Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
autoimmune disease that is associated with progressive disability
and systemic complications (1,2). RA
is able to initiate in any joint, however, it commonly begins in
the smaller joints of the fingers, hands and wrists. Other joints
that are commonly affected include the hips, knees, ankles, feet,
neck, shoulders and elbows. In addition to joint pain and
inflammation, individuals suffering from RA may experience fatigue,
occasional fevers and a general sense of ill health (2–4). The
cellular and molecular pathology of RA involves chronic
inflammation of the synovium as well as synovial proliferation and
infiltration by macrophages, memory T cells and plasma cells.
Furthermore, marked hyperplasia of the synovium and progressive
cartilage destruction occurs, which are mediated by
cytokine-induced degradative enzymes (4,5).
Regardless of recent improvements in the treatment options for RA,
the pathology underlying inflammatory arthritis and its causative
factors have not been well described.
Selection of the appropriate medication for RA is
difficult due to the range of factors that contribute to the
development of the disease. The drugs used for RA treatment include
disease-modifying anti-rheumatic drugs, such as methotrexate,
sulfasalazine, hydroxychloroquine sulfate and azathioprine
(5–7). These may also be described as
slow-acting anti-rheumatic drugs; they suppress inflammation and
may impede the development of joint erosion. The mechanism by which
the drugs act in patients with arthritis is not currently well
understood; the majority of these drugs do not effect the
progression of the disease; however, they may relieve the arthritic
symptoms. The administration of these drugs is often limited due to
an increased risk of cardiovascular events and upper
gastrointestinal complications, such as gastric ulcers (4–6). The
long-term effects of the anti-inflammatory therapeutic agents on
the joint require further investigation; there is significant
interest in supplements, nutraceuticals and novel therapeutic
agents, which have the potential to reduce arthritic symptoms and
impede the progression of the disease.
Vitamin E includes a family of lipophilic
micronutrients consisting of four forms of tocopherols and
tocotrienols (α, β, γ and δ), which consist of a chromanol ring and
a side chain. Tocopherols and tocotrienols are found in various
components of the human diet (8–9);
tocopherols are primarily present in nuts and vegetable oils, while
tocotrienols are minor plant constituents particularly abundant in
rice bran, cereal grains and palm oil. Tocopherols have a saturated
phytyl tail, whereas tocotrienols have an unsaturated phytyl tail.
The individual isoforms of tocopherols and tocotrienols differ in
the number and position of the methyl groups attached to the
aromatic ring (10). The specific
forms of vitamin E exhibit different biopotency; in the vitamin E
group, α-tocopherol demonstrates the highest biological activity
(11–12). Tocopherols have previously been
investigated for their antioxidative, anti-inflammatory, anticancer
and antineurodegenerative effects; however, investigations
concerning the antioxidant and anti-inflammatory effects of
tocotrienols are limited. γ-tocotrienols have recently become a
point of interest due to improved therapeutic potential when
compared with tocopherols. This specific isomer has been identified
as exhibiting significant physiological activity within cell line
and animal studies and γ-tocotrienol possesses antioxidant,
anti-inflammatory, cardioprotective and neuroprotective properties
(13,14). Previous studies have identified
that the γ-tocotrienols and tocotrienol-rich fractions exhibit
significant anti-inflammatory properties (13,15,16);
however, to the best of our knowledge, no studies exist concerning
the antioxidant and anti-inflammatory effects of γ-tocotrienols in
arthritis. The present study investigated the anti-inflammatory and
antioxidant properties of γ-tocotrienols against collagen-induced
arthritis in Dark Agouti rats.
Materials and methods
Chemicals
Complete Freund’s adjuvant (CFA), type II collagen
and γ-tocotrienol were purchased from Sigma-Aldrich (St. Louis, MO,
USA). ELISA kits, obtained for the determination of superoxide
dismutase (SOD) and total glutathione (GSH), were purchased from
the Cayman Chemical Company (Ann Arbor, MI, USA). A C-reactive
protein (CRP) assay kit was purchased from AssayPro (St. Charles,
MI, USA) and a tumor necrosis factor (TNF)-α assay kit was obtained
from eBioscience (San Diego, CA, USA). The remaining chemicals and
reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animals
Female dark Agouti rats (age,10 weeks; weight,
120–140 g) were obtained from the Institute of Medical Research
(Kuala Lumpur, Malaysia). The rats were housed in individual
ventilation cages with food and water provided ad libitum.
The experimental procedures were conducted according to
internationally approved ethical guidelines for the care of
laboratory animals and study gained approval from the International
Medical University, Kuala Lumpur research and ethics committee.
The animals were randomly assigned into the
following groups: i) Control; ii) arthritis; iii) γ-tocotrienol
alone; and iv) arthritis with γ-tocotrienol.
Collagen-induced arthritis
Following an acclimatization period, the rats were
injected with collagen that was emulsified in CFA, as reported in
previous investigations (17,18).
Briefly, 5 mg collagen was dissolved in 2.5 ml cold, 0.1 M acetic
acid. This mixture was emulsified with 2.5 ml CFA and the solution
was mixed using a glass homogenizer (Fisher Scientific, Kuala
Lumpur, Malaysia) for ~15 min. The procedure for preparing this
solution was conducted on ice to ensure the proteins in the
emulsion were not denatured. Prior to receiving the injection of
the collagen-CFA mixture, the rats were anesthetized with diethyl
ether (Sigma-Aldrich, St Louis, MO, USA) and 4 mg/kg body weight
collagen-CFA emulsion was injected intradermally into the four paws
of the rat as well as in to the base of its tail.
γ-tocotrienol treatment
The rats in the γ-tocotrienol group were fed orally
with 5 mg/kg of γ-tocotrienol from day 21 of the experiment and
this treatment continued, by daily gavage, until day 45. The rats
were fed normally and had access to water ad libitum.
Evaluation of arthritis
The severity of arthritis was assessed via
measurement of paw thickness, the changes were recorded from eight
joints using a digital vernier caliper (TESA, Ludwigsburg,
Germany). The thickness of two joints was measured in each of the
four paws and the changes were recorded on alternating days.
CRP assay
The CRP level in the plasma of the experimental
animals was quantified using a rat CRP ELISA kit, in accordance
with the manufacturer’s instructions. Briefly, the lyophilised
biotinylated rat CRP was dissolved in 4 ml of enzyme immunoassay
diluent solution and 25 μl of the diluted samples were added to the
respective wells in duplicates. Subsequently, 25 μl diluted
biotinylated rat CRP was added to each of the wells. The plate was
incubated for 20°C 2 h; 50 μl diluted streptavidin peroxidase
conjugate was added to each of the wells in addition to 50 μl
chromogen substrate, which was added to each well to enable color
development. The stop solution was added to each well and the
absorbance was read at 450 nm using a microplate reader (TECAN,
Männedorf, Switzerland); the CRP concentration of each sample was
calculated based on the standard curve obtained.
