Introduction
During the initiation and progression of
hypertension, the vascular endothelium is constantly exposed to
elevated angiotensin II (Ang II) levels, and certain
endotheliocytes undergo oxidative stress, which contributes to
endothelial dysfunction.
Reactive oxygen species (ROS), including the
hydroxyl radical (HO•), superoxide anion
(O2−) and hydrogen peroxide
(H2O2), are continuously produced as a result
of cellular oxidation-reduction processes stimulated by Ang II
(1). Moreover, the high levels of
Ang II-induced inflammatory factors may be mediated through
oxidative stress (2). However,
whether fractalkine (FKN), an important chemokine involved in
endothelial dysfunction, is induced by Ang II remains unclear.
Plumula Nelumbinis is a type of traditional Chinese
herbal medicine. The major components are alkaloids that exhibit a
nerve-blocking effect. Thus, Plumula Nelumbinis may be used to
treat hypertension (3). However,
the role of the flavonoid components of Plumula Nelumbinis in the
prevention of hypertension has not previously been studied.
Flavonoids are present in a large number of plants and are strong
antioxidants that scavenge various types of radicals through their
H+-donating properties in aqueous and organic
environments (4–8). Previous studies have indicated that
flavonoids protect cells against ROS-induced inflammation by
increasing the activity of antioxidant enzymes.
The present study investigated the protective role
of total flavonoids from Plumula Nemlumbinis (TFPN) in Ang
II-induced oxidative stress injury in human umbilical vein
endothelial cells (HUVECs). To the best of our knowledge, this is
the first time such a study has been conducted. In addition, the
molecular mechanisms by which TFPN affect the production of ROS and
malondialdehyde (MDA), the activity of superoxide dismutase (SOD)
and the expression of nicotinamide adenine dinucleotide phosphate
(NADPH) oxidase, IκB-α and FKN were investigated.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were purchased from Hyclone (Logan, UT, USA).
Penicillin and streptomycin were obtained from Gibco-BRL (Grand
Island, NY, USA). The mouse anti-p47phox and anti-IκB-α primary
antibodies were obtained from Anbo Biotechnology, Inc. (Sunnyvale,
CA, USA). The mouse anti-FKN primary antibody and rabbit anti-mouse
secondary antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). The bicinchoninic acid (BCA) reagent,
enhanced chemiluminescence (ECL) kit for western blotting and the
fluorescent ROS detection kit were purchased from Beyotime
Biotechnology (Shanghai, China). Reagent kits for measuring MDA and
SOD were purchased from Jiancheng Bioengineering Institute
(Nanjing, Jiangsu, China). Ang II, apocynin, pyrrolidine
dithiocarbamate (PDTC), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were
purchased from Sigma-Aldrich (St. Louis, MO, USA). The TFPN were
extracted from Plumula Nelumbinis by the Department of
Pharmacology, Xiangya Hospital of Central South University
(Changsha, China).
Cell culture and treatment
HUVECs were purchased from the Cell Culture Center,
Xiangya Medical College of Central South University. The HUVECs
were cultured in DMEM supplemented with 10% FBS, 10 mmol/l HEPES,
100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in a
humidified atmosphere of 5% CO2 and 95% air. Endothelial
cells in an actively growing condition from the fourth to sixth
passages were used for the experiments. One day prior to treatment,
80–85% confluent cells were incubated with serum-free media for 24
h to synchronize cell growth. The cells were pretreated with the
following drug interventions: 0.05, 0.1 or 0.2 mg/ml TFPN, 200 μM
apocynin (an NADPH oxidase inhibitor) and 50 μM PDTC (an NF-κB
inhibitor), for 4 h. Next, 10−7 mol/l Ang II was added
to the medium containing the various drugs for an additional 24 h.
Each treatment compound was individually dissolved in DMEM, and the
cells cultured in the serum-free medium served as controls. The
cells were incubated prior to protein isolation or the collection
of cultured supernatant for chemical colorimetry. Each individual
experiment was replicated at least three times.
Effect of TFPN on HUVEC viability
The effect of TFPN on HUVEC viability was measured
by the MTT assay, as previously described (9). HUVECs were counted and seeded into
96-well culture plates at a density of 0.6–1×104
cells/well. The cells were pretreated with various concentrations
of TFPN (0.001, 0.01, 0.025, 0.05, 0.1, 0.2, 0.4 or 0.6 mg/ml) for
4 h. Next, 10−7 mol/l Ang II was added to the medium,
which was incubated for an additional 24 h. Each well was washed
twice with PBS, then, for each well, 20 μl MTT solution (5 mg/ml)
combined with 180 μl serum-free DMEM was added. Following
incubation of the plate for 4 h, insoluble formazan crystals were
dissolved in 150 μl DMSO. The optical density of each well was
measured using an ELISA multiplate reader (Infinite 200 PRO
multimode reader; Tecan Group Ltd., Männedorf, Switzerland) at 492
nm.
MDA and SOD assays
The cells were cultured in 50-ml tissue culture
flasks at a density of 5×105 cells/ml and allowed to
grow to confluence. Following cell treatment as described in Cell
culture and treatment, supernatants of the cultures were collected
for chemical colorimetry. The cells were washed with PBS,
trypsinized for 1 min at 37°C, harvested by centrifugation (3,000 ×
g for 10 min) and resuspended in 2 ml PBS. Following
ultrasonication at 4°C, the protein content was determined using
the BCA method. The levels of MDA and SOD were determined using
assay kits, according to the manufacturer’s instructions.
