Introduction
Systemic lupus erythematosus (SLE) is a multisystem
autoimmune disease. The etiology and pathogenesis of SLE are
possibly multifactorial; however, the mechanism of pathogenesis has
not been fully elucidated. Genetic susceptibility and estrogen, as
well as environmental triggers including viral infection,
ultraviolet light exposure and drug use, may be involved in the
immune dysfunction of SLE (1),
among which viral infection has attracted the majority of attention
(2–4) as it induces the production of
antibodies (5). Since Epstein-Barr
virus (EBV) was first reported by Evans et al (6) in 1971, the relevance of EBV infection
in SLE has been continuously investigated. Thus far, the majority
of the evidence suggesting EBV infection is involved in the
pathogenesis of SLE has been obtained from viral antigens, the EBV
genome or serological detection in the peripheral circulation of
patients with SLE (7–13). The kidney is the most commonly
involved organ in patients with SLE, which is subsequently named
lupus nephritis (LN). To the best of our knowledge, whether renal
EBV infection is involved in the pathogenesis of LN has not been
reported. In the present study, the renal expression of gene and
protein markers of EBV in patients with LN were detected.
Materials and methods
All study methods were approved by the Ethics
Committee of The Affiliated Hospital of Guangdong Medical College
(Zhanjiang, China). Written consent of participation was signed by
every subject enrolled in the study.
Clinical data
In total, 58 renal tissue samples from patients with
LN, seven normal renal tissue samples from patients with
non-glomerular hematuria and 37 renal tissue samples from patients
with minimal change nephropathy were collected by the Institute of
Nephrology, Guangdong Medical College (Zhanjiang, China). All 58
patients with LN met the diagnostic criteria for SLE published by
the American College of Rheumatology in 1997 (14) and manifested renal involvement,
which was confirmed by clinical proteinuria and/or renal failure.
Of those 58 patients, 52 were female and six were male, with a mean
age of 27.5±1.0 years (range, 10–56 years). The duration of disease
was between seven days and three years. The SLE disease activity
index of the 58 patients was >10. All seven normal renal tissue
samples were collected from patients with persistent unexplained
hematuria, and it was identified by renal biopsy that the hematuria
was of non-glomerular origin. Serum were also collected at the time
of biopsy from patients with LN for autoantibody determination.
The patients with LN were divided into an initial
onset group for those who had never received any immunosuppressants
and a relapse group for those who had received immunosuppressant
treatment. The LN patients were also divided into a non-infection
group and a concurrent infection group for those who had suffered
from respiratory infection, gastrointestinal infection, urinary
tract infection, skin infection or other type of infection within 3
months prior to renal biopsy.
Detection of EBV-latent membrane
protein-1 (EBV-LMP1) expression using immunohistochemistry
(IHC)
Briefly, 3-μm-thick formalin-fixed,
paraffin-embedded sections of the renal tissue samples were
deparaffinized and rehydrated. Antigens were retrieved by treatment
with high-pressure steam for 10 min. Subsequently, endogenous
peroxidase was quenched with 0.3% H2O2 in the
dark for 30 min. The sections were incubated with monoclonal mouse
anti-EBV-LMP1 (0.2 μg/ml; DakoCytomation Corporation, Carpinteria,
CA, USA) overnight at 4°C. Subsequently, the sections were
incubated with rabbit anti-mouse horseradish peroxidase-conjugated
IgG (IgG-HRP; Beijing Zhongshan Golden Bridge Biotechnology Co.
Ltd., Beijing, China) for 30 min at room temperature. Between the
steps, the sections were washed in phosphate-buffered saline (PBS)
with three changes. Color was developed with a diaminobenzidine
(DAB) kit (Wuhan Boster Biological Technology Ltd., Wuhan, China).
Negative control tests were performed by replacing the primary
antibody with a non-specific mouse monoclonal antibody (Biolegend,
San Diego, CA, USA). Known EBV-positive undifferentiated
nasopharyngeal carcinoma (NPC) specimens which were collected from
the Department of Pathology (the Affiliated Hospital of Guangdong
Medical College) were set as the positive controls. The sections
were counterstained with hematoxylin prior to mounting.