TNF-α assay
The concentration of TNF-α in the plasma was
quantified using a rat TNF-α ELISA kit. Briefly, the plate was
coated with the appropriately diluted capture antibody
(pretitrated, purified antibody) one night prior to conducting the
assay. On the following day, 100 μl standard solution and the
sample was added to the respective wells in duplicate. Following
this, 100 μl diluted detection antibody solution was added to the
wells and the plate was incubated for 1 h. Diluted
avidin-horseradish peroxidase (100 μl) was added to the wells and
incubated for 30 min in the dark followed by the addition of 100 μl
substrate solution. Stop solution, 2N (H2SO4;
50 μl), was added to the wells and the absorbance was read at 450
nm using a microplate reader. The standard curve was used to
calculate the TNF-α levels in each sample.
SOD assay
The SOD concentration was quantified using a SOD
assay kit, in accordance with the manufacturer’s instructions.
Briefly, the assay buffer, sample buffer, radical detector,
xanthine oxidase (XO), plasma samples and standard solutions were
prepared in accordance with the manufacturer’s instructions.
Subsequently, 200 μl diluted radical detector was added to the
wells and 10 μl standard solution was added to the relevant wells
in duplicate. Diluted plasma samples (10 μl) were added to the
relevant sample wells and the reaction was initiated by the
addition of 20 μl XO into each well. The absorbance was read at 450
nm using a microplate reader. The standard curve was used to
calculate the SOD level of each sample.
GSH assay
The total GSH activity in the plasma of the
experimental rats was quantified using a total GSH assay kit.
Briefly, sample deproteination was conducted and 50 μl standard
solution and 50 μl sample was added to the respective wells.
Following this, 150 μl of the assay cocktail (mixture of
N-morpholino ethanesulphonic acid buffer, NADP+ and
glucose 6-phosphate, glutathione reductase and glucose 6-phosphate
dehydrogenase mixture and 5,5′dithio-bis-2-nitrobenzoic acid) was
added to each well. The absorbance was measured at time intervals
of 5 min for a total of 30 min, using a microplate reader with a
450-nm filter. The absorbance value of the standard solution was
subtracted from the values obtained from the standard and the
sample solution. A graph was plotted with the corrected absorbance
values of each of the standard solutions as a function of the
concentration of total GSH, thus the total GSH value for each
sample was calculated.
Histopathological analysis
Following the sacrifice of the rats with overdose of
anaesthesia (pentobarbital sodium), their joints were harvested,
the flesh was removed from the bone and the joint samples were
stored in 10% formalin solution for three weeks in specimen
bottles. Blocks were prepared following the decalcification of the
joints for 48 hours, the blocks were sectioned at 3–4 μm thickness
and slides were prepared and stained with hematoxylin and eosin
(H&E). The joints were evaluated and analyzed according to the
grading system adopted in a previous study (19).
Statistical analysis
Values were expressed as the mean ± standard error
of the mean. The differences were analyzed for significance using
one-way analysis of variance with Bonferroni post hoc multiple
comparisons, which were used to assess the differences observed
between the independent groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
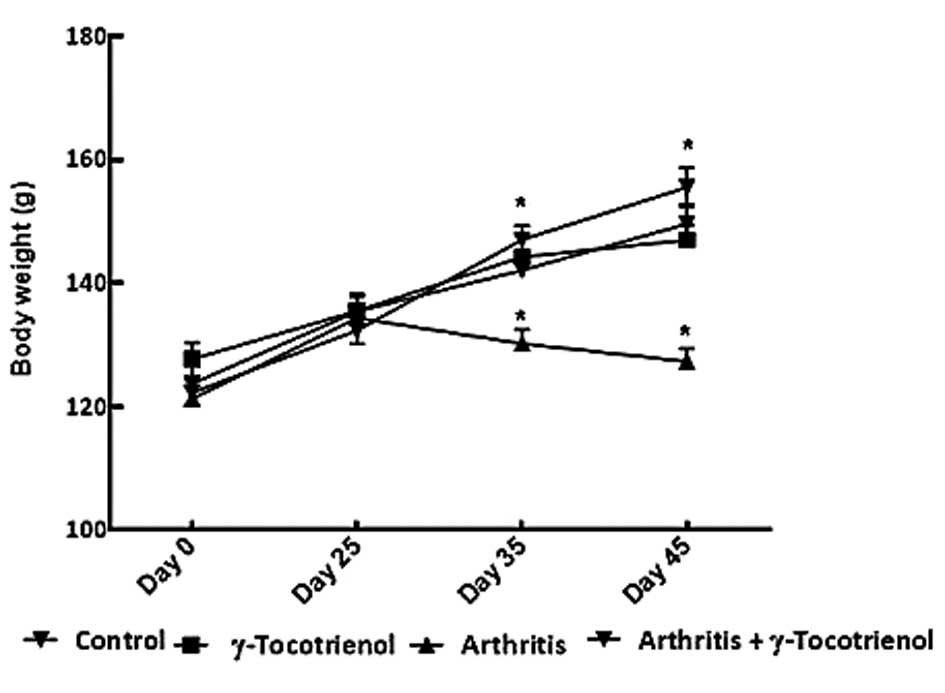
Body weight
There was a significant increase in body weight in
the normal control group and in the group with γ-tocotrienol alone
(P<0.05). The arthritis alone group exhibited a significant
decrease in body weight throughout the duration of the experiment
(P<0.05). On day 35 and 45, the arthritis group exhibited a
significant decrease in body weight compared with the other groups
(P<0.05; Fig. 1).
