to p47phox, p22phox, p67phox, gp91phox and GAPDH.
Results of (B) p22phox, (C) p47phox, (D) p67phox and (E) gp91phox
were quantified by densitometry analysis of the bands from (A) and
then normalized against GAPDH in the heart tissue. Sham
(sham-operated control; n =12), MI + vehicle (orally administered
vehicle; n=12), MI + GXSTCH (orally administered 0.2 g/kg GXSTC;
n=12) and MI + GXSTCL (orally 0.1 g/kg administered GXSTC; n=12).
*P<0.05 and **P<0.01, vs. MI + vehicle.
GXSTC, Guanxin Shutong capsule; MI, myocardial infarction. |
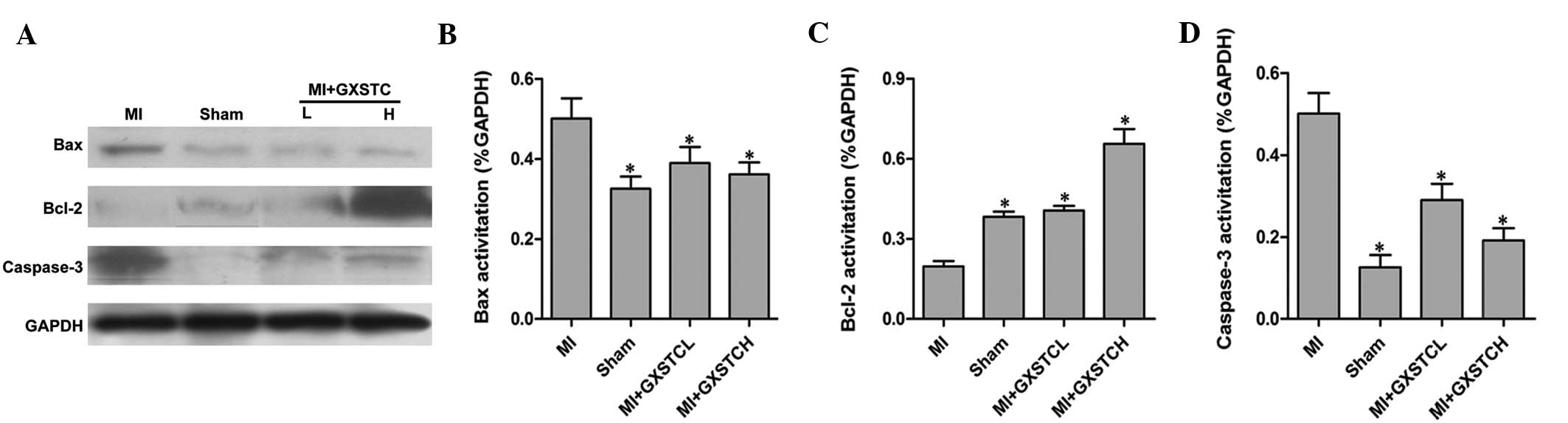
Effect of GXSTC on Bcl-2, Bax and
caspase-3 proteins
The expression of the apoptosis-related proteins
Bcl-2, Bax and caspase-3 was studied by western blot analysis.
Following the induction of MI, the expression of Bax and caspase-3
markedly increased, whereas the expression of Bcl-2 decreased
(Fig. 5). The MI-induced changes
in protein levels were significantly reversed by GXSTC. These
results indicated that GXSTC may modulate MI-induced apoptosis via
Bcl-2, Bax and caspase-3 proteins (Fig. 5).