Detection of EBV-encoded RNA 1 (EBER-1)
expression using in situ hybridization (ISH)
An ISH for EBER-1 test kit was purchased from
Triplex International Biosciences (China) Co., Ltd. (Fuzhou,
China). The detection procedures were conducted strictly according
to the manufacturer’s instructions, which included the following
four steps sequentially: i) Hybrid pre-treatment: 4-μm-thick
sections were routinely dewaxed and hydrated, then digested by
proteinase K (25 μg/ml) at room temperature for 4.5 min; ii)
hybridization: Following washing in distilled water for 1 min,
15–20 μl EBER-1 probe was added to the sections, which were then
covered with coverslips (provided in the kit), degenerated at 70°C
for 15 min and annealed for 10 min on ice. Subsequently, the slices
were incubated at 37°C for 16 h in a humidity chamber containing
30% formamide solution. iii) Hybrid post-processing: The sections
were soaked in 48°C PBS for 5 min to remove the coverslips,
followed by washing in 48°C PBS for 5 min three times. The sections
were incubated with mouse anti-digoxin antibody [Triplex
International Biosciences (China) Co., Ltd. (Fuzhou, China)] at
37°C for 2 h in a humidity chamber containing distilled water, then
with polymer enhancer solution for 40 min at room temperature, and
with polymerized HRP-anti-mouse IgG for 1 h at room temperature.
Between steps, the sections were washed in PBS at room temperature
for 2 min three times. iv) Coloring and mounting process: Color was
developed with DAB solution for 2–10 min and the reaction was
stopped according to microscopic evaluation. The sections were
counterstained with hematoxylin and then mounted with neutral gum.
Negative controls tests were conducted by adding hybridization
solution without a probe. Known EBV-positive undifferentiated NPC
specimens were used as the positive controls.
Serum autoantibody determination
Anti-nuclear antibodies (ANA) in the serum of
patients with LN were determined using an ELISA kit (Medibiotech
Ltd., Tianjin, China). The anti-extractable nuclear antigen
(anti-ENA) profiles, including anti-RNP, anti-SSA, anti-SSB,
anti-Jo-1, anti-Sm and anti-ds-DNA, were determined using an ENA
Profile ELISA kit (Medibiotech Ltd.).
Statistical analysis
The statistical software SPSS version 15.0 (SPSS.
Inc., Chicago, IL, USA) was used to perform the statistical
analysis of the data. The equivalence of EBV-LMP1 detection by IHC
and EBER-1 detection by ISH was assessed by the McNemar and κ
tests. Comparison of the rate of EBV-LMP1 or EBER-1 expression was
performed using the χ2 test. To investigate the
association of kidney-expressed EBV-LMP1/EBER-1 with serum
autoantibodies, the patients were divided into renal EBV-expressing
and non-expressing groups. The proportions of the patients
exhibiting sera autoantibodies were compared between groups using
the χ2 test.
Results
Distribution of EBV markers in renal
tissue
EBV-LMP1 was mainly expressed in the cytoplasm of
the renal tubular epithelial cells and was expressed at lower
levels in the cytoplasm of the podocytes, mesangial cells and
endothelial cells of the glomeruli. EBER-1 was mainly expressed in
the nuclei of the renal tubular epithelial cells, podocytes,
mesangial cells and endothelial cells. However, as identified by
ISH and IHC, there was less positive staining in the renal tissue
samples than in the undifferentiated NPC specimens under the same
experimental conditions (Fig.
1).