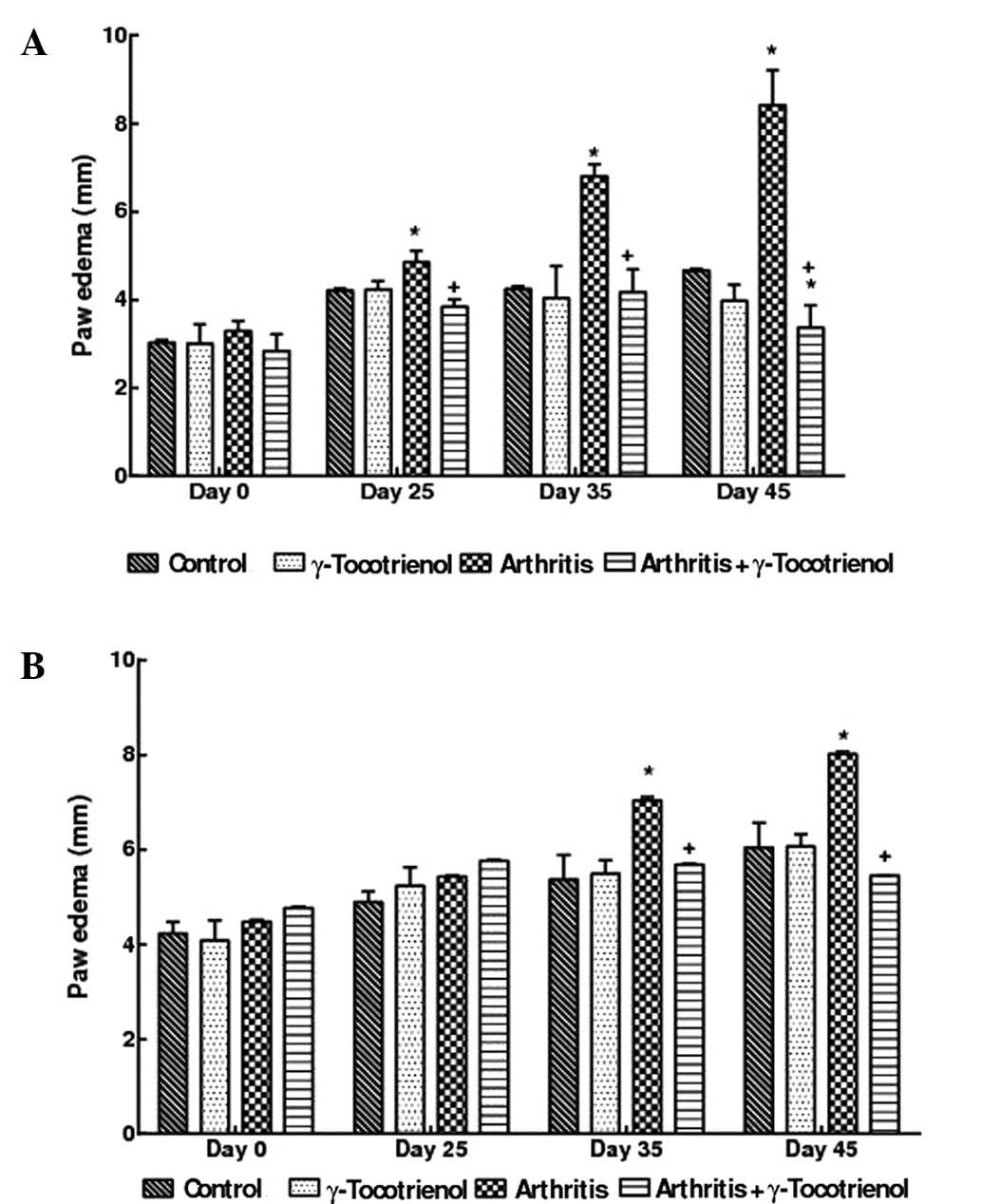
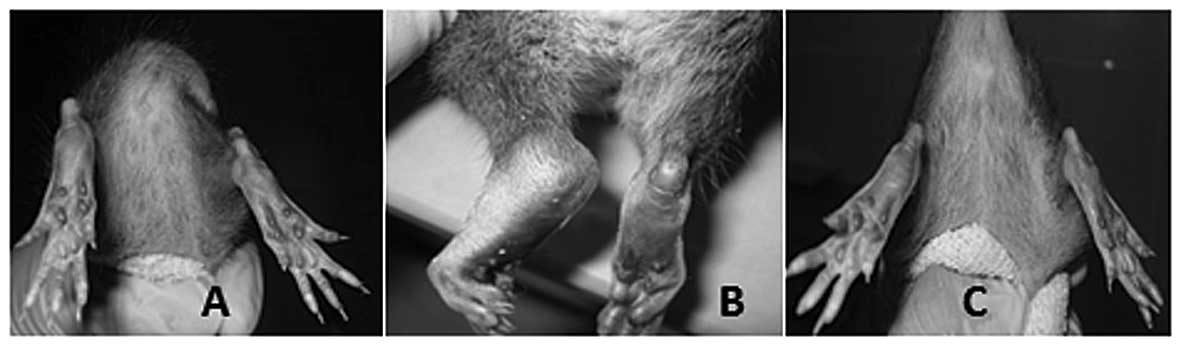
Paw thickness
The arthritis alone group showed significant,
macroscopic signs of severe arthritis such as swelling, redness,
deformity and ankylosis in the hind paw and ankle joints; however,
these symptoms were less pronounced in the forelimbs. There was a
significant decrease in the hind paw thickness and edema of the
g-tocotrienol treated arthritis rats (P<0.05) compared to
arthritis alone rats. At the end of the experimental period, the
γ-tocotrienol treated arthritis rats exhibited a hind paw thickness
that was analogous to that of the normal control rats. No
significant changes in paw thickness were observed in the
