Introduction
Breast cancer is a tumor with heterogeneity, which
differs among ethnic groups and individuals (1). Ethnicity has been reported to be an
independent factor affecting the prognosis of breast cancer
(2). Estrogen receptor (ER) β, the
newly identified ER subtype, has potentially important clinical
value for the research of biological characteristics and prognosis
evaluation of breast cancer. ERα and human epidermal growth factor
receptor-2 (Her-2) are commonly used immunohistochemistry
indicators in clinical practice. These receptors are important for
the guidance of postoperative chemotherapy and endocrine therapy of
breast cancer, as well as for the evaluation of breast cancer
prognosis. Breast cancer may be divided into various molecular
subtypes based on the expression levels of ERβ, progesterone
receptor and Her-2. Different molecular subtypes among various
ethnicities have their own characteristics (3). In the present study, the expression
levels of ERβ, ERα and Her-2, as well as the distributions of
various molecular subtypes, were compared between Uygur and Han
patients with breast cancer in Xinjiang, China.
Materials and methods
Patient data
A total of 730 specimens from patients with
pathologically confirmed invasive breast cancers were used in the
study. Twenty-one cases were censored, since certain patients
succumbed to other causes or were lost to follow-up. The patients
were diagnosed and underwent surgery in the First Affiliated
Hospital of Xinjiang Medical University (Urumchi, China) between
January 2000 and December 2010. The tumors were pathologically
confirmed as clinical stage I–II invasive non-specific breast
cancer. The clinical data were complete (Table I). Among the cases, there were 446
Han patients and 263 Uygur patients. The patients were followed-up
for 2–10 years and 21 cases were censored. The patients that
succumbed to other causes or were lost to follow-up at the time of
last contact or prior to the study cut-off were censored. According
to breast cancer molecular subtypes, 519 cases were selected,
including 188 cases of luminal A (36.2%; ERα+,
PR+, Her-2−), 128 cases of luminal B (24.7%;
ERα+, PR+, Her-2+), 97 cases of
Her-2 overexpression (18.7%; ERα−, PR−,
Her-2+) and 106 cases of basal-like types (20.4%; ERα
−, PR−, Her-2−).
 | Table IClinical data of Uygur and Han breast
cancer patients. |
Table I
Clinical data of Uygur and Han breast
cancer patients.
| Clinical
features | Cases, n (%) |
|---|
| Ethnicity |
| Uygur | 263 (37.1) |
| Han | 446 (62.9) |
| Age, years |
| ≤49 | 377 (53.2) |
| ≥50 | 332 (46.8) |
| Tumor size, cm |
| ≤2 | 257 (36.2) |
| >2≤5 | 352 (49.6) |
| >5 | 100 (14.1) |
| Histological
grade |
| Grade I | 125 (17.6) |
| Grade II | 396 (55.9) |
| Grade III | 188 (26.5) |
| Pathological
stage |
| Stage I | 312 (44.0) |
| Stage II | 397 (56.0) |
| Lymph node
metastasis |
| Negative | 412 (58.1) |
| Positive | 297 (41.9) |
| ERβ expression |
| (−) | 380 (53.6) |
| (+) | 201 (28.3) |
| (++) | 69 (9.7) |
| (+++) | 59 (8.3) |
| ERα expression |
| (−) | 302 (42.6) |
| (+/++) | 169 (23.8) |
| (+++) | 238 (33.6) |
| Her-2 expression |
| (−) | 391 (55.1) |
| (+) | 88 (12.4) |
| (++) | 55 (7.8) |
| (+++) | 175 (24.7) |
| Molecular
subtype |
| Luminal A | 188 (26.5) |
| Luminal B | 128 (18.1) |
| Her-2
overexpression | 97 (13.7) |
| Basal-like | 106 (15.0) |
Prior written and informed consent was obtained from
every patient and the study was approved by the Ethics Review Board
of Xinjiang Medical University.
