Introduction
Metformin is widely used for the treatment of type
II diabetes; it promotes lower blood glucose levels by increasing
muscle glucose uptake, decreasing insulin resistance and improving
insulin sensitivity. Metformin has been suggested to be useful for
the treatment of diseases other than type II diabetes, including
polycystic ovary syndrome and non-alcoholic fatty liver disease,
and for reducing the risk of cardiovascular disease (1). Furthermore, a number of studies have
shown that metformin plays a role in reducing the risk of certain
central nervous system diseases, specifically Parkinson’s and
Alzheimer’s diseases (2,3). Studies have increasingly focused on
the association between metformin and cancer (4) due to data showing that metformin may
reduce the risk of cancer in and improve the prognosis of type II
diabetes (5,6). The inhibitory effect of metformin on
cancer development and tumor growth is not yet clearly understood,
but it may be due to the induction of reductions in systemic
glucose and insulin levels (4).
Conversely, numerous studies have demonstrated that metformin
causes apoptosis and may directly inhibit cell proliferation and
induce cell death (7–9). These effects of metformin are
explained by its activation of the adenosine
monophosphate-activated protein kinase (AMPK)-liver kinase B1
(LKB1) signaling pathway, downregulation of cyclin D1 and
inhibition of mammalian target of rapamycin (mTOR) activity
(10–13).
Glioblastoma multiforme is the most devastating type
of cancer of the central nervous system. The median survival time
is generally one year from the time of diagnosis. Despite advances,
chemotherapeutics have not been successful due to their high
toxicity, limited efficacy and problems with drug delivery
(14,15). Novel approaches are required for
glioblastoma treatment, including chemotherapy, radiotherapy, and
the targeting of apoptosis and cell survival regulatory machinery
(16). Given the aforementioned
characteristics of metformin, it may be a good candidate for the
treatment of glioblastoma. Furthermore, metformin crosses the
blood-brain barrier when administered orally and exerts a direct
effect on the central nervous system (17).
In the present study, based on epidemiological,
clinical and preclinical investigations, the effect of metformin on
the human T98G glioblastoma multiforme cell line was examined. The
viability of the T98G cells was assessed using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Apoptosis was induced by H2O2 and
monitored by measuring caspase-3 levels, as well as by terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and
acridine orange/ethidium bromide staining.
Materials and methods
Cell culture
T98G cells were obtained from Dr Ayhan Bilir,
Histology Department, Faculty of Medicine, Istanbul University
(Istanbul, Turkey). The T98G glioblastoma cell line was maintained
in growth medium [Dulbecco’s modified Eagle’s medium (DMEM)/F12;
Gibco-BRL, Carlsbad, CA, USA]. The medium was supplemented with 10%
fetal bovine serum (FBS; Gibco-BRL) and 1% antibiotic-antimycotic
solution (Gibco-BRL). The cells were incubated in a humidified
atmosphere at 37°C and 5% CO2. For cell harvesting,
0.05% trypsin-ethylenediamine tetraacetic acid (EDTA; Biological
Industries, Kibbutz Beit Haemek, Israel) was used. Metformin
(Sigma-Aldrich, St. Louis, MO, USA) was used at concentrations of
1, 5, 10, 50 or 100 mM and H2O2 (Acros
Organics, Fair Lawn, NJ, USA) was used at a concentration of 2.5 mM
when applied alone or in combination. Consecutive dilutions were
prepared with DMEM/F12 medium.
MTT assay
The MTT assay is based on the cleavage of yellow
tetrazolium salt MTT to purple formazan crystals by metabolically
active cells. The MTT test [Cell Proliferation kit I (MTT); Roche
Applied Science, Penzberg, Germany] was used to measure
cytotoxicity. The T98G cells were seeded in culture plates at a
density of 3×104 cells per well in 96-well plates for 24
h. The cells were grown in the 96-well plates in a final volume of
100 μl culture medium supplemented with 10% FBS and 1%
antibiotic-antimycotic solution per well. The cells were incubated
for 24 h to allow cell adhesion. The total volume was then removed,
and the cells were treated with metformin (1, 5, 10, 50 or 100 mM),
H2O2 (2.5 mM), H2O2 and
metformin, or culture medium alone and incubated for 24 h.
Following the incubation period, 10 μl MTT labeling reagent (final
concentration, 0.5 mg/ml) was added to each well. The 96-well
plates were incubated for 4 h. Subsequently, 100 μl solubilization
solution was added to each well. The 96-well plates were allowed to
stand overnight in an incubator in a humidified atmosphere.