γ-tocotrienol alone group and compared with the γ-tocotrienol alone
group, the rats with arthritis and γ-tocotrienol (treatment from
day 1) exhibited a significant reduction in paw thickness
(P<0.05; Figs. 2A,B and
3).
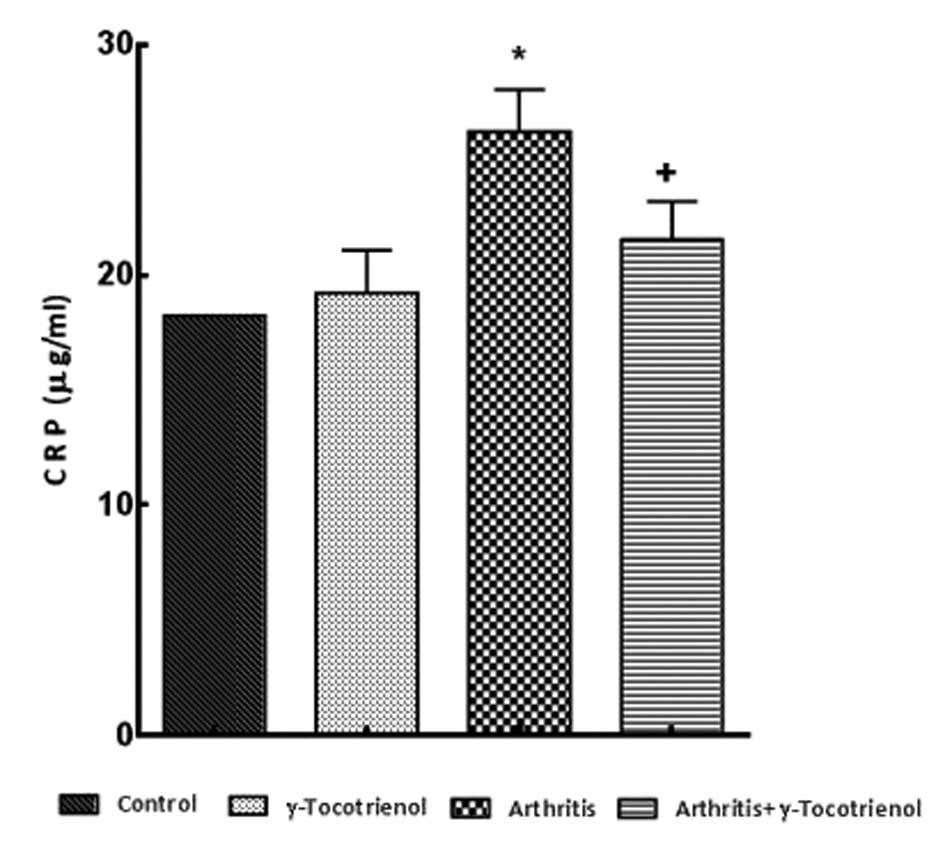
Plasma levels of CRP, TNF-α, SOD and
GSH
ELISAs were performed to quantify the CRP levels in
the plasma. There was a significantly elevated CRP concentration
observed in the untreated arthritis group and the arthritis group
treated with γ-tocotrienol, when compared with the control rats
(P<0.05). However, the CRP level was significantly decreased in
the γ-tocotrienol group when compared with the CRP levels of the
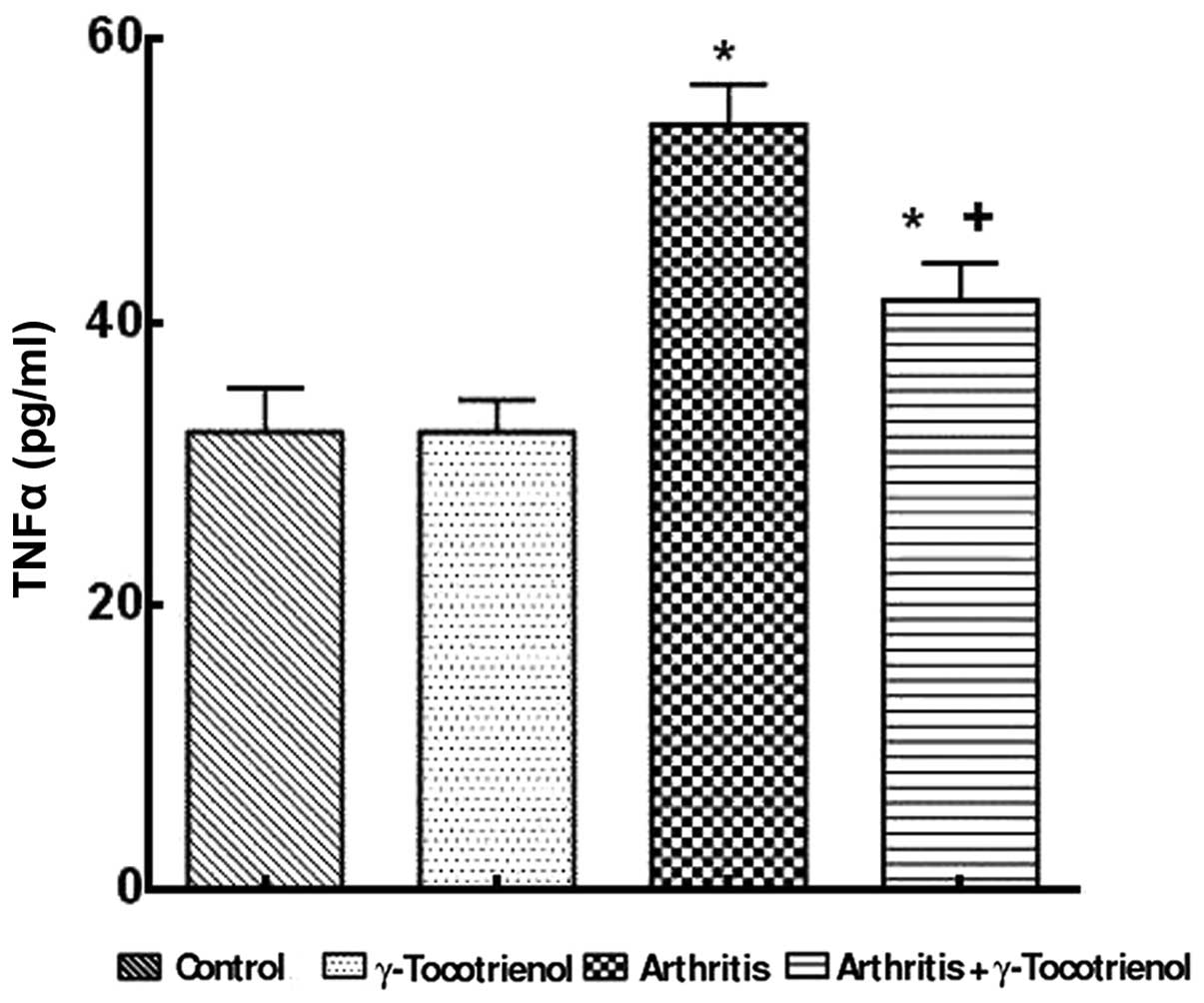
untreated arthritis group (P<0.05; Fig. 4). The untreated arthritis group