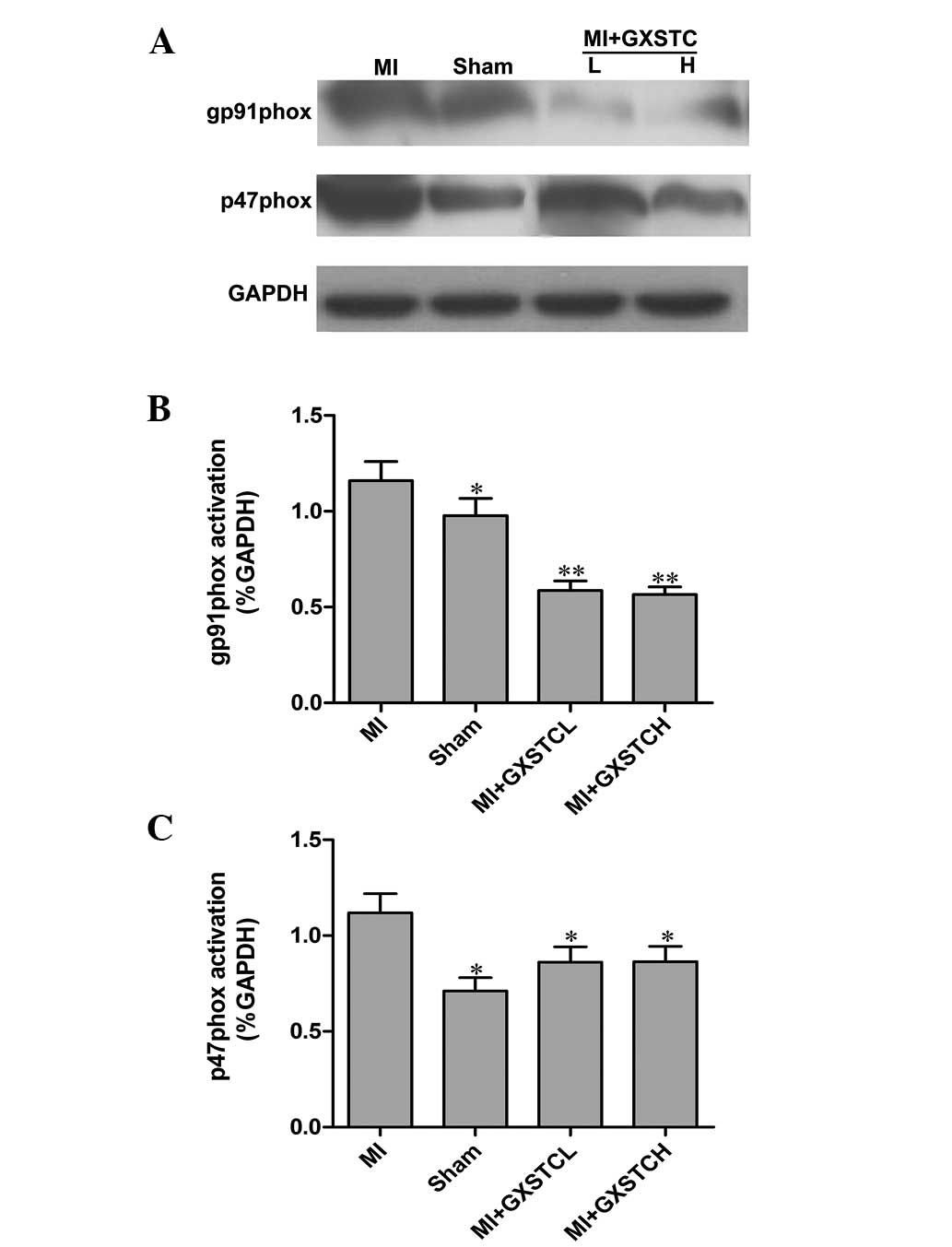
Effect of GXSTC on p47phox and gp91phox
proteins
Western blot analysis was performed on heart
homogenates to determine the protein expression levels of the NADPH
oxidase subunits gp91phox and p47phox. As shown in Fig. 6, the protein levels of gp91phox and
p47phox decreased significantly in the hearts of rats with MI
following treatment with GXSTC.
Discussion
GXSTC has been reported to be effective in treating
MI in animal models and cardiovascular diseases in humans (18). Results of the present study show
that GXSTC dose-dependently reduced the size of the infarct caused
by ischemic injuries in rats, which is the most reliable indicator
of myocardial protection. In addition, it was demonstrated that
GXSTC effectively reduced CK-MB, LHD, MDA and SGOT levels in rats
with MI, as well as the protein and mRNA expression of NADPH
oxidase subunits, but enhanced CATA and SOD activities and GSH
levels. Furthermore, protein expression of proapoptotic Bax and
caspase-3 were significantly downregulated, while the antiapoptotic
protein, Bcl-2, was upregulated in the GXSTC treated groups when
compared with the MI + vehicle group.