Measurement of O2−
generation in intact cells
Changes in intracellular ROS levels were determined
by measuring the oxidative conversion of 2′,7′-dichlorofluorescein
diacetate (DCFH-DA; Invitrogen Life Technologies, Carlsbad, CA,
USA), which is cell-permeable, to the fluorescent DCF in a
SpectraMax M5 Muti-Mode Microplate Reader (Molecular Devices,
Sunnyvale, CA, USA). The treated cells were washed with serum-free
media and incubated with 10 mM DCFH-DA at 37°C for 20 min. DCF
fluorescence was then detected using a fluorospectrophotometer.
Fluorescence analysis was measured at 485 nm excitation and 535 nm
emission. Fluorescence data are expressed as the percentage
increase in fluorescence over that of untreated samples.
Western blot analysis
The cells were lysed for 30 min at 4°C in a lysis
buffer. The total cell protein concentration was determined using
BCA reagent. Total protein (50 μg) was resolved by
SDS-polyacrylamide gel electrophoresis, transferred to a PVDF
membrane and subjected to immunoblot analysis. The primary
antibodies for FKN (1:400), p47phox (1:500), IκB-α (1:500) and
β-actin (1:1,000) and rabbit anti-mouse secondary antibodies were
used. The bands were visualized using ECL reagents and the relative
intensities were determined using a bio-imaging analyzer (ChemiDoc™
MP System 170-8280; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The densities of the products were quantified using Image Lab
3.0beta (version 3.0; Bio-Rad). All results were
representative of at least three independent experiments.
Statistical analysis
Data are expressed as the mean ± SD and were
analyzed by ANOVA, followed by the Newman-Student-Keuls test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of TFPN on HUVECs damaged by Ang
II
To evaluate whether TFPN protect against oxidative
stress, HUVECs were pretreated with various concentrations of TFPN
(0.001, 0.01, 0.025, 0.05, 0.1, 0.2, 0.4 or 0.6 mg/ml) for 4 h, and
10−7 mol/l Ang II was added to the medium for an
additional 24 h. Next, cell viability was measured by the MTT
assay. As shown in Fig. 1, Ang II
markedly decreased the viability of the endothelial cells
(P<0.01), while TFPN, at concentrations between 0.01 and 0.2
mg/ml, protected the cells from Ang II-induced cytotoxicity in a
concentration-dependent manner (P<0.01). The lowest
concentration of TFPN (0.001 mg/ml) did not have a protective
effect against Ang II-induced damage on HUVECs (P>0.05). Higher
concentrations of TFPN (0.4 and 0.6 mg/ml) increased the cell
survival rate (P<0.01), but were slightly inferior to 0.2 mg/ml
TFPN. The results indicate that TFPN protected HUVECs from Ang
II-induced cellular injury.
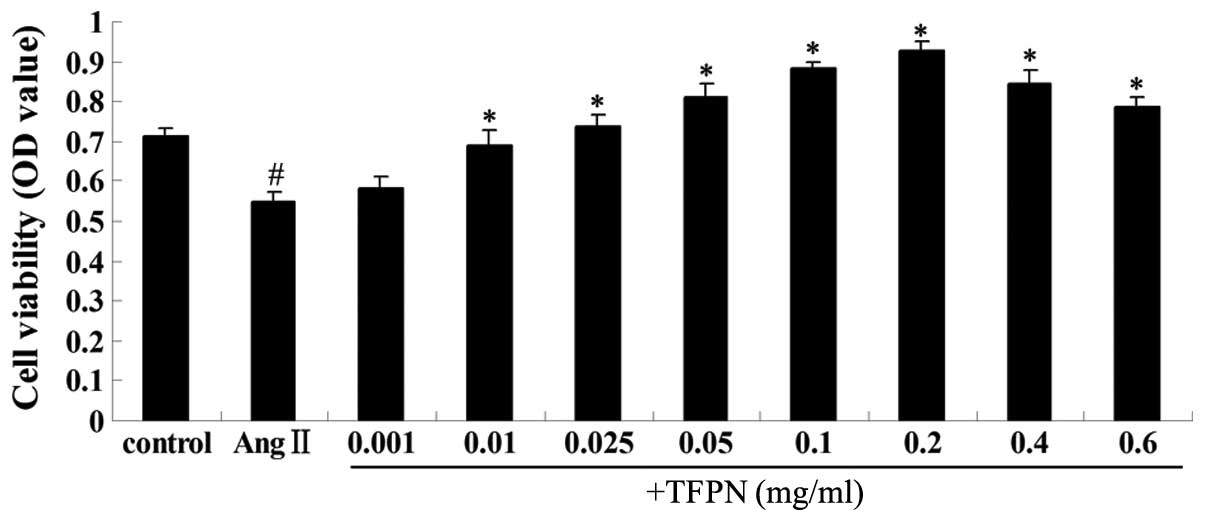 | Figure 1Effect of TFPN on the viability of
HUVECs injured by Ang II. Cells were pretreated with various
concentrations of TFPN (0.001, 0.01, 0.025, 0.05, 0.1, 0.2, 0.4 or
0.6 mg/ml) for 4 h. Ang II (10−7 mol/l) was subsequently
added to the medium for an additional 24 h. Cell viability was
measured by the MTT assay at 492 nm. #P<0.01, vs.
control; *P<0.01, vs. Ang II; (n=4). +TFPN, Ang II +
TFPN; TFPN, total flavonoids of Plumula Nelumbinis; HUVECs, human
umbilical vein endothelial cells; Ang II, angiotensin II; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide. |
Effect of TFPN on the level of MDA
To determine the effect of TFPN on the MDA level in
Ang II-damaged endothelial cells, the cells were pretreated with
TFPN, PDTC or apocyin for 4 h and then exposed to 10−7
mol/l Ang II for 24 h. As shown in Fig. 2, incubation of the cells with
10−7 mol/l Ang II increased the level of MDA
(P<0.05). Compared with the MDA level in Ang II-treated cells,
pretreatment with various concentrations of TFPN (0.05, 0.1 or 0.2
mg/ml) reduced the level of MDA in a concentration-dependent manner
(P<0.05). Pretreatment with 50 μM PDTC and 100 μM apocynin also
reduced the level of MDA (P<0.05).