Complete solubilization of the purple formazan crystals was then
assessed. The 96-well plates were subjected to agitation, and the
spectrophotometric absorbance of the samples was run using a
(ELISA) microplate reader (Thermo Fisher Scientific, Vantaa,
Finland) at 570 nm with a 630-nm reference. The mean absorbance of
the control wells served as 100% viability, and the absorbance of
sample wells was calculated from the following equation: Viability
(%) = optical density in the sample well/optical density in the
control well × 100.
Caspase-3 assay
A caspase-3 colorimetric protease assay (ApoTarget™
Caspase Colorimetric Protease Assay Sampler kit; Novex®,
Invitrogen Life Technologies, Carlsbad, CA, USA) was performed to
detect caspase-3 activity. In this assay, upon cleavage of the
substrate by caspase-3, light absorbance by free
p-nitroanilide (pNA) was quantified using a microplate
reader (Thermo Fisher Scientific). The T98G cells were seeded in
25-cm2 flasks and incubated for 24 h at 37°C and in 5%
CO2 to allow cell adhesion. The cells were then treated
with metformin (1, 5, 10, 50 or 100 mM), H2O2
(2.5 mM), H2O2 and metformin, or culture
medium alone, and incubated for 24 h. Following the incubation
period, the cells were detached and centrifuged to obtain a pellet.
The supernatant was removed, and the cells were resuspended in 50
μl of chilled cell lysis buffer and incubated on ice for 10 min.
The tubes were then centrifuged for 1 min in a microcentrifuge
(10,000 × g). The cytosolic extract was transferred to a fresh tube
and put on ice. The protein concentration was assayed using the
biuret method (Biuret solution; Norateks Kimya San. Tic. Ltd. Şti.,
Istanbul, Turkey). Each cytosolic extract was diluted to an
equalized protein concentration with cell lysis buffer.
Dithiothreitol (DTT; ApoTarget™ Caspase Colorimetric Protease Assay
Sampler kit; Invitrogen Life Technologies) was added to the
reaction buffer immediately prior to use. A total of 50 μl 2X
reaction buffer containing 10 mM DTT was added to each sample.
Subsequently, 5 μl Ac-Asp-Glu-Val-Asp-pNA (DEVD-pNA) substrate was
added, and the samples were incubated at 37°C for 2 h in the dark.
The absorbance of each sample was read at 405 nm. All samples and
controls were run in duplicate. The fold increase in caspase-3
activity was determined by comparison of the absorbance of pNA from
the metformin groups with that from the control group.
Acridine orange/ethidium bromide
staining
The T98G cells were seeded in 24-well plates and
treated with metformin (1, 5, 10, 50 or 100 mM),
H2O2 (2.5 mM), H2O2 and
metformin, or culture medium alone, and then incubated for 24 h.
Subsequently, diluted acridine orange (100 μg/ml; Invitrogen Life
Technologies) and ethidium bromide (100 μg/ml; Sigma-Aldrich) in
phosphate-buffered saline were added to the wells. The samples and
controls were incubated in the dark. After 10 min, the wells were
examined by fluorescence microscopy (Olympus CKX41, Olympus
U-RFLT50-20, Japan).
TUNEL assay
The T98G cells were seeded in 24-well plates and
treated with metformin (1, 5, 10, 50 or 100 mM),
H2O2 (2.5 mM), H2O2 and
metformin, or culture medium alone, and then incubated for 24 h.
Subsequently, labeling of apoptotic cells in the samples was
performed using a TUNEL kit (ApopTag; Millipore, Billerica, MA,
USA), which involves modifying DNA fragments by utilizing terminal
deoxynucleotidyl transferase. Specific staining allowed for the
detection of apoptotic cells.
Statistical analysis
All samples were run at least in triplicate. A
one-way analysis of variance was used when multiple comparisons
were made. The significance between two groups was determined using
Tukey’s test. Data are expressed as the mean ± standard error.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MTT assay
Metformin at concentrations ≥5 mM significantly
reduced cell viability compared with that of the control group at
24 h after treatment (Fig. 1A).