LDH and CK, which are released into plasma from
myocardial tissues, are representative of cardiac cellular damage
during MI (19). In the present
study, GXSTC significantly increased the serum levels of SOD,
indicating that GXSTC possesses a potent antioxidant property. In
addition, it was observed that the level of LDH, a biochemical
indicator of cellular damage, was also dose-dependently decreased.
Previous studies have shown that acute myocardial ischemia
generates numerous free radicals, causing damage to cellular
membranes as a result of lipid peroxidation. MDA, formed by the
breakdown of lipid peroxides, is often used to quantify the extent
of lipid peroxidation (20),
whereas serum CK-MB is a cardiac-specific marker of acute MI or an
indicator for myocardial tissue injury. The present study
demonstrated that GXSTC significantly prevented an increase of
serum MDA and CK-MB levels caused by acute ischemic injury,
indicating that GXSTC may exert its protective effect against
myocardial ischemic injury by reducing lipid peroxidation.
Choerospondiatis and Salvia miltiorrhiza contain
polyphenolic compounds with antioxidant properties, which are
considered to provide the pharmacological efficacy against heart
ischemia (21). The mechanisms of
action of choerospondiatis and Salvia miltiorrhiza are
distinct and complementary when combined together. Therefore, GXSTC
may be synergistic and more effective than the individual
components.
MDA, the degradation product of oxygen-derived free
radicals and lipid oxidation, reflects the damage caused by ROS
(22). Studies on the antioxidant
system have shown that changes in SOD activity and MDA levels are
always negatively correlated (23). In the present study, GXSTC also
increased the serum levels of SOD when administered at doses of 0.1
and 0.2 g/kg/day. However, MDA production was low which implied
that the formula may affect the level of endogenous antioxidants
and/or oxidative stress. One possible explanation is that the
elevated SOD activities resulted in the scavenging of excessive ROS
and attenuated lipid peroxidation. GSH is an important cellular
reductant that offers protection against free radicals, peroxides
and toxic compounds, which is reformed from glutathione disulfide
(24). The results showed that
administration of GXSTC caused a significant increase in GSH
levels, which may be due to the compounds present in GXSTC.
Previous animal studies and clinical observations
have indicated that the balance between antiapoptotic Bcl-2 and
proapoptotic Bax and caspase-3 proteins plays a major role in
regulating apoptosis (25,26). Overexpression of Bax and caspase-3
in cells leads to apoptotic death in response to apoptosis signals.
In contrast, overexpression of Bcl-2 inhibits apoptosis and
decreases the ROS formation and lipid peroxidation initiated by
various stimuli (26). In
pathological conditions, including MI, diabetes and stroke, the
production of free radicals may override the scavenging effects of
antioxidants, leading to oxidative stress (27). Thus, targeting myocardial apoptosis
is a reasonable therapeutic strategy for reducing the risk of
ischemic injury. It had been indicated that Bcl-2 inhibits the
apoptosis induced by ROS through an antioxidative pathway (25). Analysis of the expression and
regulation of Bcl-2 family proteins may provide insight as to their
role in ischemia-induced apoptosis and stress adaptation. The
present study observed that GXSTC treatment increased Bcl-2 protein
expression during MI, as determined by western blot analysis.