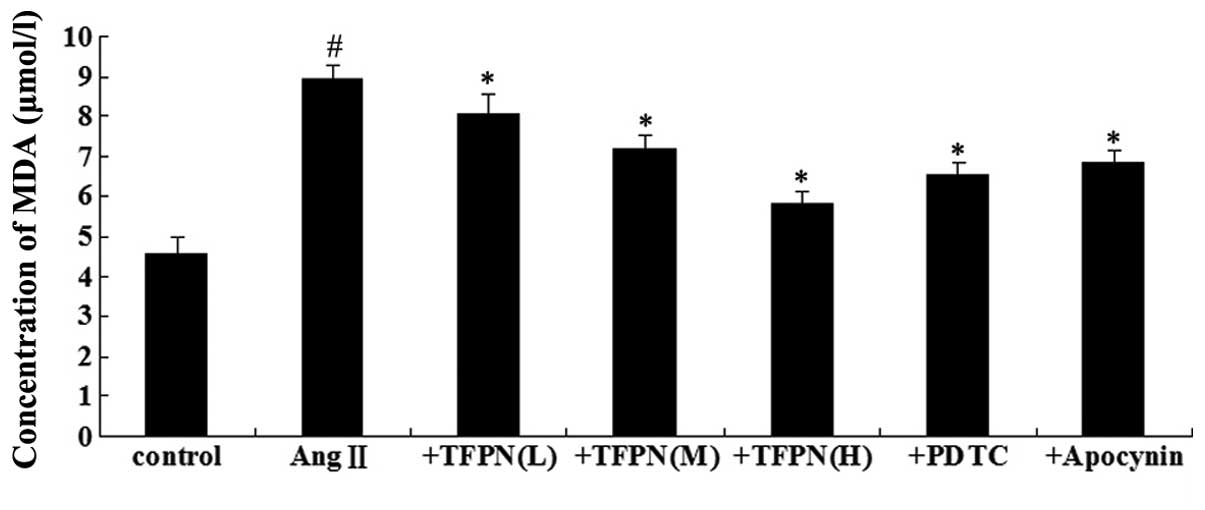 | Figure 2Effect of TFPN on the level of MDA.
Cells were pretreated with various concentrations of TFPN (0.05,
0.1 or 0.2 mg/ml), 50 μM PDTC or 100 μM apocynin for 4 h. Ang II
(10−7 mol/l) was added to the medium containing the
various drugs for an additional 24 h prior to protein isolation.
#P<0.05, vs. control; *P<0.05, vs. Ang
II; (n=4). +TFPN(L), Ang II + 0.05 mg/ml TFPN; +TFPN(M), Ang II +
0.1 mg/ml TFPN; +TFPN(H), Ang II + 0.2 mg/ml TFPN; +PDTC, Ang II +
PDTC; +Apocynin, Ang II + apocynin; TFPN, total flavonoids of
Plumula Nelumbinis; MDA, malondialdehyde; Ang II, angiotensin II;
PDTC, pyrrolidine dithiocarbamate. |
Effect of TFPN on SOD activity
To determine the effect of TFPN on SOD activity in
Ang II-damaged endothelial cells, the cells were pretreated with
TFPN, PDTC or apocyin for 4 h and then exposed to 10−7
mol/l Ang II for 24 h. As shown in Fig. 3, incubation of the cells with
10−7 mol/l Ang II decreased the SOD activity
(P<0.05). Compared with the SOD activity in the Ang II-treated
cells, pretreatment with various concentrations of TFPN (0.05, 0.1
or 0.2 mg/ml) increased the SOD activity in a
concentration-dependent manner (P<0.05). Pretreatment with 50 μM
PDTC and 100 μM apocynin also increased SOD activity
(P<0.05).
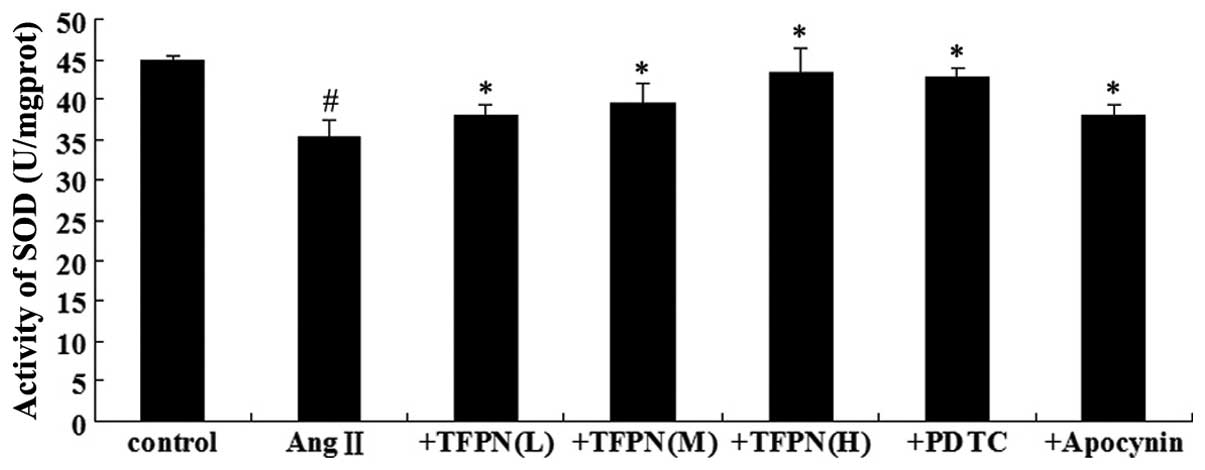 | Figure 3Effect of TFPN on SOD activity. Cells
were pretreated with various concentrations of TFPN (0.05, 0.1 or
0.2 mg/ml), 50 μM PDTC or 100 μM apocynin for 4 h. Ang II
(10−7 mol/l) was added to the medium containing the
various drugs for an additional 24 h prior to protein isolation.