Immunohistochemistry
Breast cancer tissue samples were fixed with 10%
formaldehyde for 24 h, embedded in paraffin and sliced into 3-μm
thick sections. Following dewaxing with xylene, the sections were
treated with antigen retrieval reagents. After blocking, the
sections were incubated with primary antibodies at 37°C in the dark
for 1 h. The samples were then washed with phosphate-buffered
saline (PBS) and secondary antibodies were added and incubated in
the dark for 30 min. The sections were developed with
3,3′-diaminobenzidine chromogenic reagent. Finally, the sections
were counterstained with hematoxylin and eosin. A positive sample
was set up as a positive control and PBS, instead of primary
antibody, was used as a negative control. ERβ antibodies and the
working solution were purchased from Fuzhou Maixin Biotechnology
Development Co., Ltd. (Fuzhou, China). ERα and Her-2 antibodies
were obtained from Gene Tech (Shanghai) Co., Ltd. (Shanghai,
China). Primary ERβ rabbit anti-human polyclonal antibody and
HRP-polymer rabbit anti-mouse antibody were purchased from Yueyan
Biotech Company (Shanghai, China). Primary ERα rabbit anti-human
monoclonal antibody, primary PR rabbit anti-human monoclonal
antibody, primary Her-2 rabbit anti-human monoclonal antibody and
HRP-polymer rabbit anti-human antibody were purchased from ZSGB
Biotech Company (Beijing, China).
Determination of expression levels
For the determination of ERβ expression, cells with
brown or yellow staining in the nucleus were considered as
ERβ-positive cells. ERβ-positive cells were then counted. The
ERβ-positive rate was the ratio of the number of ERβ-positive cells
to the total number of cells. An ERβ-positive rate of <1% was
defined as ERβ (−), a positive rate between 1 and 10% was defined
as ERβ (+), an ERβ-positive rate between >10 and 50% was defined
as ERβ (++) and an ERβ-positive rate of >50% was ERβ (+++).
For the determination of ERα expression, cells with
brown or yellow particles in the nucleus were considered as
ERα-positive cells. Expression levels of ERα were divided into four
levels: ERα (−), positive rate <30%; ERα (+), positive rate
between 30 and 40%; ERα (++), positive rate between >40 and 60%;
ERα (+++), positive rate >60% (4).
For the determination of Her-2 expression, the Her-2
Detection Guide published by the Chinese Journal of Pathology in
2009 was used (5). Her-2
expression was defined as follows: Her-2 (−), no staining; Her-2
(+), weak or incomplete cell membrane staining; Her-2 (++), >10%
of invasive cancer cells showing weak to moderate intensity with
complete but non-uniform membrane staining or <30% of invasive
cancer cells showing; Her-2 (++), >30% of invasive cancer cells
showing strong, complete and uniform membrane staining.
Statistical analysis
Data were analyzed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA) and differences were compared using a
χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of ERβ, ERα and Her-2
in breast cancer
To determine the expression levels of ERβ, ERα and
Her-2 in breast cancer tissue, immunohistochemical staining was
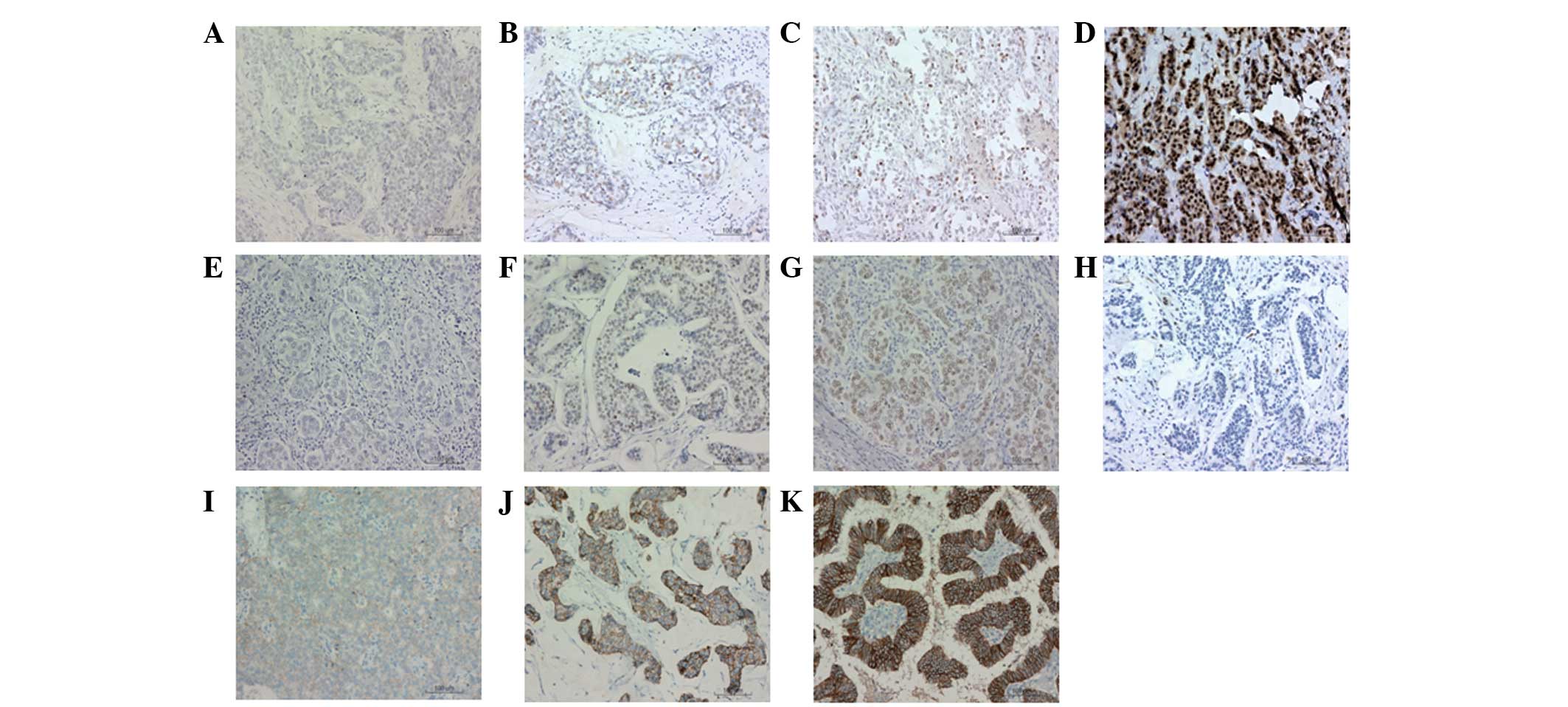
performed. Representative results are shown in Fig. 1. Cells with blue staining were
classified as negative expression cells and cells with brown
staining were classified as positive expression cells. ERβ
expression levels were divided into ERβ (−), (+), (++) and (+++)
(Fig. 1A–D, respectively). ERα
expression levels were divided into ERα (−), (+/++ ) and (+++)
(Fig. 1E–G, respectively). Her-2
expression levels were divided into Her-2 (−), (+), (++) and (+++)
(Fig. 1H–K, respectively).
Molecular subtypes of breast cancer were defined based on the
expression levels of ERα and Her-2.
Positive ERβ expression is higher in
Uygur breast cancer patients
To compare the difference in ERβ expression levels
between Uygur and Han patients, a χ2 test was performed.
The results are shown in Table
II. The percentage of ERβ (−) in Uygur patients (47.5%) was
lower than that in Han patients (57.2%). However, percentages of
ERβ (+), (++) and (+++) in Uygur patients were 29.3, 11.8 and
11.4%, respectively, which were higher than those in Han patients
(27.8, 8.5 and 6.5%, respectively). Statistically, the ERβ-positive
expression rate in Uygur patients was significantly higher when
compared with Han patients (P<0.05). This result indicates that
Uygur patients exhibited higher levels of ERβ expression.
 | Table IIComparison between ERβ expression
levels in Uygur and Han breast cancer patients. |
Table II
Comparison between ERβ expression
levels in Uygur and Han breast cancer patients.
| ERβ expression | Uygur, n (%) | Han, n (%) | χ2
value | P-value |
|---|
| (−) | 125 (47.5) | 255 (57.2) | 9.596 | 0.022a |
| (+) | 77 (29.3) | 124 (27.8) | | |
| (++) | 31 (11.8) | 38 (8.5) | | |
| (+++) | 30 (11.4) | 29 (6.5) | | |
Positive ERα expression is lower in Uygur
breast cancer patients
Differences in ERα expression levels between Uygur
and Han patients were also compared with a χ2 test. As
shown in Table III, in Uygur
patients, the percentage of ERα (−) expression (48.7%) was higher
than that in Han patients (39.0%), whereas percentages of ERα
(+/++) and (+++) expression (22.1 and 29.3%, respectively) were
lower when compared with Han patients (24.9 and 36.15%,
respectively). Statistically significant differences were observed
in ERα expression levels between Uygur and Han patients
(P<0.05). This result demonstrated that Uygur patients had lower
levels of ERα expression.
 | Table IIIComparison between ERα expression
levels in Uygur and Han breast cancer patients. |
Table III
Comparison between ERα expression
levels in Uygur and Han breast cancer patients.