showed a significantly higher concentration of TNF-α compared with
the γ-tocotrienol-treated group (P<0.05). Treatment with
γ-tocotrienol to arthritis rats resulted in a significant reduction
in TNF-α when compared with the untreated arthritis group
(P<0.05; Fig. 5). There was a
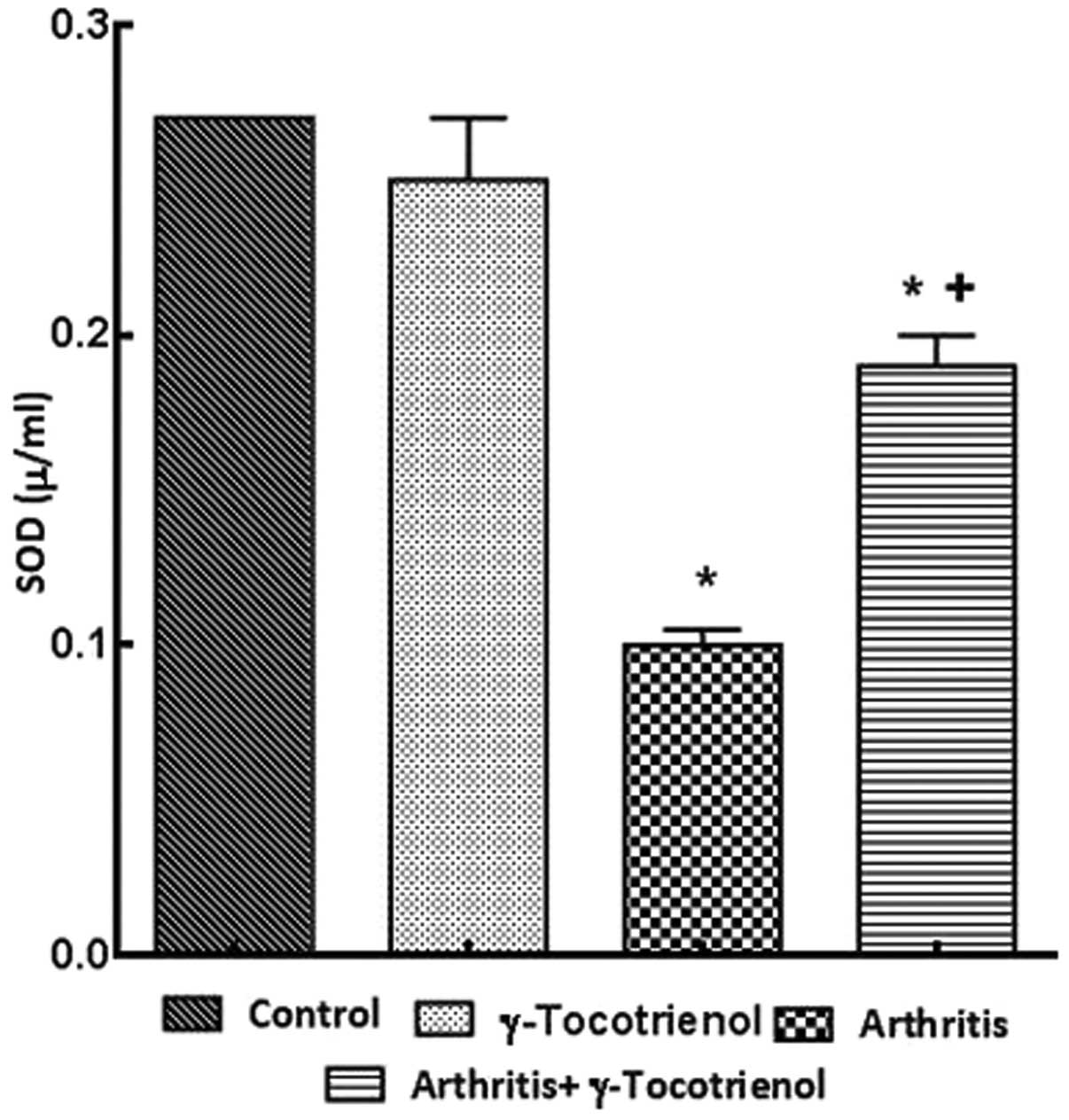
significant decrease in the SOD in the arthritis group and the
γ-tocotrienol-treated group indicated high levels of SOD
concentration when compared with the arthritis only group (Fig. 6). The γ-tocotrienol-treated
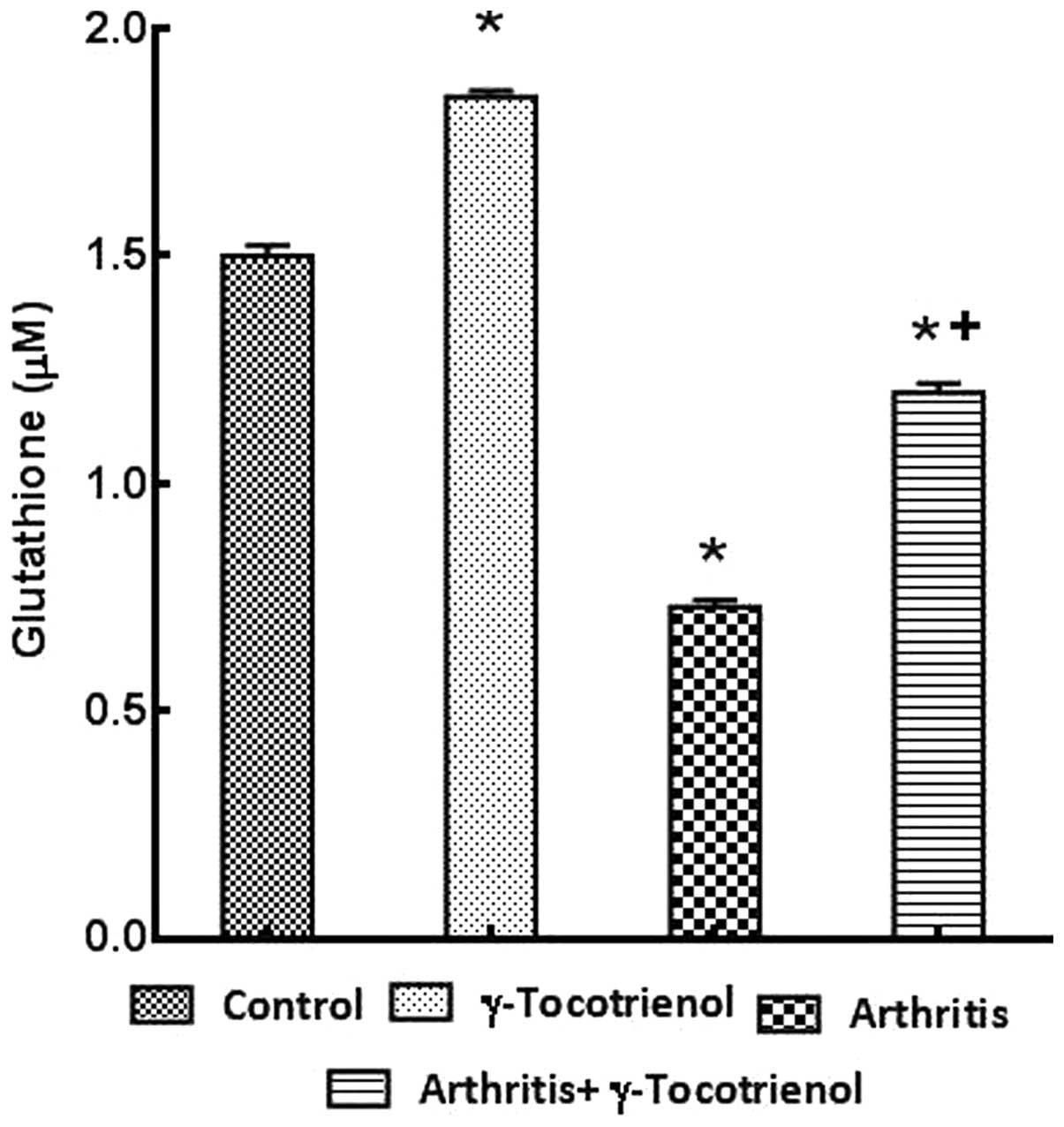
arthritis group exhibited significantly elevated levels of total
GSH when compared with the arthritis group (P<0.05; Fig. 7).