#P<0.05, vs. control; *P<0.05, vs. Ang
II; (n=4). +TFPN(L), Ang II + 0.05 mg/ml TFPN; +TFPN(M), Ang II +
0.1 mg/ml TFPN; +TFPN(H), Ang II + 0.2 mg/ml TFPN; + PDTC, Ang II +
PDTC; +Apocynin, Ang II + apocynin; TFPN, total flavonoids of
Plumula Nelumbinis; SOD, superoxide dismutase; Ang II, angiotensin
II; PDTC, pyrrolidine dithiocarbamate. |
Effect of TFPN on ROS levels
To determine the effect of TFPN on the level of ROS
in Ang II-damaged endothelial cells, the cells were pretreated with
TFPN, PDTC or apocyin for 4 h and then exposed to 10−7
mol/l Ang II for 24 h. As shown in Fig. 4, incubation of the cells with
10−7 mol/l Ang II increased the ROS levels (P<0.01).
Pre-treatment with various concentrations of TFPN (0.05, 0.1 or 0.2
mg/ml) reduced the levels of ROS in a concentration-dependent
manner compared with those in the Ang II-treated cells (P<0.01).
Pretreatment with 50 μM PDTC and 100 μM apocynin also reduced the
levels of ROS (P<0.01).
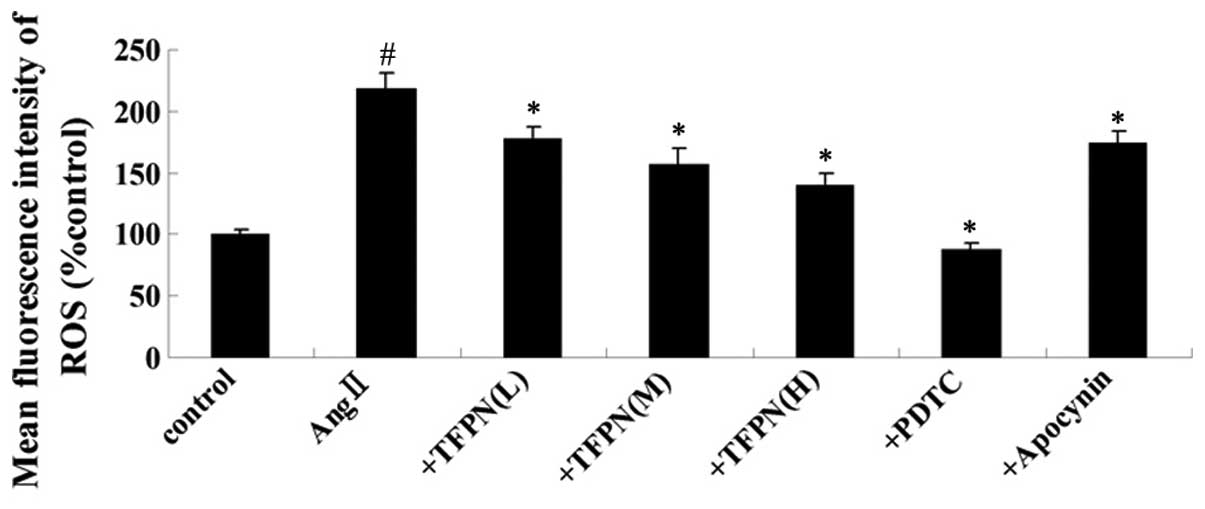 | Figure 4Effect of TFPN on the ROS levels.
Cells were pretreated with various concentrations of TFPN (0.05,
0.1 or 0.2 mg/ml), 50 μM PDTC or 100 μM apocynin for 1 h. Ang II
(10−7 mol/l) was added to the medium containing the
various drugs for an additional 4 h prior to mean fluorescence
intensity testing. #P<0.01, vs. control;
*P<0.01, vs. Ang II; (n=4). +TFPN(L), Ang II + 0.05
mg/ml TFPN; +TFPN(M), Ang II + 0.1 mg/ml TFPN; +TFPN(H), Ang II +
0.2 mg/ml TFPN; +PDTC, Ang II + PDTC; +Apocynin, Ang II + apocynin;
TFPN, total flavonoids of Plumula Nelumbinis; ROS, reactive oxygen
species; Ang II, angiotensin II. |
FKN relative protein expression
Western blotting was used to investigate whether FKN
protein was upregulated in endothelial cells following treatment
with Ang II, and to analyze whether TFPN inhibited this
upregulation. As shown in Fig. 5,
10−7 mol/l Ang II significantly increased the protein
expression level of FKN (P<0.01). With the exception of 0.05
mg/ml TFPN, pretreatment with TFPN (0.1 and 0.2 mg/ml) and
pretreatment with 50 μM PDTC and 100 μM apocynin markedly blocked
the Ang II-induced expression of FKN (P<0.05).