Systematic reviews have shown that oxidative stress
may powerfully induce programmed cell death. ROS are implicated in
myocardial hypertrophy, apoptosis, contractile dysfunction and
fibrosis (28). Increases in NADPH
oxidase activity, oxidative stress and myocyte apoptosis have been
observed concurrently in failing hearts (29). In cardiac myocytes, the in
vitro inhibition of NADPH oxidase reduces apoptosis. NADPH
oxidase subunits, gp91phox, p22phox, p40phox, p47phox, p67phox and
rac1, have been found to be expressed in endothelial cells,
vascular smooth muscle cells, cardiomyocytes and fibroblasts
(30). The increased expression of
NADPH oxidase subunits, gp91phox and p22phox, has been associated
with lipid peroxidation levels following acute MI in rats (31). The present study shows that GXSTC
is able to reduce the increased protein and mRNA expression of
NADPH oxidase subunits and oxidative stress associated with remote
infarct myocardium in rats. In response to the treatment, the
animals’ natural defensive system was activated to cope with the
unwanted and toxic species, including increased production of SOD,
GSH and CATA. The present study indicates that orally administered
GXSTC possesses antioxidative properties. The results are
consistent with those of a previous study demonstrating that NADPH
oxidase activation mediates ROS production in cardiac hypertrophy
and failure (3,30), which is also implicated in myocyte
apoptosis.
In conclusion, the present study demonstrates that
GXSTC has significant cardioprotective effects against ischemic
myocardial injury in rats, which is likely to be due to its
antioxidant and antiapoptotic properties. Therefore, GXSTC may be
used as an effective and promising medicine for the prophylaxis and
treatment of IHD.
Acknowledgements
The study was supported by a grant from the Ministry
of National Science and Technology during the Significant New Drugs
Creation Special Project (no. 2011ZX09401-308-6).
References
|
1
|
Marzilli M, Affinito S and Focardi M:
Changing scenario in chronic ischemic heart disease: therapeutic
implications. Am J Cardiol. 98:3J–7J. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Razavi HM, Hamilton JA and Feng Q:
Modulation of apoptosis by nitric oxide: implications in myocardial
ischemia and heart failure. Pharmacol Ther. 106:147–162. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar D, Lou H and Singal PK: Oxidative
stress and apoptosis in heart dysfunction. Herz. 27:662–668. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qin F, Simeone M and Patel R: Inhibition
of NADPH oxidase reduces myocardial oxidative stress and apoptosis
and improves cardiac function in heart failure after myocardial
infarction. Free Radic Biol Med. 43:271–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qin F, Liu YX, Zhao HW, Huang X, Ren P and
Zhu ZY: Chinese medicinal formula Guan-Xin-Er-Hao protects the
heart against oxidative stress induced by acute ischemic myocardial
injury in rats. Phytomedicine. 16:215–221. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matkowski A, Zielińska S, Oszmiański J and
Lamer-Zarawska E: Antioxidant activity of extracts from leaves and
roots of Salvia miltiorrhiza Bunge, S. przewalskii
Maxim, and S verticillata L. Bioresour Technol.
99:7892–7896. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takemura G and Fujiwara H: Role of
apoptosis in remodeling after myocardial infarction. Pharmacol
Ther. 104:1–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singal PK, Khaper N, Palace V and Kumar D:
The role of oxidative stress in the genesis of heart disease.
Cardiovasc Res. 40:426–432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huo Y, Yao TM, Liang Z, Li J, You Y and
Han YL: Effects of Guanxin Shutong capsule on lipid metabolism and
hemorrheology in rat model with experimental atherosclerosis.
Journal of Liaoning University of TCM. 13:248–250. 2011.(In
Chinese).
|
|
10
|
Lee KG and Shibamoto T: Antioxidant
property of aroma extract isolated from clove buds [Syzygium
aromaticum (L.) Merr. et Perry]. Food Chem. 74:443–448.
2001.
|
|
11
|
Qi JX, Zhao L, Kong LG, Wang ML, Wang P,
Ren J and Wang L: Influence of Guanxin Shutong containing serum on
vascular smooth muscle cells proliferation induced by advanced
glycation end products. Pharmacology and Clinics of Chinese Materia
Medica. 28:155–158. 2012.(In Chinese).
|
|
12
|
Lam FF, Yeung JH, Chan KM and Or PM:
Dihydrotanshinone, a lipophilic component of Salvia
miltiorrhiza (danshen), relaxes rat coronary artery by
inhibition of calcium channels. J Ethnopharmacol. 119:318–321.