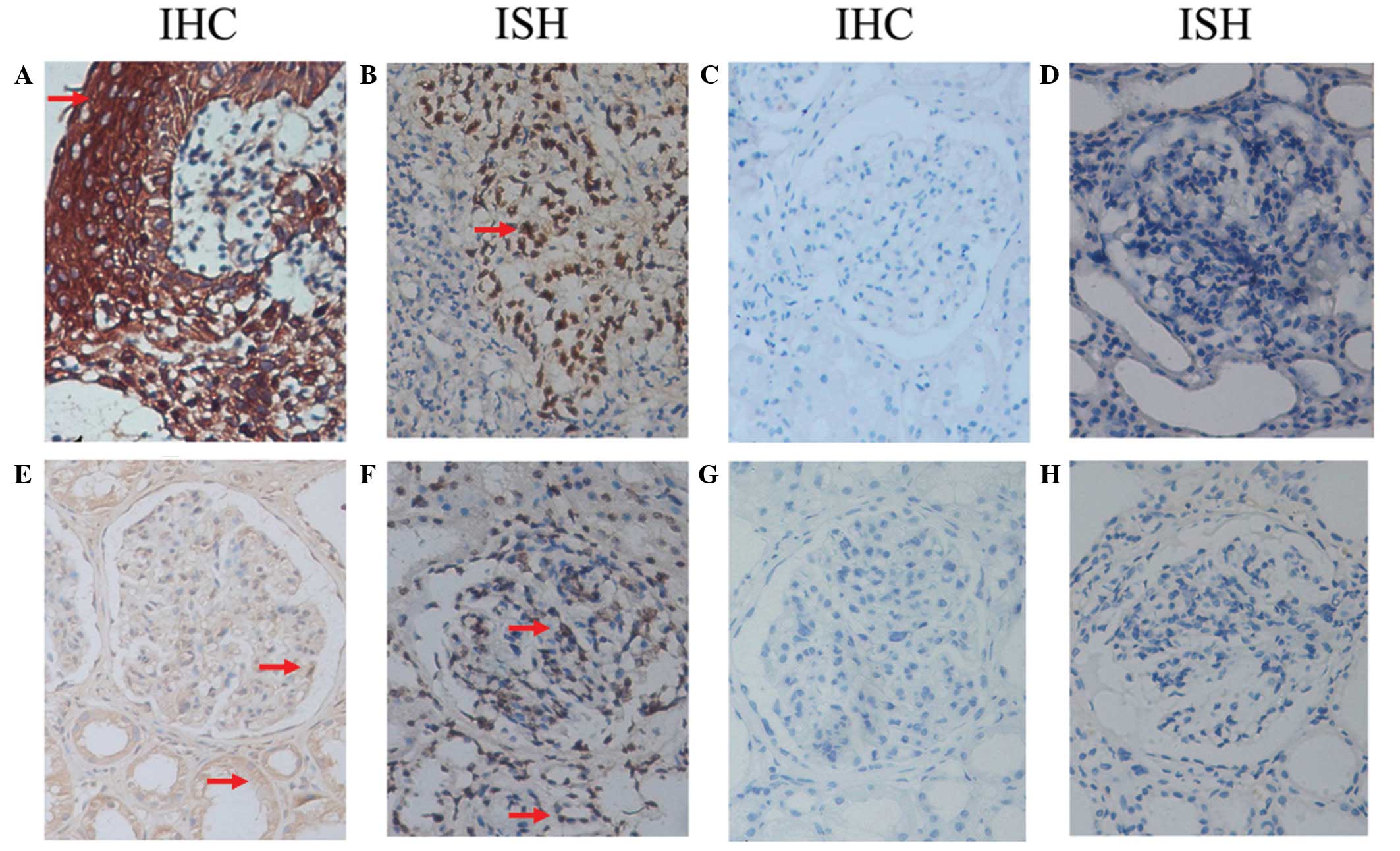 | Figure 1Distribution of EBV-LMP1 and EBER-1 in
renal tissue. (A) EBV-LMP1 was strongly expressed in the cytoplasm
and (B) EBER-1 was strongly expressed in the nuclei in the
undifferentiated NPC specimens. (C and D) Renal tissues that were
negative for EBV-LMP1 and EBER-1 expression, respectively. In the
renal tissue in which positive expression was observed, (E)
EBV-LMP1 was mainly expressed in the cytoplasm of renal tubular
epithelial cells and less expressed in the cytoplasm of the
podocytes, mesangial cells and endothelial cells of the glomeruli,
(F) while EBER-1 was mainly expressed in the nuclei of the renal
tubular epithelial cells, podocytes, mesangial cells and
endothelial cells. (G) When the primary antibody was replaced with
a non-specific mouse monoclonal antibody, EBV-LMP1 expression was
not detected in the positively expressing renal tissue. (H) When
hybridization solution without a probe was added, EBER-1 was not
detected in the positively expressing renal tissue. IHC,
immunohistochemistry; ISH, in situ hybridization; EBV,
Epstein-Barr virus; LMP1, latent membrane protein-1; EBER-1,
EBV-encoded RNA 1; NPC, nasopharyngeal carcinoma tissues. Red
arrows indicate EBV-LMP1-positive expression in IHC staining and
EBER-1 positive expression in ISH staining. |
Agreement of the results of IHC and
ISH
Of the total 102 renal tissue samples, 42 cases were
identified as expressing EBV-LMP1, while 41 renal tissue samples
were identified as expressing EBER-1. A total of 37 renal tissue
samples were positive for EBV-LMP1 and EBER-1 while 56 renal tissue
samples were negative for both. The McNemar test revealed that
there was not a statistically significant difference between the
results of the two detection methods (P=1.00) and the κ coefficient
was 0.817 (P<0.001). These results showed that there was a high
degree of consistency between the two detection methods.
Positive rates of renal EBER-1 and
EBV-LMP1 expression
EBV-LMP1 was identified in 34 (58.6%) of the renal