| ERα expression | Uygur, n (%) | Han, n (%) | χ2
value | P-value |
|---|
| (−) | 128 (48.7) | 174 (39.0) | 6.472 | 0.039a |
| (+/++) | 58 (22.1) | 111 (24.9) | | |
| (+++) | 77 (29.3) | 161 (36.1) | | |
Positive Her-2 expression is higher in
Uygur breast cancer patients
Her-2 expression, an additional immunohistochemical
indicator of breast cancer, was further analyzed. The difference in
Her-2 expression levels between Uygur and Han individuals was
similar to ERβ expression (Table
IV). In Uygur patients, the percentage of Her-2 (−) was 49.8%,
which was lower than in Han patients (58.3%). The percentages of
Her-2 (+), (++) and (+++) in Uygur patients were 11.4, 9.9 and
28.9%, respectively, which were higher than those in Han patients
(13.0, 6.5 and 22.2%, respectively). The differences between Uygur
and Han patients were statistically significant (P<0.05), thus,
the results indicated that Uygur patients had higher levels of
Her-2 expression.
 | Table IVComparison between Her-2 expression
levels in Uygur and Han breast cancer patients. |
Table IV
Comparison between Her-2 expression
levels in Uygur and Han breast cancer patients.
| Her-2 expression | Uygur, n (%) | Han, n (%) | χ2
value | P-value |
|---|
| (−) | 131 (49.8) | 260 (58.3) | 7.951 | 0.047a |
| (+) | 30 (11.4) | 58 (13.0) | | |
| (++) | 26 (9.9) | 29 (6.5) | | |
| (+++) | 76 (28.9) | 99 (22.2) | | |
Differences in molecular subtypes between
Uygur and Han patients
As previously described, there are four molecular
subtypes of breast cancer, including luminal A, luminal B, Her-2
overexpression and basal-like types. The distributions of these
molecular subtypes in Uygur and Han patients were further
investigated. As shown in Table V,
when compared with Han patients, Uygur patients had a lower
percentage of luminal A (33.3 vs. 37.8%) and luminal B types (20.2
vs. 27.1%), however, had higher levels of Her-2 overexpression
(25.1 vs. 15.2%) and basal-like types (21.3 vs. 19.9%). These
differences between Uygur and Han patients were statistically
significant (P<0.05). Thus, the distributions of molecular
subtypes in Uygur breast cancer patients were different from those
in Han breast cancer patients.
 | Table VComparison between molecular subtypes
in Uygur and Han breast cancer patients. |
Table V
Comparison between molecular subtypes
in Uygur and Han breast cancer patients.
| Molecular
subtype | Uygur, n (%) | Han, n (%) | χ2
value | P-value |
|---|
| Luminal A | 61 (33.3) | 127 (37.8) | 9.092 | 0.028a |
| Luminal B | 37 (20.2) | 91 (27.1) | | |
| Her-2
overexpression | 46 (25.1) | 51 (15.2) | | |
| Basal-like | 39 (21.3) | 67 (19.9) | | |
Discussion
In the present study, the differences among ERβ, ERα
and Her-2 expression levels, as well as breast cancer molecular
subtype distribution, in Uygur and Han patients were compared.
ERβ was first identified in mice and rats in 1996
(6). In 1997, Dotzlaw et al
(7) observed the expression levels
of ERβ in human tumor tissues. Since then, the clinical
significance of ERβ expression has gained much attention. Järvinen
et al (8) detected 55 cases
of ERβ expression out of 92 cases (59.8%) of breast cancer; Mann
et al (9) detected 78 cases
(66%) out of 118 cases of breast cancer; Fuqua et al
(10) detected 184 cases (76%) out
of 242 cases; Han et al (11) detected 66 cases (42.6%) out of 155
cases. However, there are few studies investigating the difference
in ERβ expression between Uygur and Han populations. In the present
study, ERβ expression levels were compared in 263 Uygur and 446 Han
breast cancer patients. The results demonstrated that the
percentages of ERβ (−) in Uygur and Han patients were 47.5 and
57.2%, respectively, which was statistically significant
(P<0.05). In addition, ERβ-positive expression levels in Uygur
patients were significantly higher when compared with Han patients
(P<0.05). It has been reported that in other tumors, including
esophageal (12) and cervical
cancers (13), incidence and gene
expression differ among various ethnic groups. These differences
may be associated with genetic background, environmental factors,
lifestyle, diet and cultural level. The results of the present
study indicated that there was also a difference in ERβ expression
in breast cancer between Uygur and Han patients.