Histopathological analysis
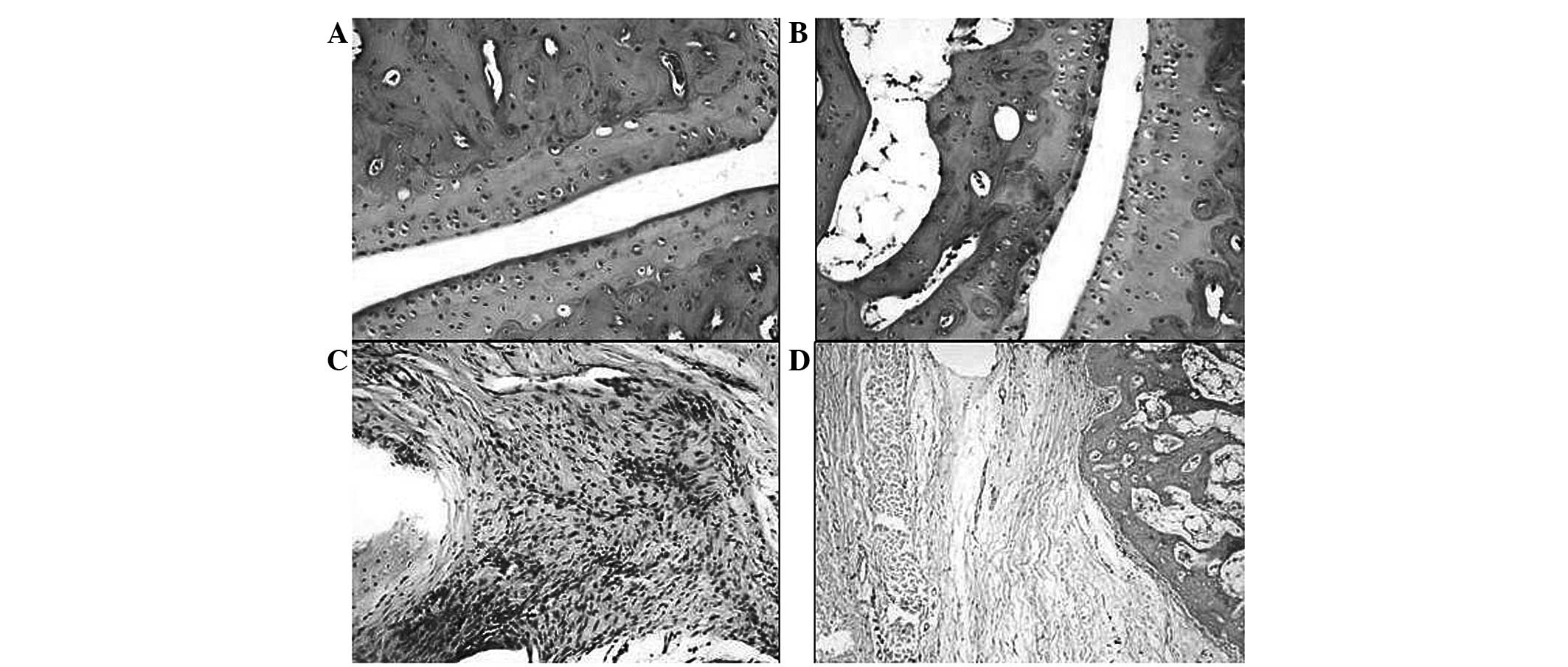
To evaluate the treatment of γ-tocotrienol against
collagen-induced arthritis, histopathological analysis was
conducted using an adapted method from a previous study (19). The arthritis only group showed a
severity of grade three, while the γ-tocotrienol-treated arthritis
group exhibited the characteristics of grade two severity. The
significance between these groups, in terms of pathological
conditions based on their grading, was calculated against the
untreated arthritis group. The γ-tocotrienol-treated arthritis
group exhibited a significant reversal in the histopathological
changes compared with the untreated group (Fig. 8).
Synovial hyperplasia was observed in the untreated
arthritis group; the rats appeared to develop extensive edema
resulting in a narrowing of the joint space. There was
inflammation, to the extent of forming panni, observed in numerous
locations within the rats in the untreated arthritis group. The
panni were composed of a granulomatous accumulation of chronic
inflammatory cells, such as lymphocytes, plasma cells, macrophages
and multinucleated giant cells. In the arthritis treated with
γ-tocotrienol group, it was observed that synovial hyperplasia was
moderately present in addition to inflammation and vascular
dilation. The inflammation was moderate and was observed as
scattered clusters of chronic inflammatory cells, with few focal
attempts at granuloma formation. In the control group the fore- and
hind limbs were identified as exhibiting a normal joint
orientation. There was no evidence of edema, cellular infiltration,
joint narrowing, synovial hyperplasia, fibrosis or erosion. In the
untreated arthritis rats, the joints exhibited extensive edema with
narrowing of the joint spaces and the surface of the joint margins
exhibited degenerative changes. In the arthritis group treated with
γ-tocotrienol there was narrowing of the joint space, however, it
was to a lesser extent than that in the untreated arthritis group
(Fig. 8).
Discussion
The present study demonstrated that γ-tocotrienols
are an effective inhibitor of arthritis-induced oxidative stress
and TNF-α secretion. To the best of our knowledge, this is the
first study to identify the anti-arthritic effect of
γ-tocotrienols, against collagen-induced arthritis, in Dark Agouti
rats. Female Dark Agouti rats, aged 6–10 weeks, were injected with
type II collagen emulsified with CFA, which induced an
immunological hypersensitivity reaction to the collagen within the
rats, leading to the development of chronic inflammatory arthritis.
The arthritis developed within 2–3 weeks of primary immunization
and exhibited characteristic arthritic pathology comparable to that
of human RA (20,21). A significant decrease in the body
weight of the rats was observed in the arthritis group compared
with the other groups, which confirmed the observations in previous
studies where collagen-induced arthritis significantly decreased
body weight, and where the body weight was reduced following three
weeks of immunization (22,23).
Body weight loss is the hallmark symptom of inflammatory arthritis,
where a gradual decrease in weight gain is observed as the disease
progresses (24). The
γ-tocotrienol-treated arthritis group experienced a significant
recovery of body weight following the second immunization.
Therefore the γ-tocotrienol supplement to arthritis rats may have
decreased the production of reactive oxygen species within the
tissues and inhibited the metabolic rate of arthritic rats;
γ-tocotrienol was able to impede the metabolism of the body, thus
favoring fat accumulation (25).
CRP is an inflammatory marker, which is a member of
the group of acute phase proteins and the level of CRP increases in
response to inflammation (26,27).
The CRP assay is used as an optimal laboratory test for the
observation of inflammation resulting from RA and other
inflammatory diseases. It is an effective indicator of tissue
damage and the concentration of CRP in serum is associated with
disease activity (27,28). In the present study, an increased
CRP level was observed in the circulation of rats with arthritis
and treatment with γ-tocotrienol significantly inhibited the
arthritis-induced CRP changes observed. The higher level of CRP
observed in the arthritis group confirmed the pathology of the
joint and the CRP production may have increased as a result of the
activated macrophages and fibroblasts within the synovium of the
inflamed joints. The production of CRP is also controlled by
inflammatory mediators within the joints including IL
(interleukin)-1 and IL-6, thus the reversal of CRP levels following
supplementation indicates a significant decrease in the activation
of synovial macrophages and fibroblasts (29).