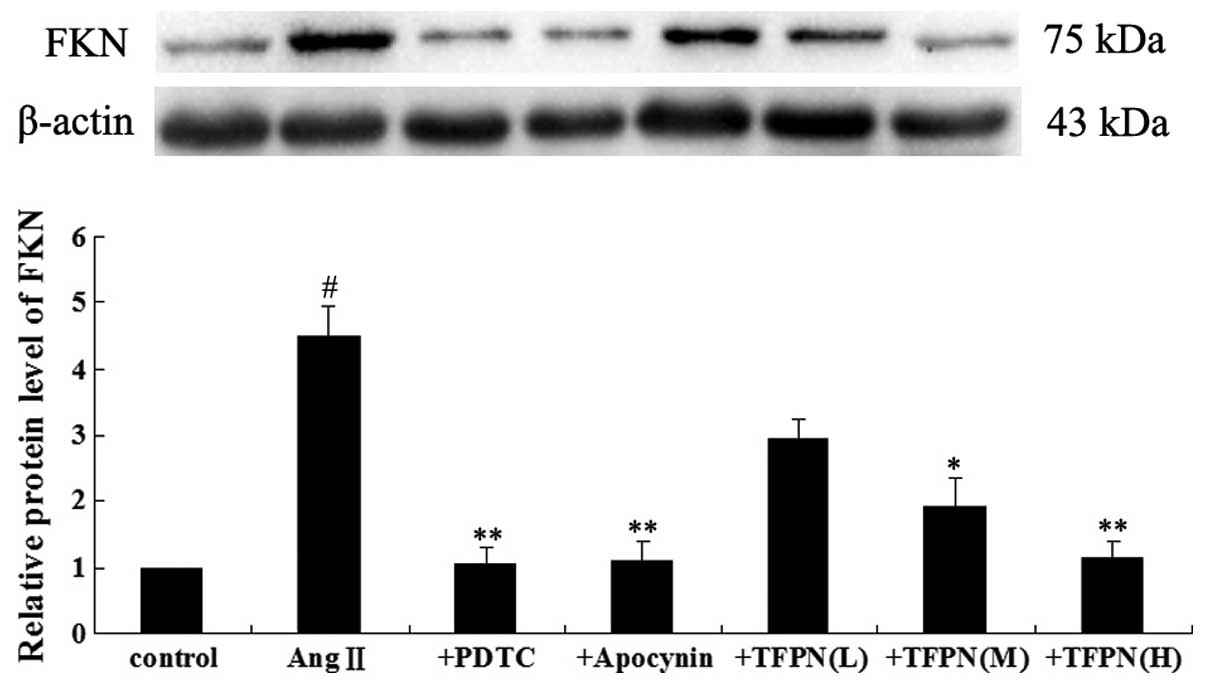 | Figure 5Cells were pretreated with 50 μM PDTC,
100 μM apocynin and various concentrations of TFPN (0.05, 0.1 or
0.2 mg/ml) for 4 h. Ang II (10−7 mol/l) was added to the
medium containing the various drugs for an additional 24 h prior to
protein isolation. The gels represent the western blot analysis
results of FKN protein expression. β-actin was used as the internal
control. #P<0.01, vs. control; *P<0.05,
vs. Ang II; **P<0.01, vs. Ang II; (n=3). +PDTC, Ang
II + PDTC; +Apocynin, Ang II + apocynin; +TFPN(L), Ang II + 0.05
mg/ml TFPN; +TFPN(M), Ang II + 0.1 mg/ml TFPN; +TFPN(H), Ang II +
0.2 mg/ml TFPN; TFPN, total flavonoids of Plumula Nelumbinis; FKN,
fractalkine; Ang II, angiotensin II; PDTC, pyrrolidine
dithiocarbamate. |
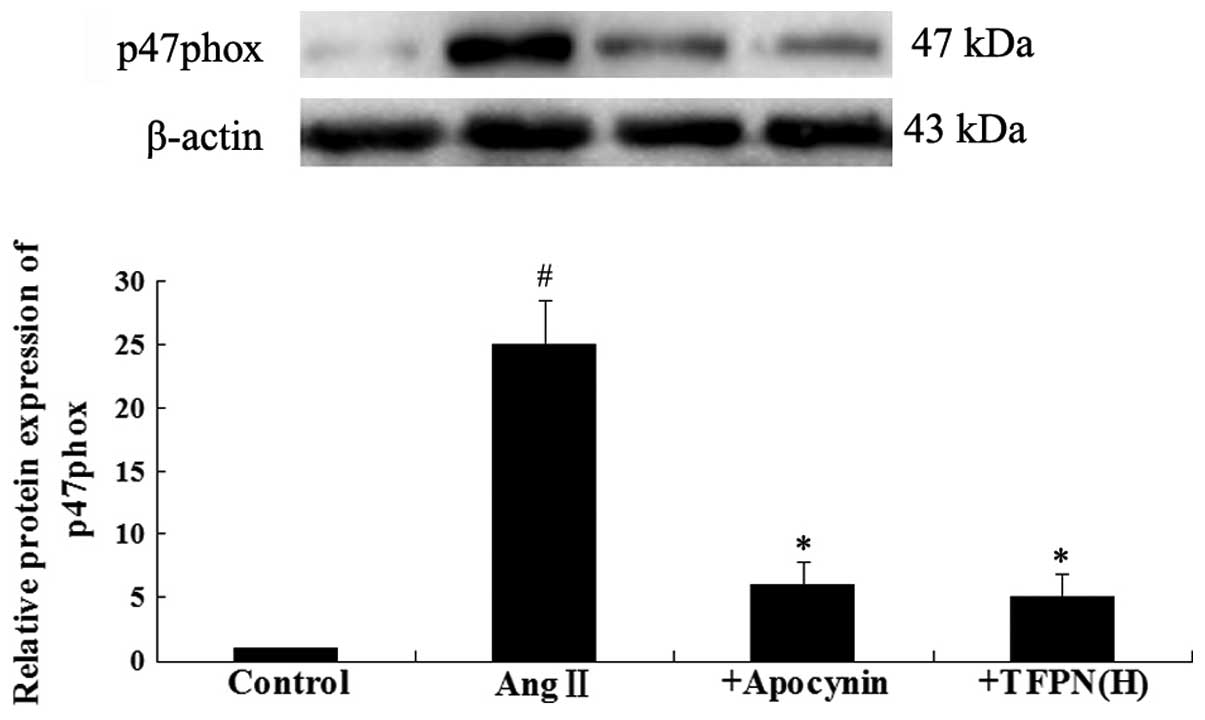
p47phox relative protein expression
To clarify whether oxidase activation is involved in
the effect of 0.2 mg/ml TFPN on Ang II-induced FKN elevation,
western blotting for p47phox (a subunit of NADPH oxidase) was
performed. As shown in Fig. 6,
10−7 mol/l Ang II significantly increased the protein
expression of p47phox (P<0.01). Pretreatment with 0.2 mg/ml TFPN
and 100 μM apocynin markedly blocked the Ang II-induced expression
of p47phox (P<0.05).