Metformin concentrations of 10, 50 and 100 mM reduced the cell
viability from 100±1.6 to 76.8±3.3, 31.8±5.3 and 16.7±0.7%,
respectively. Similar results were observed in the presence of 2.5
mM H2O2. H2O2 reduced
the viability of the cells by ~50%. In the presence of 2.5 mM
H2O2, metformin reduced the cell viability
compared with that of the control group at concentrations ≥1 mM.
This reduction was significant at metformin concentrations of 50
and 100 mM compared with the reduction caused by 2.5 mM
H2O2 alone (Fig.
1B).
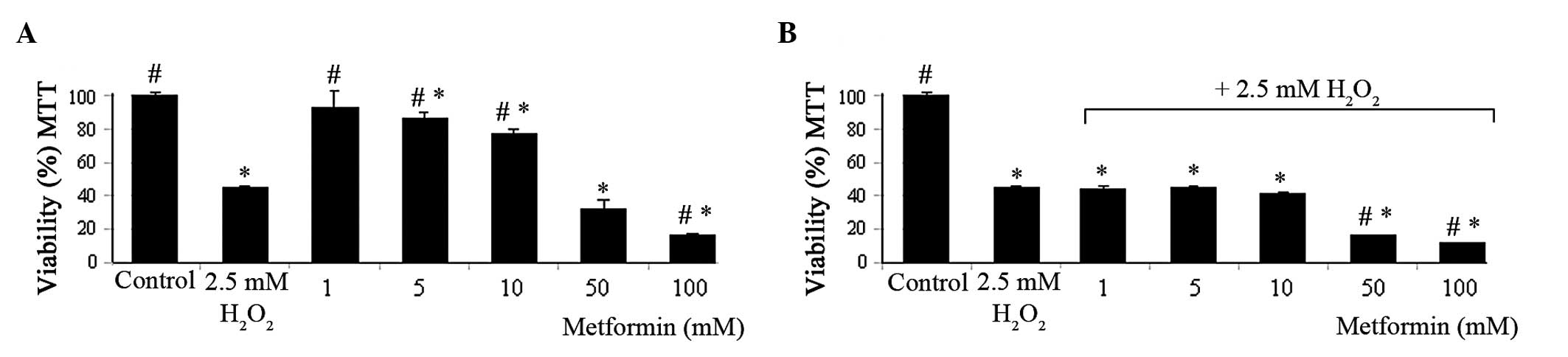 | Figure 1MTT assay viability results in T98G
cells. Viability of the T98G cells in the 24 h presence of (A)
metformin (1, 5, 10, 50 and 100 mM) and (B) metformin (1, 5, 10, 50
and 100 mM) + 2.5 mM H2O2 combination. All
data are expressed as the mean and SE from three independent
experiments. *P<0.001 vs. control,
#P<0.001 vs. 2.5 mM H2O2. MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
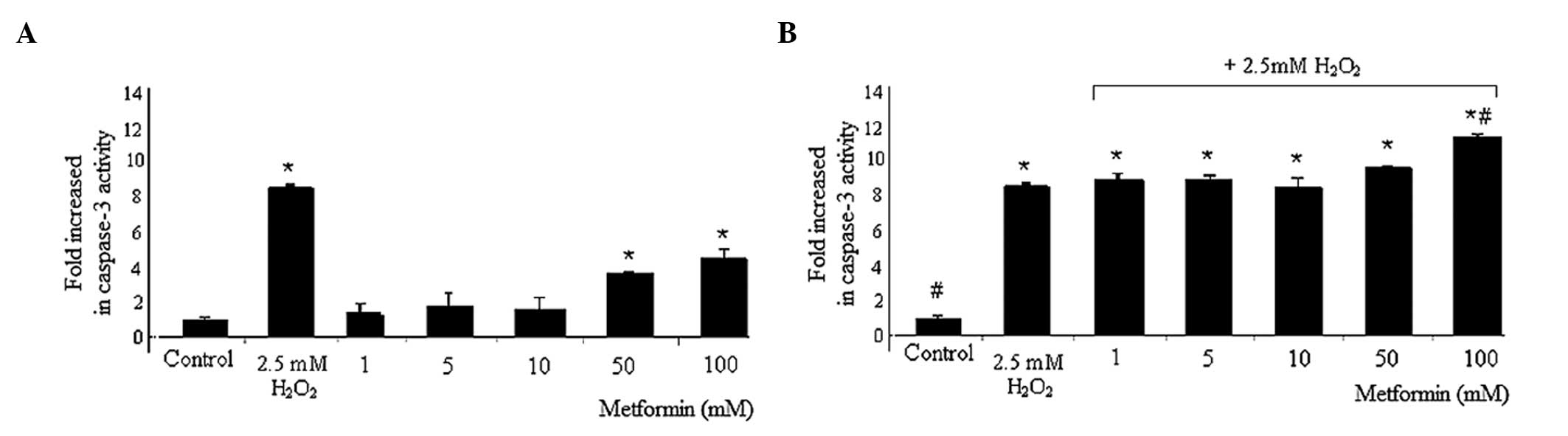
Caspase-3 assay
Compared with the caspase-3 levels in the control
cells, metformin treatment increased caspase-3 levels significantly
at 50 and 100 mM, from 1±0.20 to 3.7±0.1 and 4.9±0.6%,
respectively. However, 2.5 mM H2O2 alone also
caused a significant increase in the caspase-3 levels compared with