Various inflammatory mediators are released, which
are responsible for pain in addition to swelling in the joints
observed in cases of severe arthritis. The most common inflammatory
mediators are IL-1β and TNF-α (30,31).
A series of inflammatory changes develop following the
administration of collagen in arthritic rats; joint swelling,
infiltration of inflammatory cells, bone destruction and cartilage
erosion were the significant arthritic changes that were observed
in the present study. In inflammatory arthritis, CD4+ T
helper cells are activated in the joints that stimulate the
production of cytokines and other inflammatory mediators. TNF-α is
produced by macrophages and the synovial lining, and is present at
higher concentration in individuals suffering from arthritis; TNF-α
modulates the secretion of proinflammatory cytokines (IL-1 and
IL-6) within the synovial joints (31–33).
TNF-α acts synergistically with IL-1β in the production of matrix
metalloproteinases, the expression of cell adhesion molecules and
the secretion of prostaglandins and these changes result in the
joint destruction that is associated with arthritis. The present
study demonstrated that γ-tocotrienol supplementation in arthritic
conditions attenuated the arthritis-induced elevation of the TNF-α
level. Furthermore, activation of transcription factor, nuclear
factor kappa-light-chain-enhancer of activated B cells (NF-κB) is
considered to be key in TNF-α-induced inflammatory processes,
including the upregulation of IL-6. In previous studies,
γ-tocotrienol was shown to exhibit an inhibitory effect on the
NF-κB activation pathway (33–35).
In addition, Wu et al (2008) identified that the
tocotrienol-rich fraction was capable of inhibiting proinflammatory
cytokines in human monocyte cells (36). Non-steroidal anti-inflammatory
drugs, glucocorticoids and other immunosuppressants, that are
commonly used in the treatment of RA, inhibit the NF-κB pathway and
the expression of different inflammatory-associated genes. At
present, inhibitors of NF-κB are considered to be the optimum
anti-inflammatory drug in the therapeutic treatment of arthritis
(33,37). In the present study, γ-tocotrienol
significantly inhibited the TNF-α level observed in the circulation
of the rats, which may be a result of its suppressive effect on the
activation of the NF-κB pathway within the joints. The findings
provide support for the use of γ-tocotrienol as an
anti-inflammatory candidate for the treatment of arthritis;
moreover, to the best of our knowledge, there are no known side
effects as a result of prolonged treatment.
Free radicals are significant in the induction of RA
(38); activation of mono- and
polymorphonuclear cells in the articular joints result in oxidative
damage within the joints. Increased oxidative stress is indicated
by decreased concentrations of SOD and total GSH; two significant
antioxidant enzymes within the circulation. A case of chronic
inflammatory arthritis reduces the antioxidant capacity of the body
and leads to an imbalance in the oxidant-antioxidant system
(39,40). The significant decline in the level
of SOD and GSH in the present study indicated an increase in the
accumulation of the reactive oxygen species within the synovium and
that these antioxidant enzymes were depleted due to quenching of
the free radicals (41).
Tocotrienols possess a potent antioxidant property, thus treatment
with γ-tocotrienol enabled an increase in SOD and total GSH levels
in the blood, which aided with reducing oxidant-induced joint
tissue damage. Furthermore, tocotrienols exhibit superior
antioxidant and anti-lipid peroxidation effects when compared with
tocopherols, therefore tocotrienols have gained interest. Previous
studies identified that low doses of tocotrienols were exhibiting
an improved antioxidant and free radical scavenging effect, when
compared with α-tocopherols (42,43).
γ-tocotrienol exhibits significant antioxidant activity due to an
ability for greater distribution within the membrane bilayer
(15,41,43).
It exhibits an improved ability to trap free radicals as a result
of the unsaturated double bonds within the chemical structure. The
restoration of the two antioxidant enzyme levels with γ-tocotrienol
supplementation may be attributed to the ability of γ-tocotrienol
to elevate the mRNA expression of these enzymes.
The histopathology of collagen-induced arthritis in
Dark Agouti rats indicated cartilage destruction and extensive
pannus formation, bone resorption and synovitis. Histopathological
and biomarker changes correlated with the changes observed in paw
edema. The suppression of vascularity, congestion, pannus formation
and joint space narrowing, as a result of treatment, indicated the
anti-arthritis effect of γ-tocotrienol. The γ-tocotrienols may have
suppressed the progression of arthritis by inhibiting the chronic
inflammatory phase and decreasing the free radical accumulation
within the joints, thus reducing the incidence of cartilage
destruction (6).
In conclusion, the results of the present study
indicated that γ-tocotrienol was capable of reducing the oxidative
stress and inflammation that was observed in the collagen-induced
arthritic rats. The γ-tocotrienol treatment increased the
antioxidant enzyme levels and decreased the TNF-α levels observed
in arthritic rats, which provided protection against
arthritis-induced joint damage. Histopathology indicated that the
administration of γ-tocotrienol protected the joints and prevented
the destruction of cartilage, thus significantly improving the
arthritic symptoms. Therefore, γ-tocotrienol may be an effective,
long-term anti-arthritic agent for reducing the serious side
effects of synthetic, anti-arthritis drugs.
Acknowledgements
The present study was supported by grants from the
International Medical University (Kuala Lumpur, Malaysia).
References
|
1
|
Navarro-Cano G, Del Rincón I, Pogosian S,
et al: Association of mortality with disease severity in rheumatoid
arthritis, independent of comorbidity. Arthritis Rheum.
48:2425–2433. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee DM and Weinblatt ME: Rheumatoid
arthritis. Lancet. 358:903–911. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmona L, Cross M, Williams B, Lassere M
and March L: Rheumatoid arthritis. Best Pract Res Clin Rheumatol.
24:733–745. 2010. View Article : Google Scholar
|
|
4
|
Smolen JS, Aletaha D and Redlich K: The
pathogenesis of rheumatoid arthritis: new insights from old
clinical data? Nat Rev Rheumatol. 8:235–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. N Engl J Med.
344:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herman S, Krönke G and Schett G: Molecular
mechanisms of inflammatory bone damage: emerging targets for
therapy. Trends Mol Med. 14:245–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soliman MM, Ashcroft DM, Watson KD, et al;
British Society for Rheumatology Biologics Register. Impact of
concomitant use of DMARDs on the persistence with anti-TNF
therapies in patients with rheumatoid arthritis: results from the
British Society for Rheumatology Biologics Register. Ann Rheum Dis.
70:583–589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brigelius-Flohé R and Traber MG: Vitamin
E: function and metabolism. FASEB J. 13:1145–1155. 1999.
|
|
9
|
Sokol RJ: Vitamin E and neurologic
function in man. Free Radic Biol Med. 6:189–207. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ricciarelli R, Zingg JM and Azzi A:
Vitamin E 80th anniversary: a double life, not only fighting
radicals. IUBMB Life. 52:71–76. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hosomi A, Arita M, Sato Y, Kiyose C, Ueda
T, Igarashi O, Arai H and Inoue K: Affinity for alpha-tocopherol
transfer protein as a determinant of the biological activities of
vitamin E analogs. FEBS Lett. 409:105–108. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weimann BJ and Weiser H: Functions of
vitamin E in reproduction and in prostacyclin and immunoglobulin
synthesis in rats. Am J Clin Nutr. 53(4 Suppl): 1056S–1060S.