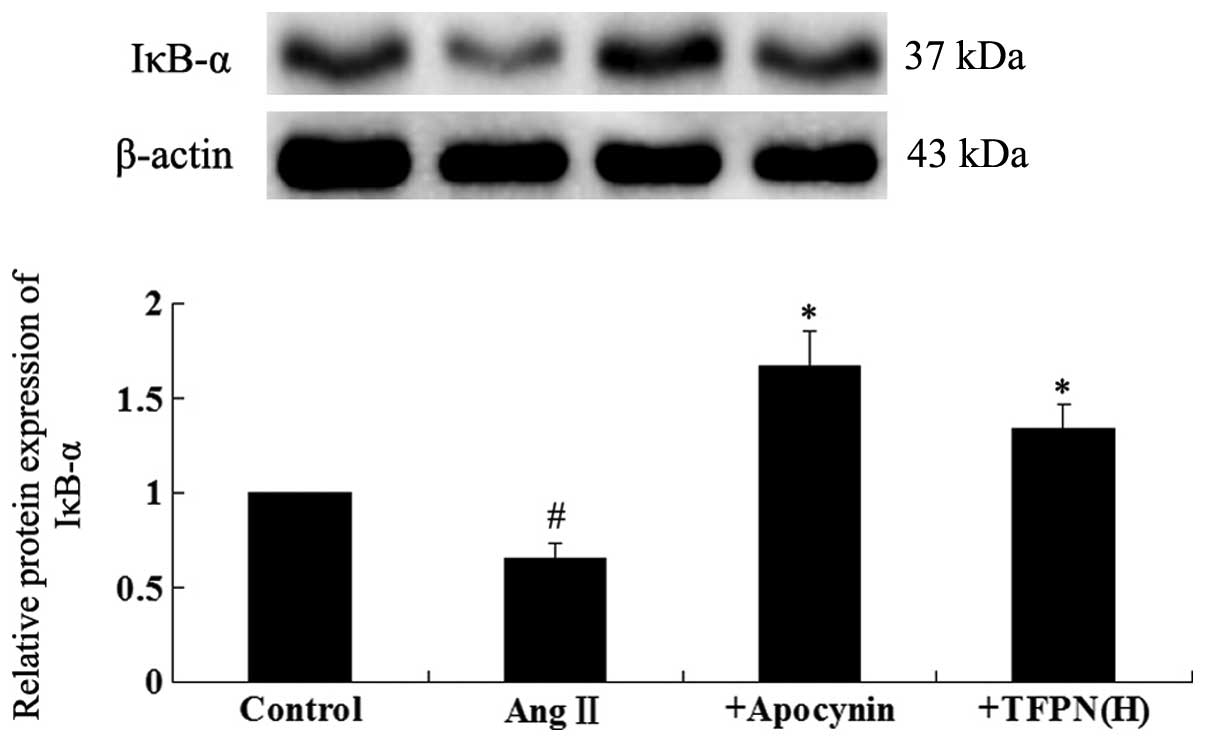
IκB-α relative protein expression
To clarify the mechanism of the TFPN-associated
reduction in FKN protein, western blotting for IκB-α (an endogenous
inhibitor of NF-κB) was applied. As shown in Fig. 7, 10−7 mol/l Ang II
significantly decreased the protein expression level of IκB-α
(P<0.01). Pretreatment with 0.2 mg/ml TFPN inhibited the
reduction of the IκB-α level induced by Ang II (P<0.01) and the
same effect was also observed when the cells were pretreated with
50 μM PDTC (P<0.01).
Discussion
The present study showed that Ang II activated
p47phox and increased DCF-sensitive cellular ROS, MDA and FKN
expression in HUVECs. However, SOD and IκB-α expression decreased
following treatment with Ang II. The results of the present study
indicate that the expression of FKN was regulated through the
generation of NF-κB activated by ROS. The administration of each of
TFPN, PDTC and apocynin alone significantly reduced the cellular
oxidative responses to Ang II.
Endothelial dysfunction is the foundation and
initial step of numerous cardiovascular diseases, and promotes
abnormal vascular growth, leading to end-organ damage (10,11).
However, the underlying molecular mechanisms remain poorly
understood. Increasing evidence supports the critical roles of
oxidative stress in endothelial dysfunction (12). Endothelial cells are involved in
oxidative injury and serve as an important source of arterial
oxidative stress (13).
Endothelial oxidative stress increases cell apoptosis and the
expression of inflammatory cytokine genes, inducing the arteries to
fail, relax or dilate, resulting in increased tension of the
arterial wall (14). Vascular
smooth muscle cells proliferate due to endothelial dysfunction,
which further increases the stiffness of the arteries (15). As a result, blood pressure
increases.
Xanthine oxidase, uncoupled nitric oxide synthase
and NADPH oxidase are likely potential endothelial ROS sources,
with NADPH oxidase being the most significant (16). NADPH oxidase is an inducible
electron transport system, which transfers reducing equivalents
from NADPH to molecular oxygen via flavins, resulting in ROS
generation (17). The expression
and activation of NADPH oxidase has been demonstrated in a number
of cell lines, including endothelial cells, vascular smooth muscle
cells and cardiomyocytes (18). In
endothelial cells, the upregulation of NADPH oxidase may be
required for the expression of pro-inflammatory factors or
chemokines induced by a neuroendocrine factor, such as Ang II
(19).