those in the control group (from 1±0.20 to 8.67±0.18%). In the
presence of 2.5 mM H2O2, metformin at 1, 5,
10, 50 and 100 mM increased the levels of caspase-3 from 1±0.20 to
9±0.4, 9±0.2, 8.6±0.5, 9.7±0.1 and 11.5±0.2%, respectively,
compared with those in the control group. Additionally, 100 mM
metformin + 2.5 mM H2O2 significantly
increased the levels of caspase-3 activity compared with those in
the 2.5 mM H2O2 group (11.5±0.2 vs.
8.67±0.18%, respectively; Fig.
2).
Acridine orange and ethidium bromide
staining
Apoptotic cells were not observed in the control
group. However, apoptotic changes were observed in the presence of
10, 50 and 100 mM metformin and these changes increased as the
concentration of metformin increased. Apoptotic changes were also
observed following treatment with 2.5 mM H2O2
alone and with all concentrations of metformin in the presence of
2.5 mM H2O2. Apoptotic cells are marked with
arrows in Fig. 3.
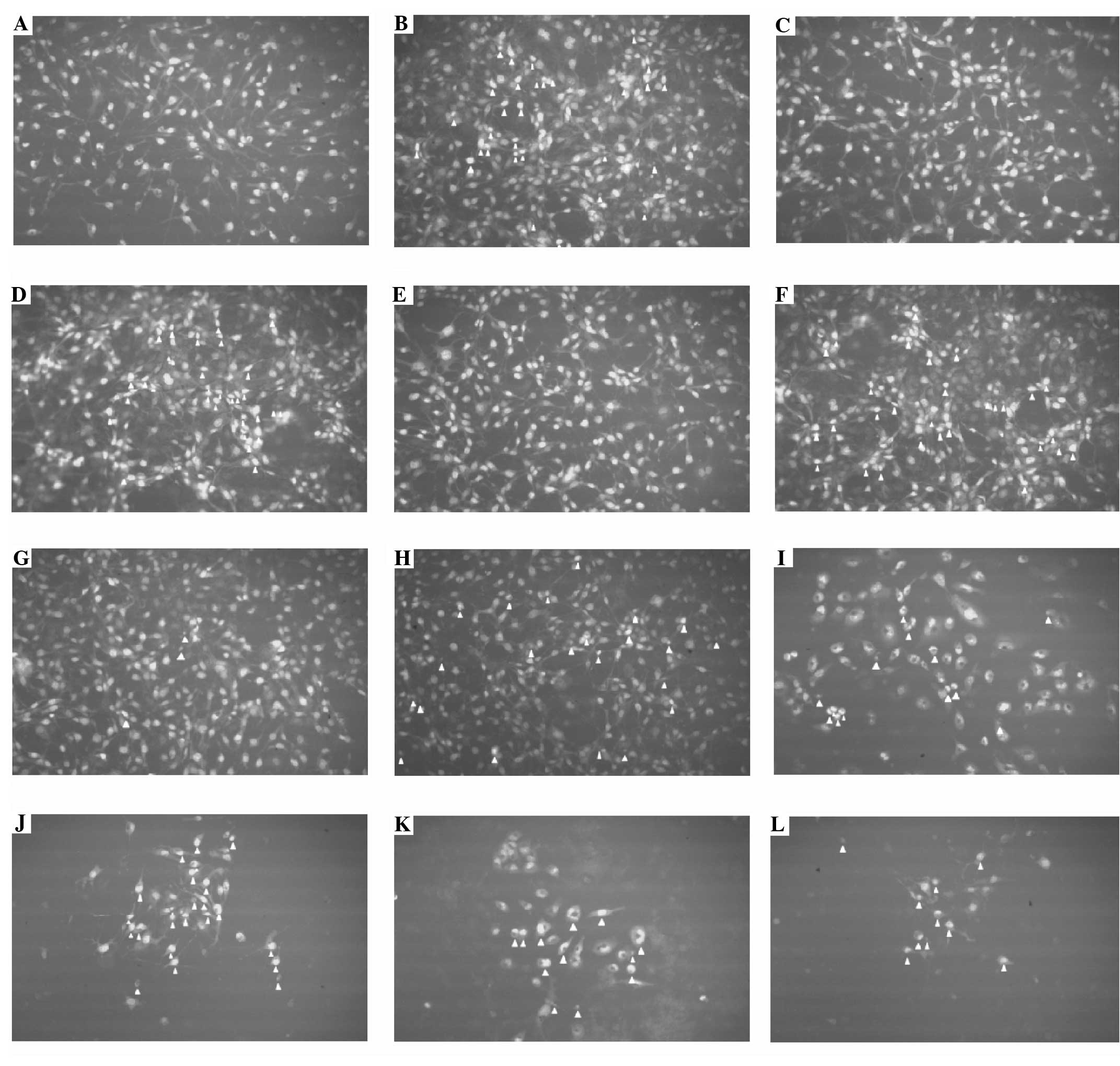 | Figure 3Ethidium bromide and acridine orange
fluorescein dye in the T98G cell line (magnification ×400).
Apoptotic cells are indicated with an arrowhead. (A) Control, (B)
2.5 mM H2O2, (C) 1 mM metformin, (D) 1 mM
metformin + 2.5 mM H2O2, (E) 5 mM metformin,
(F) 5 mM metformin + 2.5 mM H2O2, (G) 10 mM
metformin, (H) 10 mM metformin + 2.5 mM H2O2,