tissue samples from patients with LN, while there was only one
(14.3%) renal tissue sample in the normal group and seven (18.9%)
renal tissue samples in the minimal change nephropathy group in
which the expression of EBV-LMP1 was identified. The positive rate
of renal EBV-LMP1 expression in the LN group was significantly
higher than those of the normal and minimal change nephropathy
groups (P<0.001). EBER-1 was identified in 35 (60.3%) of the
renal tissue samples with LN and six (16.2%) renal tissue samples
with minimal change nephropathy, while no normal renal tissue
samples (0.0%) were identified to express EBER-1. The positive rate
of renal EBER-1 expression of the LN group was significantly higher
than those of the normal and minimal change nephropathy groups
(P<0.001), while no significant difference was identified
between the normal and minimal change nephropathy groups
(P>0.05; Table I).
 | Table IPositive rates of EBER-1 and EBV-LMP1
expression in the renal tissues of patients with LN, MCN and
non-nephropathy. |
Table I
Positive rates of EBER-1 and EBV-LMP1
expression in the renal tissues of patients with LN, MCN and
non-nephropathy.
| EBER-1 | EBV-LMP1 |
|---|
|
|
|
|---|
| Condition | Negative | Positive | Positive rate
(%) | Negative | Positive | Positive rate
(%) |
|---|
| Normal | 7 | 0 | 0.0 | 6 | 1 | 14.3 |
| MCN | 31 | 6 | 16.2 | 30 | 7 | 18.9 |
| LN | 23 | 35 | 60.3a | 24 | 34 | 58.6a |
Renal expression of EBV-LMP1 and EBER-1
in patients with LN and different clinical statuses
The positive rate of EBV-LMP1 and EBER-1 expression
in the renal tissue samples with LN was not observed to be
statistically different between the initial onset (non-treated) and
recurrent patients (immunosuppressant-treated) and between the
patients with and without concurrent infection (P>0.05; Table II).
 | Table IIPositive rates of renal EBER-1 and
EBV-LMP1 expression in patients with LN and different clinical
statuses. |
Table II
Positive rates of renal EBER-1 and
EBV-LMP1 expression in patients with LN and different clinical
statuses.