The results of the present study showed that
apocynin prevented the induction of FKN expression by Ang II in
endothelial cells, indicating that FKN is responsive to NADPH
oxidase activation with the potential involvement of ROS. PDTC was
also found to reduce NF-κB-dependent FKN expression, which is
consistent with results reported in previous studies (20–22).
In endothelial cells, there are several transcriptional
factor-binding sites in the cytokine promoter, including one for
NF-κB (23). Activated NF-κB may
bind to the cytokine promoter, which is critically involved in
cytokine gene regulation by various stimuli, such as Ang II. ROS
have been hypothesized to be secondary messengers leading to NF-κB
activation in response to extracellular stimuli and then
upregulating the gene expression of chemokines (24). Although PDTC is a well-known
inhibitor of NF-κB, it has been shown that PDTC suppresses NF-κB
activation partly through its antioxidant property, which may
account for the reduced ROS formation (25). In the present study, Ang II
increased the production of intracellular ROS and induced FKN
expression in the endothelial cells; these effects were suppressed
by antioxidant agents.
Flavonoids are broad-spectrum antioxidants and
anti-inflammatory agents that are known to have efficacy in a
number of inflammatory disease models, including coronary heart
disease and hypertension (4–8).
Moreover, the ethanol extracts from Plumula Nelumbinis have
antioxidant and anti-inflammatory functions (26). The current study also indicated the
antioxidant property of TFPN, which are the total flavonoids
extracted from Plumula Nelumbinis. It was observed that TFPN
inhibited the expression of the p47phox subunit of NADPH oxidase,
ROS and MDA, while increasing the production of SOD, an oxyradical
scavenger, and IκB-α, an endogenous NF-κB inhibitor. Oxygen
radicals are hypothesized to participate in the regulation of
NF-κB, inflammatory factors and chemokines through a NADPH
oxidase-dependent pathway. Moreover, the results indicate that the
effects of TFPN against NF-κB and FKN are associated with their
antioxidant properties. The antioxidative effect of TFPN may be
significant in the mechanism by which they prevent arterial
inflammation and endothelial dysfunction.
The suppressive effects of TFPN on cellular
oxidative stress and the inflammatory response to Ang II are
significant. A series of studies regarded Ang II, which was
selected as the cytokine stimulator of ROS in the present study, as
one of the important inductive agents of oxidative stress and
endothelial dysfunction in vitro and in vivo
(1,2,27–29).
ROS generation is one of the major mechanisms involved in Ang
II-induced tissue damage (28).
Ang II also contributes to ROS-dependent vascular smooth muscle
cell proliferation in hypertension (29). These results indicate that the
effects of Ang II on superoxide production are mediated through
NADPH oxidase.
In the present study, TFPN were observed to
attenuate the FKN expression induced by Ang II in endothelial cells
in a concentration-dependent manner. Furthermore, TFPN decreased
FKN expression at similar levels to apocynin and PDTC, indicating
that the antioxidant effects of TFPN may be mediated via multiple
mechanisms.
Inflammation and oxyradicals contribute to
hypertension. FKN, a pleomorphic chemokine, contributes to
endothelial dysfunction by inducing inflammatory responses in
atherosclerotic disease (30).
Previously, FKN levels were found to be increased in spontaneously
hypertensive rats, indicating that FKN plays a role in hypertension
(31). Furthermore, FKN has been
shown to induce ROS production in arteries in vitro
(32). The suppressive action of
TFPN on FKN implies the prospect of the application of TFPN in the
treatment of hypertension.
In conclusion, for the first time to the best of our
knowledge, the present study reported that TFPN attenuate Ang
II-induced ROS production and thus, ROS-induced NF-κB and FKN
expression. TFPN exhibit these effects in HUVECs by a
radical-scavenging mechanism through an NADPH oxidase-dependent
process, indicating that TFPN may become a promising agent for the
prevention of endothelial dysfunction.
Acknowledgements
The study was supported by a grant from the Natural
Science Foundation of Hunan Province, China (no. 12JJ5070).
References
|
1
|
Rajagopalan S, Kurz S, Münzel T, et al:
Angiotensin II-mediated hypertension in the rat increases vascular
superoxide production via membrane NADH/NADPH oxidase activation.
Contribution to alterations of vasomotor tone. J Clin Invest.
97:1916–1923. 1996. View Article : Google Scholar
|
|
2
|
Touyz RM and Briones AM: Reactive oxygen
species and vascular biology: implications in human hypertension.
Hypertens Res. 34:5–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Hu X, Yin W and Cai H: Alkaloids
of plumula Nelumbinis. Zhongguo Zhong Yao Za Zhi. 16:673–675.
7031991.(In Chinese).
|
|
4
|
Lebeau J, Furman C, Bernier JL, Duriez P,
Teissier E and Cotelle N: Antioxidant properties of
di-tert-butylhydroxylated flavonoids. Free Radic Biol Med.