(I) 50 mM metformin, (J) 50 mM metformin + 2.5 mM
H2O2, (K) 100 mM metformin, (L) 100 mM
metformin + 2.5 mM H2O2. |
TUNEL assay
Incorporation of the TUNEL dye into apoptotic cells
was assessed 24 h after treatment. Metformin induced apoptotic
changes in the 50 and 100 mM groups. Apoptosis-specific
morphological changes were observed in the presence of 2.5 mM
H2O2 alone and with all concentrations of
metformin. Apoptotic cells are marked with arrows in Fig. 4.
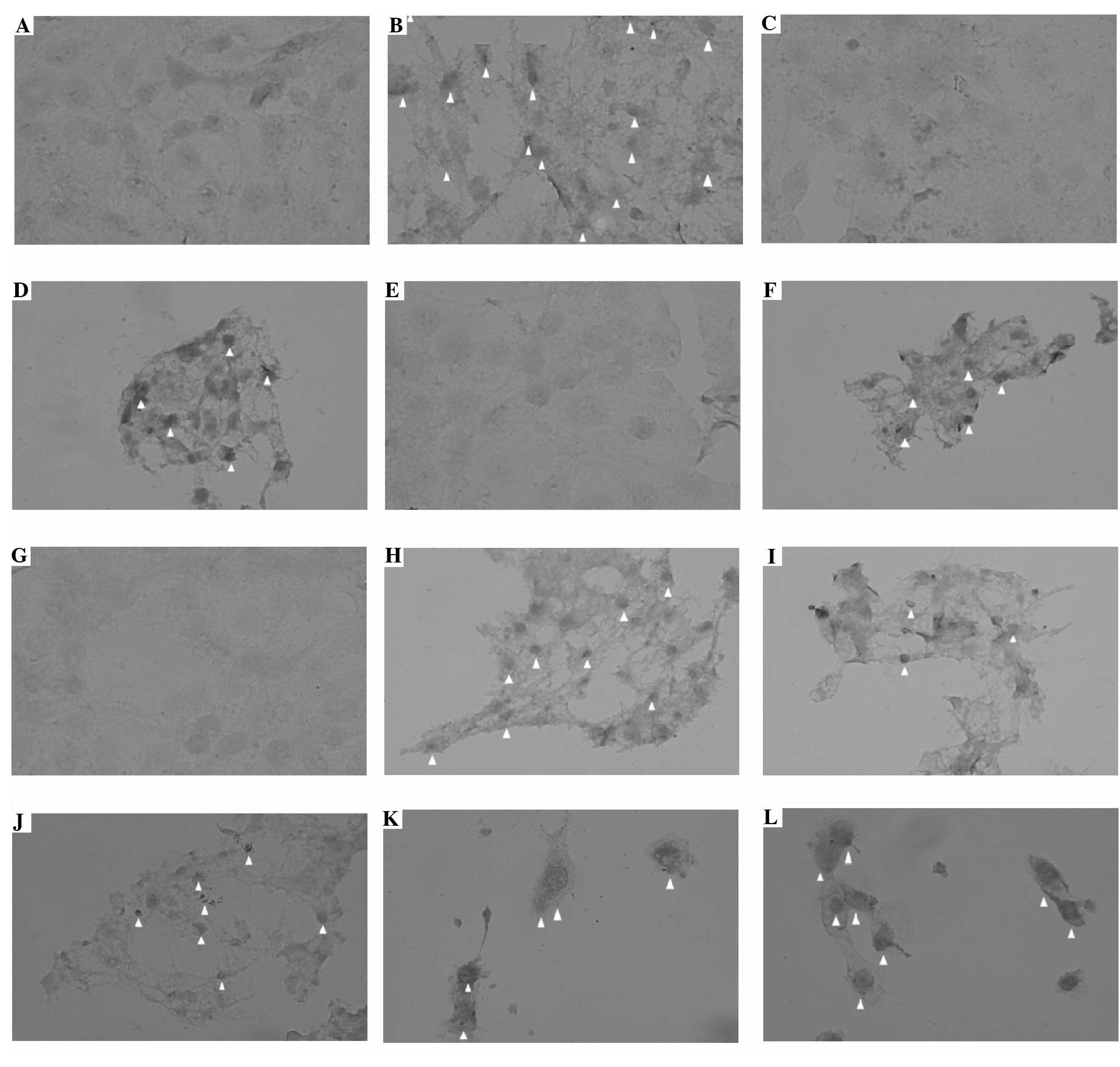 | Figure 4Apoptosis by TUNEL assay of the T98G
cell line (magnification ×400). Apoptotic cells are indicated with
an arrowhead. (A) Control, (B) 2.5 mM H2O2,
(C) 1 mM metformin, (D) 1 mM metformin + 2.5 mM
H2O2, (E) 5 mM metformin, (F) 5 mM metformin
+ 2.5 mM H2O2, (G) 10 mM metformin, (H) 10 mM
metformin + 2.5 mM H2O2, (I) 50 mM metformin,
(J) 50 mM metformin + 2.5 mM H2O2, (K) 100 mM
metformin, (L) 100 mM metformin + 2.5 mM
H2O2. TUNEL, terminal deoxynucleotidyl
transferase dUTP nick end labeling. |
Discussion
Glioblastoma is the most common and aggressive
primary brain tumor of the human central nervous system. The median
survival time is approximately one year for patients with advanced
tumors. Despite intensive efforts to identify more effective
therapies against glioblastoma, the possibility of successful
treatment is extremely poor. New approaches have demonstrated that
the induction of apoptosis in malignant cells may be a promising
strategy in cancer therapy (18).
Furthermore, metformin easily crosses the blood-brain barrier
(17). In the present study, the
effectiveness of metformin as an antiglioma agent was investigated
from this perspective. The results show that metformin exerted
apoptotic and antiproliferative effects in a
concentration-dependent manner, and that these effects were
increased in the presence of H2O2.
In the present study, it was determined using an MTT
assay that metformin inhibited the proliferation of the T98G cells,
and that the effect was more pronounced following the addition of
H2O2. This effect occurred in a
concentration-dependent manner for 1–100 mM metformin. However, it
was not possible to use this method to determine how the cells died
(19). Thus, different assays were
used to determine whether metformin exerts an apoptotic effect.
The nuclear morphology and light emission rates of
the T98G cells were evaluated by staining with acridine orange and
ethidium bromide. The data showed that apoptotic changes occurred
in the cells following treatment with 10, 50 and 100 mM metformin
and ≥1 mM metformin in the presence of H2O2.