1991.PubMed/NCBI
|
|
13
|
Sen CK, Khanna S and Roy S: Tocotrienols:
Vitamin E beyond tocopherols. Life Sci. 78:2088–2098. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schaffer S, Müller WE and Eckert GP:
Tocotrienols: constitutional effects in aging and disease. J Nutr.
135:151–154. 2005.PubMed/NCBI
|
|
15
|
Kamat JP, Sarma HD, Devasagayam TP, et al:
Tocotrienols from palm oil as effective inhibitors of protein
oxidation and lipid peroxidation in rat liver microsomes. Mol Cell
Biochem. 170:131–137. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsunaga T, Shoji A, Gu N, et al:
γ-Tocotrienol attenuates TNF-α-induced changes in secretion and
gene expression of MCP-1, IL-6 and adiponectin in 3T3-L1
adipocytes. Mol Med Rep. 5:905–909. 2012.
|
|
17
|
Trentham DE: Collagen arthritis as a
relevant model for rheumatoid arthritis. Arthritis Rheum.
25:911–916. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Asquith DL, Miller AM, McInnes IB and Liew
FY: Animal models of rheumatoid arthritis. Eur J Immunol.
39:2040–2044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KH, Chen YS, Judson JP, et al: The
effect of water extracts of Euphorbia hirta on cartilage
degeneration in arthritic rats. Malays J Pathol. 30:95–102.
2008.
|
|
20
|
Joe B and Wilder RL: Animal models of
rheumatoid arthritis. Mol Med Today. 5:367–369. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Phadke K, Fouts R and Parrish JE:
Collagen-induced and adjuvant-induced arthritis in rats.
Post-immunization treatment with collagen to suppress or abrogate
the arthritic response. Arthritis Rheum. 27:797–806. 1984.
|
|
22
|
Nagatomo F, Gu N, Fujino H, et al: Effects
of exposure to hyperbaric oxygen on oxidative stress in rats with
type II collagen-induced arthritis. Clin Exp Med. 10:7–13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tudave D, Radhakrishnan A, Chakravarthi S
and Haleagrahara N: Modulation of C-reactive protein and tumour
necrosis factor-alpha in collagen-induced arthritis in Dark Agouti
rats: impact of collagen concentration on severity of arthritis.
Inflamm Res. 60:897–907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trentham DE, Townes AS and Kang AH:
Autoimmunity to type II collagen an experimental model of
arthritis. J Exp Med. 146:857–868. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ima-Nirwana S, Norazlina M, Abd Gapor MT,
et al: Vitamin E deficiency impairs weight gain in normal and
ovariectomized growing female rats. Med J Islamic Acad Sci.
11:99–105. 2000.
|
|
26
|
Kamezaki F, Yamashita K, Kubara T, et al:
Derivatives of reactive oxygen metabolites correlates with
high-sensitivity C-reactive protein. J Atheroscler Thromb.
15:206–212. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rhodes B, Fürnrohr BG and Vyse TJ:
C-reactive protein in rheumatology: biology and genetics. Nat Rev
Rheumatol. 7:282–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Poole CD, Conway P, Reynolds A and Currie
CJ: The association between C-reactive protein and the likelihood
of progression to joint replacement in people with rheumatoid
arthritis: a retrospective observational study. BMC Musculoskelet
Disord. 9:1462008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jones NR, Pegues MA, McCrory MA, et al:
Collagen-induced arthritis is exacerbated in C-reactive
protein-deficient mice. Arthritis Rheum. 63:2641–2650. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Madhok R, Crilly A, Watson J and Capell
HA: Serum interleukin 6 levels in rheumatoid arthritis:
correlations with clinical and laboratory indices of disease
activity. Ann Rheum Dis. 52:232–234. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Goldring SR: Pathogenesis of bone erosions
in rheumatoid arthritis. Curr Opin Rheumatol. 14:406–410. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Filippin LI, Vercelino R, Marroni NP and
Xavier RM: Redox signaling and the inflammatory response in
rheumatoid arthritis. Clin Expt Immunol. 152:415–422. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feldmann M, Brennan FM, Foxwell BM and
Maini RN: The role of TNF alpha and IL-1 in rheumatoid arthritis.
Curr Dir Autoimmun. 3:188–199. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McInnes IB and Schett G: Cytokines in the
pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 7:429–442.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo YJ, Chen J, Xiong XG, Wu D, Zhu H and
Liang QH: Effect of Bizhongxiao decoction and its dismantled
formulae on IL-1 and TNF levels in collagen-induced arthritis in
rat synovial joints. Theor Biol Med Model. 9:472012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu SJ, Liu PL and Lean-Teik N:
g-Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory
property by suppressing the expression of inflammatory mediators in
human monocytic cells. Mol Nutr Food Res. 52:921–929. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okazaki Y, Sawada T, Nagatani K, et al:
Effect of nuclear factor-kappaB inhibition on rheumatoid
fibroblast-like synoviocytes and collagen induced arthritis. J
Rheumatol. 32:1440–1447. 2005.PubMed/NCBI
|
|
38
|
Taysi S, Polat F, Gul M, Sari RA and Bakan
E: Lipid peroxidation, some extracellular antioxidants, and
antioxidant enzymes in serum of patients with rheumatoid arthritis.
Rheumatol Int. 21:200–204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Blake DR, Hall ND, Treby DA, et al:
Protection against superoxide and hydrogen peroxide in synovial
fluid from rheumatoid patients. Clin Sci (Lond). 61:483–486.
1981.PubMed/NCBI
|
|
40
|
Kasama T, Kobayashi K, Sekine F, et al:
Follow-up study of lipid peroxides, superoxide dismutase and
glutathione peroxidase in the synovial membrane, serum and liver of
young and old mice with collagen-induced arthritis. Life Sci.
43:1887–1896. 1988. View Article : Google Scholar
|
|
41
|
Mahajan A and Tandon VR: Antioxidants and
rheumatoid arthritis. J Indian Rheumatol Assoc. 12:139–142.
2004.
|
|
42
|
Kamat JP, Sarma HD, Devasagayam TP,
Nesaretnam K and Basiron Y: Tocotrienols from palm oil as effective
inhibitors of protein oxidation and lipid peroxidation in rat liver
microsomes. Mol and Cell Biochem. 170:131–137. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kamat JP and Devasagayam TP: Tocotrienols
from palm oil as potent inhibitors of lipid peroxidation and
protein oxidation in rat brain mitochondria. Neurosci Lett.
195:179–182. 1995. View Article : Google Scholar : PubMed/NCBI
|