29:900–912. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Owen RW, Haubner R, Mier W, et al:
Isolation, structure elucidation and antioxidant potential of the
major phenolic and flavonoid compounds in brined olive drupes. Food
Chem Toxicol. 41:703–717. 2003. View Article : Google Scholar
|
|
6
|
Bergman M, Perelman A, Dubinsky Z and
Grossman S: Scavenging of reactive oxygen species by a novel
glucurinated flavonoid antioxidant isolated and purified from
spinach. Phytochemistry. 62:753–762. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dugas AJ Jr, Castañeda-Acosta J, Bonin GC,
Price KL, Fischer NH and Winston GW: Evaluation of the total
peroxyl radical-scavenging capacity of flavonoids:
structure-activity relationships. J Nat Prod. 63:327–331. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mishra B, Priyadarsini KI, Kumar MS,
Unnikrishnan MK and Mohan H: Effect of O-glycosylation on the
antioxidant activity and free radical reactions of a plant
flavonoid, chrysoeriol. Bioorg Med Chem. 11:2677–2685. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia SJ, Jiang DJ, Hu CP, et al:
Lysophosphatidylcholine-induced elevation of asymmetric
dimethylarginine level by the NADPH oxidase pathway in endothelial
cells. Vascul Pharmacol. 44:143–148. 2006. View Article : Google Scholar
|
|
10
|
Park JB, Charbonneau F and Schiffrin EL:
Correlation of endothelial function in large and small arteries in
human essential hypertension. J Hypertens. 19:415–420. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tomita N, Yamasaki K, Osako MK, Komai N,
Shimosato T and Morishita R: Combination therapy based on the
angiotensin receptor blocker olmesartan for vascular protection in
spontaneously hypertensive rats. Mol Med Rep. 2:733–738. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guzik TJ, West NE, Black E, et al:
Vascular superoxide production by NAD(P)H oxidase: association with
endothelial dysfunction and clinical risk factors. Circ Res.
86:E85–E90. 2000. View Article : Google Scholar
|
|
13
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: the role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guzik TJ, Hoch NE, Brown KA, et al: Role
of the T cell in the genesis of angiotensin II induced hypertension
and vascular dysfunction. J Exp Med. 204:2449–2460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Griendling KK, Minieri CA, Ollerenshaw JD
and Alexander RW: Angiotensin II stimulates NADH and NADPH oxidase
activity in cultured vascular smooth muscle cells. Circ Res.
74:1141–1148. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi Y, Niculescu R, Wang D, Patel S,
Davenpeck KL and Zalewski A: Increased NAD(P)H oxidase and reactive
oxygen species in coronary arteries after balloon injury.
Arterioscler Thromb Vasc Biol. 21:739–745. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohtsu H, Frank GD, Utsunomiya H and Eguchi
S: Redox-dependent protein kinase regulation by angiotensin II:
mechanistic insights and its pathophysiology. Antioxid Redox
Signal. 7:1315–1326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lassègue B and Clempus RE: Vascular
NAD(P)H oxidases: specific features, expression, and regulation. Am
J Physiol Regul Integr Comp Physiol. 285:R277–R297. 2003.PubMed/NCBI
|
|
19
|
Kalinowski L and Malinski T: Endothelial
NADH/NADPH-dependent enzymatic sources of superoxide production:
relationship to endothelial dysfunction. Acta Biochim Pol.
51:459–469. 2004.PubMed/NCBI
|
|
20
|
Chandrasekar B, Mummidi S, Perla RP, et
al: Fractalkine (CX3CL1) stimulated by nuclear factor kappaB
(NF-kappaB)-dependent inflammatory signals induces aortic smooth
muscle cell proliferation through an autocrine pathway. Biochem J.
373:547–558. 2003. View Article : Google Scholar
|
|
21
|
Garcia GE, Xia Y, Chen S, et al:
NF-kappaB-dependent fractalkine induction in rat aortic endothelial
cells stimulated by IL-1beta, TNF-alpha, and LPS. J Leukoc Biol.
67:577–584. 2000.PubMed/NCBI
|
|
22
|
Zernecke A, Weber KS and Weber C: Combined
modulation of the mesangial machinery for monocyte recruitment by
inhibition of NF-kappaB. Am J Physiol Cell Physiol.
281:C1881–C1888. 2001.
|
|
23
|
Pomerantz JL and Baltimore D: Two pathways
to NF-kappaB. Mol Cell. 10:693–695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonello S, Zähringer C, BelAiba RS, et al:
Reactive oxygen species activate the HIF-1alpha promoter via a
functional NFkappaB site. Arterioscler Thromb Vasc Biol.
27:755–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yokoo T and Kitamura M: Antioxidant PDTC
induces stromelysin expression in mesangial cells via a tyrosine
kinase-AP-1 pathway. Am J Physiol. 270:F806–F811. 1996.PubMed/NCBI
|
|
26
|
Chen J, Zhang M, Zheng T-S and Tao J-H:
Study on antioxidant activities of ethanol extracts from lotus germ
in vitro. Food Science. 29:48–51. 2008.(In Chinese).
|
|
27
|
Hanna IR, Taniyama Y, Szöcs K, Rocic P and
Griendling KK: NAD(P)H oxidase-derived reactive oxygen species as
mediators of angiotensin II signaling. Antioxid Redox Signal.
4:899–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ruiz-Ortega M, Ruperez M, Esteban V and
Egido J: Molecular mechanisms of angiotensin II-induced vascular
injury. Curr Hypertens Rep. 5:73–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo Z, Teerlink T, Griendling K, Aslam S,
Welch WJ and Wilcox CS: Angiotensin II and NADPH oxidase increase
ADMA in vascular smooth muscle cells. Hypertension. 56:498–504.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wong BW, Wong D and McManus BM:
Characterization of fractalkine (CX3CL1) and CX3CR1 in human
coronary arteries with native atherosclerosis, diabetes mellitus,
and transplant vascular disease. Cardiovasc Pathol. 11:332–338.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sullivan JC, Pardieck JL, Doran D, Zhang
Y, She JX and Pollock JS: Greater fractalkine expression in
mesenteric arteries of female spontaneously hypertensive rats
compared with males. Am J Physiol Heart Circ Physiol.
296:H1080–H1088. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schäfer A, Schulz C, Fraccarollo D, et al:
The CX3C chemokine fractalkine induces vascular dysfunction by
generation of superoxide anions. Arterioscler Thromb Vasc Biol.
27:55–62. 2007.PubMed/NCBI
|