ERα is an important indicator of breast cancer
prognosis and endocrine therapy. Elledge et al (14) studied ERα expression in females of
various ethnicities, including African-American and Caucasian. The
results demonstrated that the levels of ERα expression were higher
in Caucasian and Asian females, while lower in African females. The
levels of ERα expression in Hispanic females were intermediate
(14,15). These observations indicate that ERα
expression exhibits ethnic differences. In the present study,
differences were also identified in ERα expression levels between
Uygur and Han patients. The ERα (+++) rate was 29.3% in Uygur
patients, which was significantly lower than that in Han patients
(36.1%). Schwartz et al (16) observed that the ERα-positive
expression rate of Chinese females with primary breast cancer was
lower than that of European and American females, indicating that
ERα expression may also be associated with various regions and
ethnic factors. Therefore, these observations further indicate that
the estrogen-sensitivity of breast cancer patients in Uygur
individuals may be lower than that in Han individuals, which may be
one of the reasons for the different therapeutic effects of breast
cancer in Uygur and Han individuals.
Her-2, a proto-oncogene, is a common breast cancer
gene marker that is involved in the regulation of cell growth,
proliferation and differentiation. Overexpression of Her-2 often
indicates a high degree of malignancy. Al-Abbadi et al
(17) compared Her-2 expression
levels in breast cancer between Caucasian-Americans and
African-Americans. The authors observed that there was no
significant difference between the two ethnicities. By contrast,
Yang et al (18) observed
that the Her-2 expression rate in breast infiltration ductal
carcinoma tissues of Han patients was significantly higher than in
Uygur patients, indicating that Her-2 expression exhibited ethnic
differences. Thus, whether Her-2 is differentially expressed in
different ethnicities remains controversial. In the present study,
Her-2 expression was compared between Uygur and Han individuals and
a significant difference was observed. The Her-2 (+++) expression
rate (28.9%) in Uygur individuals was significantly higher when
compared with Han individuals (22.2%; P<0.05). However, whether
the difference was caused by inherent differences in inter-ethnic
genomes or the results of different expression progression requires
further investigation (18).
Based on the differences observed in gene expression
levels in tumor tissues, Perou et al (19) divided breast cancer into five
molecular subtypes in 2000, which included luminal A, luminal B,
Her-2 overexpression and basal-like types. Different subtypes had
different prognoses. Carey et al (20) examined the immunohistochemistry
indicators of Caucasian and African-American females with breast
cancer from 24 towns and cities in eastern and central North
Carolina. The results demonstrated that there was an ethnic
difference in basal-like type distribution in this population.
Carey et al (20) compared
the molecular subtypes of African-Americans and
non-African-Americans. The authors observed that the expression
levels of basal-like and luminal A types in the two ethnicities
differed. In addition, the study revealed that Her-2 overexpression
and basal-like types had a higher histological grade and greater
cell proliferation capability as compared with other subtypes of
breast cancer, indicating poor prognosis of the Her-2
overexpression and basal-like types. The authors also found that
luminal A type cases had a better prognosis than other subtypes.
However, the differences in breast cancer subtypes of Uygur and Han
individuals in Xinjiang were not reported. In the present study,
the distribution of various breast cancer molecular subtypes were
compared in Uygur and Han patients. Luminal A type was identified
to be the main molecular subtype in Uygur and Han individuals (33.3
and 37.8%, respectively), which was similar to the results of a
previous study (16). The
percentage of luminal A type in Uygur patients was lower than that
in Han patients. However, the percentages of basal-like and Her-2
overexpression types in Uygur individuals were higher than those in
Han individuals, and the difference was statistically different
(P<0.05). Thus, the results of the present study indicate that
breast cancer in Uygur individuals has a higher degree of
malignancy than that in Han individuals.
In conclusion, in Xinjiang, the expression levels of
ERβ and Her-2 and the percentages of basal-like and Her-2
overexpression types in Uygur individuals were higher than those in
Han individuals. The expression levels of ERα and the percentages
of luminal A and luminal B types in Uygur individuals were lower
than those in Han individuals. These observations indicate that
breast cancer in Uygur patients may have a higher degree of
malignancy and poorer prognosis than that of Han patients. However,
the underlying mechanisms of these differences require further
study. The results of the present study provide experimental
evidence for evaluating prognosis and developing individualized
comprehensive treatment for Uygur and Han patients with breast
cancer.
Acknowledgements
The study was supported by a grant from the Natural
Science Foundation of Xinjiang Uygur Autonomous Region (no.
2011211A069).