However, throughout the experiments, cell washing and transfers may
have changed the cell population distribution and apoptotic and/or
necrotic cell rates (20). The
TUNEL and caspase-3 assays in the present study produced similar
results for ≥50 mM metformin when used alone. However, as shown in
a previous study, metformin-induced cell apoptosis may occur in a
caspase-independent manner (7). In
the present study, the effects of metformin on apoptosis were
observed in the presence of H2O2. The results
indicate that the apoptotic effect of metformin was increased when
it was applied in combination with H2O2.
The combination of metformin with cytotoxic drugs
has been shown to markedly inhibit tumor growth (21,22).
In this respect, metformin is used as an adjunct to cancer
chemotherapy (23,24). However, metformin has been
demonstrated to suppress cisplatin-induced apoptosis in cancer
cells (25). Although it has been
demonstrated in other cell lines, few studies in the literature
have reported the apoptotic effect of metformin on glioblastoma
cells (26,27). Epithelial growth factor receptor
(EGFR) in connection with the phosphoinositol-3 kinase (PI3K)
pathway signaling, as well as the association between the EGFR and
the mTOR signaling pathway, appears important to maintaining the
life of glioblastoma multiforme cells (28,29).
Therefore, PI3K and mTOR signaling pathway inhibitors alone or in
conjunction with EGFR have become a novel therapeutic target.
Metformin as an mTOR inhibitor may provide an important
contribution to the treatment of glioblastoma multiforme. The
findings of the present study show the effect of metformin on T98G
cells and may provide a novel perspective regarding the future
treatment of glioblastoma multiforme.
We predict that the direction of future treatment
for glioblastoma multiforme will be to optimize surgery to remove
the tumor and to use multi-targeted synergistic drug combinations.
The targets of the novel drugs are likely to be cell survival
mechanisms and apoptosis. In this respect, the apoptotic property
of metformin shown in the present study in T98G cells indicate that
metformin may be a good candidate for inclusion in treatment
protocols.
References
|
1
|
Campbell IW: Metformin - life begins at
50: A symposium held on the occasion of the 43rd Annual Meeting of
the European Association for the Study of Diabetes, Amsterdam, The
Netherlands, September 2007. (Meeting Report). Br J Diabet Vasc
Dis. 7:247–252. 2007. View Article : Google Scholar
|
|
2
|
Li J, Deng J, Sheng W and Zuo Z: Metformin
attenuates Alzheimer’s disease-like neuropathology in obese,
leptin-resistant mice. Pharmacol Biochem Behav. 101:564–574.
2012.PubMed/NCBI
|
|
3
|
Wahlqvist ML, Lee MS, Hsu CC, Chuang SY,
Lee JT and Tsai HN: Metformin-inclusive sulfonylurea therapy
reduces the risk of Parkinson’s disease occurring with Type 2
diabetes in a Taiwanese population cohort. Parkinsonism Relat
Disord. 18:753–758. 2012.PubMed/NCBI
|
|
4
|
Gallagher EJ and LeRoith D: Diabetes,
cancer, and metformin: connections of metabolism and cell
proliferation. Ann NY Acad Sci. 1243:54–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Libby G, Donnelly LA, Donnan PT, Alessi
DR, Morris AD and Evans JM: New users of metformin are at low risk
of incident cancer: a cohort study among people with type 2
diabetes. Diabetes Care. 32:1620–1625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evans JM, Donnelly LA, Emslie-Smith AM,
Alessi DR and Morris AD: Metformin and reduced risk of cancer in
diabetic patients. BMJ. 330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhuang Y and Miskimins WK: Metformin
induces both caspase-dependent and poly(ADP-ribose)
polymerase-dependent cell death in breast cancer cells. Mol Cancer
Res. 9:603–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Will MA, Palaniappan M, Peegel H,
Kayampilly P and Menon KM: Metformin: direct inhibition of rat
ovarian theca-interstitial cell proliferation. Fertil Steril.
98:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colquhoun AJ, Venier NA, Vandersluis AD,
Besla R, Sugar LM, Kiss A, Fleshner NE, Pollak M, Klotz LH and
Venkateswaran V: Metformin enhances the antiproliferative and
apoptotic effect of bicalutamide in prostate cancer. Prostate
Cancer Prostatic Dis. 15:346–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, et al: Role of AMP-activated protein kinase in
mechanism of metformin action. J Clin Invest. 108:1167–1174. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shaw RJ, Lamia KA, Vasquez D, Koo SH,
Bardeesy N, Depinho RA, Montminy M and Cantley LC: The kinase LKB1
mediates glucose homeostasis in liver and therapeutic effects of
metformin. Science. 310:1642–1646. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ben Sahra I, Regazzetti C, Robert G,
Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF,
Giorgetti-Peraldi S and Bost F: Metformin, independent of AMPK,
induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer
Res. 71:4366–4372. 2011.PubMed/NCBI
|
|
13
|
Zhuang Y and Miskimins WK: Cell cycle
arrest in Metformin treated breast cancer cells involves activation
of AMPK, downregulation of cyclin D1, and requires p27Kip1 or
p21Cip1. J Mol Signal. 3:182008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peacock KH and Lesser GJ: Current
therapeutic approaches in patients with brain metastases. Curr
Treat Options Oncol. 7:479–489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Atsina KB, Himes BT, Strohbehn GW
and Saltzman WM: Novel delivery strategies for glioblastoma. Cancer
J. 18:89–99. 2012. View Article : Google Scholar
|
|
16
|
Krakstad C and Chekenya M: Survival
signalling and apoptosis resistance in glioblastomas: opportunities
for targeted therapeutics. Mol Cancer. 9:1352010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Łabuzek K, Suchy D, Gabryel B, Bielecka A,
Liber S and Okopień B: Quantification of metformin by the HPLC
method in brain regions, cerebrospinal fluid and plasma of rats
treated with lipopolysaccharide. Pharmacol Rep. 62:956–965.
2010.PubMed/NCBI
|
|
18
|
Ferreira CG, Epping M, Kruyt FA and
Giaccone G: Apoptosis: target of cancer therapy. Clin Cancer Res.
8:2024–2034. 2002.PubMed/NCBI
|
|
19
|
Twentyman PR and Luscombe M: A study of
some variables in a tetrazolium dye (MTT) based assay for cell
growth and chemosensitivity. Br J Cancer. 56:279–285. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muppidi J, Porter M and Siegel RM:
Measurement of apoptosis and other forms of cell death. Curr Protoc
Immunol. Chapter 3(Unit 3): 172004.PubMed/NCBI
|
|
21
|
Ben Sahra I, Le Marchand-Brustel Y, Tanti
JF and Bost F: Metformin in cancer therapy: a new perspective for
an old antidiabetic drug? Mol Cancer Ther. 9:1092–1099. 2010.
|
|
22
|
Jalving M, Gietema JA, Lefrandt JD, de
Jong S, Reyners AK, Gans RO and de Vries EG: Metformin: taking away
the candy for cancer? Eur J Cancer. 46:2369–2380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iliopoulos D, Hirsch HA and Struhl K:
Metformin decreases the dose of chemotherapy for prolonging tumor
remission in mouse xenografts involving multiple cancer cell types.
Cancer Res. 71:3196–3201. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Janjetovic K, Vucicevic L, Misirkic M,
Vilimanovich U, Tovilovic G, Zogovic N, et al: Metformin reduces
cisplatin-mediated apoptotic death of cancer cells through
AMPK-independent activation of AKT. Eur J Pharmacol. 651:41–50.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rattan R, Graham RP, Maguire JL, Giri S
and Shridhar V: Metformin suppresses ovarian cancer growth and
metastasis with enhancement of cisplatin cytotoxicity in vivo.
Neoplasia. 13:483–491. 2011.PubMed/NCBI
|
|
26
|
Isakovic A, Harhaji L, Stevanovic D,
Markovic Z, Sumarac-Dumanovic M, Starcevic V, Micic D and Trajkovic
V: Dual antiglioma action of metformin: cell cycle arrest and
mitochondria-dependent apoptosis. Cell Mol Life Sci. 64:1290–1302.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vucicevic L, Misirkic M, Janjetovic K,
Harhaji-Trajkovic L, Prica M, Stevanovic D, Isenovic E, Sudar E,
Sumarac-Dumanovic M, Micic D and Trajkovic V: AMP-activated protein
kinase-dependent and -independent mechanisms underlying in vitro
antiglioma action of compound C. Biochem Pharmacol. 77:1684–1693.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kraskstad C and Chekenya M: Survival
signalling and apoptosis resistance in glioblastomas: opportunities
for targeted therapeutics. Mol Cancer. 9:1352010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao H, Lebrun DG, Yang J, Zhu VF and Li M:
Deregulated signaling pathways in glioblastoma multiforme:
molecular mechanisms and therapeutic targets. Cancer Invest.
30:48–56. 2012. View Article : Google Scholar : PubMed/NCBI
|