| EBER-1 | EBV-LMP1 |
|---|
|
|
|
|---|
| Clinical status | Negative | Positive | Positive rate
(%) | Negative | Positive | Positive rate
(%) |
|---|
| Disease course |
| Initial onset | 9 | 13 | 59.1 | 9 | 13 | 59.1 |
| Relapse | 14 | 22 | 61.1 | 15 | 21 | 58.3 |
| Concurrent
infection |
| Without | 9 | 17 | 65.4 | 11 | 15 | 57.7 |
| With | 14 | 18 | 56.3 | 13 | 19 | 59.4 |
Positive rates of renal EBV-LMP1 and
EBER-1 expression in patients with LN of different age ranges
When the LN patients were divided according to age
(0–19, 20–39 and ≥40 years), no significant differences in the
positive rates of renal EBV-LMP1 and EBER-1 expression were
identified among the three age groups (Table III).
 | Table IIIPositive rates of renal EBER-1 and
EBV-LMP1 expression in patients with LN of different age
ranges. |
Table III
Positive rates of renal EBER-1 and
EBV-LMP1 expression in patients with LN of different age
ranges.
| EBER-1 | EBV-LMP1 |
|---|
|
|
|
|---|
| Age range | Negative | Positive | Positive rate
(%) | Negative | Positive | Positive rate
(%) |
|---|
| 0–19 years | 4 | 7 | 63.6 | 4 | 7 | 63.6 |
| 20–39 years | 14 | 19 | 57.6 | 15 | 18 | 54.6 |
| ≥40 years | 5 | 9 | 64.3 | 1 | 13 | 64.3 |
Association of renal EBV-LMP1/EBER-1
expression with autoantibody production in patients with LN
The positive rate of serum anti-Sm in the LN
patients was significantly higher in the renal EBV-expressing group
than in the non-expressing group (P<0.05), while the positive
rates of serum ANA, anti-RNP, anti-SSA, anti-SSB, anti-Jo-1 and
anti-ds-DNA were not found to be significantly different between
the two groups (P>0.05) (Table
IV).
 | Table IVPositive rates of serum autoantibodies
between patients with and without renal EBER-1/EBV-LMP1
expression. |
Table IV
Positive rates of serum autoantibodies
between patients with and without renal EBER-1/EBV-LMP1
expression.
| Positive rate
(%) |
|---|
|
|
|---|
| EBV markers | ANA | anti-Sm | anti-RNP | anti-SSA | anti-SSB | anti-Jo-1 | anti ds-DNA |
|---|
| EBER-1 |
| Negative | 73.9 (17/23) | 8.7 (2/23) | 13.0 (3/23) | 13.0 (3/23) | 4.3 (1/23) | 0.0 (0/23) | 73.9 (17/23) |
| Positive | 68.6 (24/35) | 34.3 (12/35)a | 14.3 (5/35) | 8.6 (3/35) | 2.9 (1/35) | 2.9 (1/35) | 77.1 (27/35) |
| LMP1 |
| Negative | 79.2 (19/24) | 8.3 (2/24) | 16.7 (4/24) | 20.8 (5/24) | 8.3 (2/24) | 0.0 (0/24) | 70.8 (17/24) |
| Positive | 64.7 (22/34) | 35.3 (12/34)a | 11.8 (4/34) | 2.9 (1/34) | 0.0 (0/34) | 2.9 (1/34) | 70.6 (24/34) |
Discussion
EBV infection and NPC are highly prevalent in
southern China (15). Primary EBV
infection usually occurs in childhood, and is asymptomatic and
latently infectious. However, if primary infection occurs in
adolescence or adulthood, it may result in infectious mononucleosis
syndrome. Primary infection may contribute to viral persistence in
the human body, manifesting with an asymptomatic latent infection
status (16–18).