2008.PubMed/NCBI
|
|
13
|
Zhou X, Chan SW, Tseng HL, Deng Y, Hoi PM,
Choi PS, Or PM, Yang JM, Lam FF, Lee SM, et al: Danshensu is the
major marker for the antioxidant and vasorelaxation effects of
Danshen (Salvia miltiorrhiza) water-extracts produced by
different heat water-extractions. Phytomedicine. 19:1263–1269.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, He L, Meng L, Luo W and Xu X:
Suppression of tumor-induced angiogenesis by taspine isolated from
Radix et Rhizoma Leonticis and its mechanism of action in vitro.
Cancer Lett. 262:103–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou Y, Luo W, Zheng L, Li M and Zhang Y:
Construction of recombinant FGFR1 containing full-length gene and
its potential application. Plasmid. 64:60–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YM, Dai BL, Zheng L, Zhan YZ, Zhang
J, Smith WW, Wang XL, Chen YN and He LC: A novel angiogenesis
inhibitor impairs lovo cell survival via targeting against human
VEGFR and its signaling pathway of phosphorylation. Cell Death Dis.
3:e4062012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li GL and li LX: Efficacy of Guanxin
Shutong Capsules for Treatment of Chronic Seable Angina. Chinese
Journal of Integrative Medicine on Cardio/Cerebrovascular Disease.
10:1041–1042. 2012.
|
|
18
|
Zheng L, He X, Ma W, Dai B, Zhan Y and
Zhang Y: Ta1722, an anti-angiogenesis inhibitor targeted on VEGFR-2
against human hepatoma. Biomed Pharmacother. 66:499–505. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu L, Qiao H, Li Y and Li L: Protective
roles of puerarin and Danshensu on acute ischemic myocardial injury
in rats. Phytomedicine. 14:652–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Biesiada L, Pietrzak Z, Brocka U,
Oszukowski P and Krasomski G: Markers of oxidative stress in
pregnancies complicated by pregnancy induced hypertension and
intrahepatic cholestasis. Ginekol Pol. 78:956–960. 2007.(In
Polish).
|
|
21
|
Marnett LJ: Lipid peroxidation-DNA damage
by malondialdehyde. Mutat Res. 424:83–95. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng W, Huang LZ, Zhao L, Wang B, Xu HB,
Wang GY, Wang ZL and Zhou H: Superoxide dismutase activity and
malondialdehyde level in plasma and morphological evaluation of
acute severe hemorrhagic shock in rats. Am J Emerg Med. 26:54–58.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Circu ML and Aw TY: Glutathione and
modulation of cell apoptosis. Biochim Biophys Acta. 1823:1767–1777.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wattanapitayakul SK and Bauer JA:
Oxidative pathways in cardiovascular disease: roles, mechanisms,
and therapeutic implications. Pharmacol Ther. 89:187–206. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neuss M, Crow MT, Chesley A and Lakatta
EG: Apoptosis in cardiac disease - what is it - how does it occur.
Cardiovasc Drugs Ther. 15:507–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sorenson CM: Bcl-2 family members and
disease. Biochim Biophys Acta. 1644:169–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dhalla AK, Hill MF and Singal PK: Role of
oxidative stress in transition of hypertrophy to heart failure. J
Am Coll Cardiol. 28:506–514. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao L, Pimentel DR, Wang J, Singh K,
Colucci WS and Sawyer DB: Role of reactive oxygen species and
NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat
cardiac myocytes. Am J Physiol Cell Physiol. 282:C926–C934. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brandes RP, Weissmann N and Schröder K:
NADPH oxidases in cardiovascular disease. Free Radic Biol Med.
49:687–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fukui T, Yoshiyama M, Hanatani A, Omura T,
Yoshikawa J and Abe Y: Expression of p22-phox and gp91-phox,
essential components of NADPH oxidase, increases after myocardial
infarction. Biochem Biophys Res Commun. 281:1200–1206. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pardo-Andreu GL, Barrios MF, Curti C,
Hernández I, Merino N, Lemus Y, Martínez I, Riaño A and Delgado R:
Protective effects of Mangifera indica L extract (Vimang),
and its major component mangiferin, on iron-induced oxidative
damage to rat serum and liver. Pharmacol Res. 57:79–86. 2008.
|