References
|
1
|
Li CI, Malone KE and Daling JR:
Differences in breast cancer stage, treatment, and survival by race
and ethnicity. Arch Intern Med. 163:49–56. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao F, Wang J, Liu X, et al: Racial
differences in breast cancer survival in Yunnan. Zhong guo yi xue
li lun yu shi jian za zi. 1:778–779. 2003.(In Chinese).
|
|
3
|
Newman B, Moorman PG, Millikan R, et al:
The Carolina Breast Cancer Study: intergrating population-based
epidemiology and molecular biology. Breast Cancer Res Treat.
35:51–60. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hammond ME, Hayes DF, Wolff AC, et al:
American society of clinical oncology/college of american
pathologists guideline recommendations for immunohistochemical
testing of estrogen and progesterone receptors in breast cancer. J
Oncol Pract. 6:195–197. 2010. View Article : Google Scholar
|
|
5
|
Breast cancer compiling group. Guidelines
for HER2 detection in breast cancer, the 2009 version. Chinese
Journal of Pathology. 38:836–840. 2009.(In Chinese).
|
|
6
|
Kuiper GG, Enmark E, Pelto-Huikko M, et
al: Cloning of a novel receptor expressed in rat prostate and
ovary. Proc Natl Acad Sci USA. 93:5925–5930. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dotzlaw H, Leygue E, Watson PH and Murphy
LC: Expression of estrogen receptor-beta in human tumors. J Clin
Endocrinol Metab. 82:2371–2374. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Järvinen TA, Pelto-Huikko M, Holli K and
Isola J: Estrogen receptor beta is coexpressed with ERalpha and PR
and associated with nodal status, grade, and proliferation rate in
breast cancer. Am J Pathol. 156:29–35. 2000.PubMed/NCBI
|
|
9
|
Mann S, Laucirica R, Carlson N, et al:
Estrogen receptor beta expression in invasive breast cancer. Hum
Pathol. 32:113–118. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fuqua SA, Schiff R, Parra I, et al:
Estrogen receptor beta protein in human breast cancer: correlation
with clinical tumor parameters. Cancer Res. 63:2434–2439. 2003.
|
|
11
|
Han J, Wang PJ, Tang RY, et al: The
expression of estrogen receptor α, β in different breast tissues
and its relation with breast cancer. Tongji xue bao. 26:21–24.
2005.(In Chinese).
|
|
12
|
Li Y, Wu MB, Zhao XX, et al: Loss of
heterozygosity and cloning of human chromosome 3p24 in esophageal
carcinoma. Xinjiang Yi Ke Da Xue Xue Bao. 26:224–226. 2003.(In
Chinese).
|
|
13
|
Chen R, Li TF, Wang XL, et al: Clinical
epidemiological analysis of cervical carcinoma of 2417 cases in
Xinjiang. Zhonghua Zhong Liu Fang Zhi Za Zhi. 15:329–331. 2008.(In
Chinese).
|
|
14
|
Elledge RM, Clark GM, Chamness GC and
Osborne CK: Tumor biologic factors and breast cancer prognosis
among white, Hispanic, and black women in the United State. J Natl
Cancer Inst. 86:705–712. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miller BA, Hankey BF and Thomas TL: Impact
of sociodemographic factors, hormone receptor status, and tumor
grade on ethnic differences in tumor stage and size for breast
cancer in US women. Am J Epidemiol. 155:534–545. 2002. View Article : Google Scholar
|
|
16
|
Schwartz LH, Koerner FC, Edgerton SM, et
al: pS2 expression and response to hormonal therapy in patients
with advanced breast cancer. Cancer Res. 51:624–628.
1991.PubMed/NCBI
|
|
17
|
Al-Abbadi MA, Washington TA, Saleh HA, et
al: Differential expression of HER-2/NEU receptor of invasive
mammary carcinoma between Caucasian and African American patients
in the Detroit metropolitan area. Correlation with overall survival
and other prognostic factors. Breast Cancer Res Treat. 97:3–8.
2006. View Article : Google Scholar
|
|
18
|
Yang H, Shi X, Lv X and Li H: The
difference in expressions of CD44v5 and HER4 between Uygur and Han
with breast invasive duct carcinoma. Zhongguo Lao Nian Xue Za Zhi.
26:589–590. 2006.(In Chinese).
|
|
19
|
Perou CM, Sørlie T, Eisen MB, et al:
Molecular portraits of human breast tumours. Nature. 406:747–752.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carey LA, Perou CM, Livasy CA, et al:
Race, breast cancer subtypes, and survival in the Carolina Breast
Cancer Study. JAMA. 295:2492–2502. 2006. View Article : Google Scholar : PubMed/NCBI
|