The detection methods for human EBV infection
include detecting EBER-1 expression or EBV DNA by ISH, detecting
EBV DNA by Southern blotting, detecting the expression of a variety
of EBV antigens by IHC, detecting the expression of a variety of
EBV antigens by serological methods, and detecting viral particles
by electron microscopy. EBER-1 is a small RNA molecule without a
poly A tail that is not translated into proteins. Copy numbers of
EBER-1 are very high and reach 106 copies in a single
host cell nucleus, and EBER-1 is currently the most abundant viral
RNA during EBV latent infection. Detection of EBER-1 by ISH is
considered as the gold standard for the identification of EBV as it
precisely identifies the sites of expression and is highly
sensitive and specific; however, ISH is expensive (19). EBV-LMP1 is a rich protein product
of EBV. Due to relatively inexpensive detecting reagents, detection
of EBV-LMP1 by IHC is usually employed to screen for EBV infection
in clinical practice (20). The
results of the present study showed that the two aforementioned
detection methods had strong agreement, which indicated that the
results were reliable.
In the present study, it was observed that the
EBV-LMP1 expression intensity and EBER-1 hybridization in the renal
tissue samples were weaker than those in the NPC tissues (the
positive controls) under the same experimental conditions, which
suggested that the number of copies of EBV in the renal tissues was
lower than that in the NPC tissues. Additionally, EBV-LMP1, a
transmembrane protein, would theoretically be only distributed in
the cytoplasm and membrane, which was confirmed in the positive
control specimens. Unexpectedly, it was also expressed in some of
the nuclei of podocytes, glomerular mesangial cells, glomerular
endothelial cells and renal tubular epithelial cells, which
requires further investigation.
It was found that the EBV positive rate in the renal
tissue samples with LN was significantly higher than those of the
renal tissue samples with minimal change nephropathy and
non-nephropathy, suggesting that EBV infection may play a role in
the pathogenesis of LN. Two hypotheses may explain these results:
i) individuals with LN genetic susceptibility may easily develop LN
following EBV infection and ii) intrinsic immune disorder and
immunosuppressant treatment in patients with LN may lead to EBV
infection vulnerability. In order to screen out the latter
possibility, the positive rate of the expression of two virus
markers was compared between initial onset (non-treated) and
relapse (immunosuppressive agent-treated) patients, patients with
and without complicating clinical infection, as well as among
patients in different age ranges. The results indicated that the
expression of the renal EBV markers (represented by the positive
rate) was not influenced by immunosuppressive agent treatment,
concurrent infection (the majority of cases being respiratory tract
infection) or age. These results suggest that EBV infection
possibly occurs prior to the onset of LN. Certain susceptible
individuals may develop LN due to the inductive effect of EBV
infection.
To further confirm our hypotheses, the association
between renal EBV infection and autoantibody production was
analyzed in the LN patients. It was found that the positive rate of
anti-Sm was higher in the group positive for the renal EBV marker
than in the group that was negative for it. This result suggested
that anti-Sm production may be associated with EBV infection.
Anti-Sm is highly specific and the detection rate is ~20–25% in SLE
patients (21). EBNA-1 is an
important EBV nuclear antigen, which contains a region of PPPGRRP
that has been considered to be highly homologous with the Sm
antigen region PPPGMRPP (22).
Poole et al (23) reported
that rabbits and rats immunized with PPPGRRP or PPPGMRPP peptide
fragments presented lupus-like autoimmunity by producing similar
autoantibodies. These results suggest that EBV infection may result
in SLE due to molecular mimicry.
In conclusion, the present study suggests that renal
EBV infection may be involved in the pathogenesis of LN, and the
mechanism is likely to be associated with the induction of
autoantibody production.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (no. 30971370/C140405).
References
|
1
|
Simard JF and Costenbader KH: What can
epidemiology tell us about systemic lupus erythematosus? Int J Clin
Pract. 61:1170–1180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pérez-Mercado AE and Vilá-Pérez S:
Cytomegalovirus as a trigger for systemic lupus erythematosus. J
Clin Rheumatol. 16:335–337. 2010.PubMed/NCBI
|
|
3
|
Pavlovic M, Kats A, Cavallo M and
Shoenfeld Y: Clinical and molecular evidence for association of SLE
with parvovirus B19. Lupus. 19:783–792. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Sun S, Li W, Li B and Li J:
Prevalence of human herpesvirus 8 infection in systemic lupus
erythematosus. Virol J. 8:2102011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barzilai O, Ram M and Shoenfeld Y: Viral
infection can induce the production of autoantibodies. Curr Opin
Rheumatol. 19:636–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans AS, Rothfield NF and Niederman JC:
Raised antibody titres to E.B. virus in systemic lupus
erythematosus. Lancet. 297:167–168. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
James JA, Kaufman KM, Farris AD,
Taylor-Albert E, Lehman TJ and Harley JB: An increased prevalence
of Epstein-Barr virus infection in young patients suggests a
possible etiology for systemic lupus erythematosus. J Clin Invest.
100:3019–3026. 1997. View Article : Google Scholar
|
|
8
|
James JA, Neas BR, Moser KL, et al:
Systemic lupus erythematosus in adults is associated with previous
Epstein-Barr virus exposure. Arthritis Rheum. 44:1122–1126. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moon UY, Park SJ, Oh ST, et al: Patients
with systemic lupus erythematosus have abnormally elevated
Epstein-Barr virus load in blood. Arthritis Res Ther. 6:R295–R302.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CJ, Lin KH, Lin SC, et al: High
prevalence of immunoglobulin A antibody against Epstein-Barr virus
capsid antigen in adult patients with lupus with disease flare:
case control studies. J Rheumatol. 32:44–47. 2005.PubMed/NCBI
|
|
11
|
Yu SF, Wu HC, Tsai WC, et al: Detecting
Epstein-Barr virus DNA from peripheral blood mononuclear cells in
adult patients with systemic lupus erythematosus in Taiwan. Med
Microbiol Immunol. 194:115–120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu JJ, Chen DY, Hsieh CW, Lan JL, Lin FJ
and Lin SH: Association of Epstein-Barr virus infection with
systemic lupus erythematosus in Taiwan. Lupus. 16:168–175.
2007.PubMed/NCBI
|
|
13
|
Poole BD, Templeton AK, Guthridge JM,
Brown EJ, Harley JB and James JA: Aberrant Epstein-Barr viral
infection in systemic lupus erythematosus. Autoimmun Rev.
8:337–342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hochberg MC: Updating the American College
of Rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40:17251997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao SM, Simons MJ and Qian CN: The
prevalence and prevention of nasopharyngeal carcinoma in China.
Chin J Cancer. 30:114–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Odumade OA, Hogquist KA and Balfour HH Jr:
Progress and problems in understanding and managing primary
Epstein-Barr virus infections. Clin Microbiol Rev. 24:193–209.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vetsika EK and Callan M: Infectious
mononucleosis and Epstein-Barr virus. Expert Rev Mol Med. 6:1–16.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okano M: Overview and problematic
standpoints of severe chronic active Epstein-Barr virus infection
syndrome. Crit Rev Oncol Hematol. 44:273–282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lerner MR, Andrews NC, Miller G and Steitz
JA: Two small RNAs encoded by Epstein-Barr virus and complexed with
protein are precipitated by antibodies from patients with systemic
lupus erythematosus. Proc Natl Acad Sci USA. 78:805–809. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gulley ML: Molecular diagnosis of
Epstein-Barr virus-related diseases. J Mol Diagn. 3:1–10. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harley JB and James JA: Autoepitopes in
lupus. J Lab Clin Med. 126:509–516. 1995.
|
|
22
|
Kaufman KM, Kirby MY, Harley JB and James
JA: Peptide mimics of a major lupus epitope of SmB/B′. Ann NY Acad
Sci. 987:215–229. 2003.PubMed/NCBI
|
|
23
|
Poole BD, Gross T, Maier S, Harley JB and
James JA: Lupus-like autoantibody development in rabbits and mice
after immunization with EBNA-1 fragments. J Autoimmun. 31:362–371.
2008. View Article : Google Scholar : PubMed/NCBI
|















