Introduction
Hematopoietic stem cells (HSCs) are an important
index that reflect the function and status of hematopoiesis in the
bone marrow, the growth of which requires a number of stimulation
factors (1,2). It has been reported that >10 types
of cell growth factors exist with various stimulation effects. Cell
growth factors are part of a complex system that has several
influencing factors (3–5). The secretion, release and function of
cell growth factors depend on the hematopoietic microenvironment,
which is important for HSC growth and development. Bone marrow
stromal cells (BMSCs) are the main components of the hematopoietic
microenvironment. Thus, studying the effects of BMSCs on
hematopoiesis is important for investigating the hematopoietic
function and microenvironment status. BMSCs constitute the main
backbone of the bone marrow microenvironment (BMM) and perform a
variety of functions involved with extracellular matrix secretion,
cell surface adhesion, cytokine solubility and membrane
connectivity. BMSCs can regulate and control their proliferation,
differentiation and survival by adhering to HSCs (6,7).
Whether under physiological status or stress conditions, the
structural and functional integrity of BMSCs is important for
maintaining stability and reconstructing hematopoietic function in
organisms (8,9). A lack of BMSCs may result in the
apoptosis of partially immature B lymphocytes (10).
Methylthiouracil (MTU; chemical name,
6-methyl-2-thiourea pyrimidine; Aladdin Co., Ltd., Chengdu, China)
is one of the mostly commonly used medicines for the treatment of
hyperthyroidism (11). Antithyroid
drugs have been shown to damage and poison the circulatory system,
among which myelosuppression is the most severe effect (12,13).
These factors indicate that MTU affects HSCs to a varying degree.
However, there are no studies investigating whether MTU affects the
BMM, which HSCs depend on to survive, as well as the essential
components of the BMM, which are the BMSCs. Therefore, the aim of
the present study was to investigate the effects of MTU on BMSC
proliferation and apoptosis.
Materials and methods
Animals
Specific-pathogen free Sprague-Dawley (SD) rats were
provided by the Laboratory Animal Center of Guangdong Medical
College (Zhanjiang, China). The rats were aged between 3 and 4
weeks, weighed 100–120 g and were selected without gender
limitation. The study was conducted in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health. The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of Guangdong Medical College.
Isolation and cultivation of BMSCs in
vitro
SD rats weighing 100–120 g were selected for the
study. Bone marrow mononuclear cells were extracted and the
karyocyte concentration was adjusted to 2×105 cells/ml.
The samples were then inoculated in 25 cm2 plastic
culture flasks. These cells were the primary cells and designated
as the P0 generation. The P0 generation was cultivated in an
incubator with conditions of 5% CO2 under a saturated
humidity of 37°C. Following inoculation for 72 h, semiliquid
exchange was conducted and the medium was changed every 2 days
until 80–90% of the cells fused with each other, at which the
digestion passage was able to be processed. Inoculative cell
passage was then performed at a ratio of 1:2 and the cells were
designated as the P1 generation. The culture fluid of the cell
passage was changed every 2 or 3 days until the cells fused with
each other and spread over the bottom of the flask. The
aforementioned procedure was then repeated. By parity of reasoning,
the next generation was considered the second BMSC generation and
marked as the P2 generation. Subsequent cells following the P2
generation were all cultivated with 10% cell culture fluid.
Identification of BMSCs
Surface antigen markers of BMSCs were detected by
flow cytometry (FCM; Beckman-Coulter Co., Ltd., Beijing, China).
The well-grown P3 generation was obtained and then rinsed three
times with phosphate-buffered saline (PBS). Following digestion
with 0.05% trypsin, the samples were centrifuged at a speed of 100
× g for 10 min. The cell suspension was then processed with PBS and
the cells were counted simultaneously. Next, the cell suspension
concentration was adjusted to 2×106 cells/ml, equally
transferred to four eppendorf tubes for 5 min and centrifuged at
100 × g. The supernatant was removed and 100 μl CD34-fluorescein
isothiocyanate (FITC), CD45-FITC, CD90-FITC and rabbit IgG-FITC
antibody (antibody titer, 1:50) were added to each tube. A blank
control tube was prepared for comparison. Following thorough
mixing, the cells were incubated for 30 min in the dark at room
temperature. Finally, the samples were centrifuged at 100 × g for 5
min, the supernatant was removed and each tube was prepared for
detection following resuspension with 500 μl PBS.
MTT method
Well-grown P3 and P5 generations of BMSCs were
obtained for inoculation and cultivated in an incubator under
saturated humidity, 5% CO2 and 37°C for 1, 2, 3, 4, 5,
6, 7 or 8 days. Next, 10 μl MTT solution (5 mg/ml) was added to
each well and the samples were incubated for 4 h at 37°C following
mixing. The supernatant was removed and 150 μl dimethyl sulfoxide
solution was added to each well. Following oscillation using a
microdosis oscillator for 10 min, the optical density (OD) of each
group was detected with a Danny microplate reader at a wavelength
of 570 nm. The results were recorded and the BMSC growth curve was
plotted with time as the x-axis and OD as the y-axis.
Detection of BMSC proliferation
Well-grown BMSCs in a logarithmic growth phase were
collected for the experimental study. The cell concentration was
adjusted to 3–5×104 cells/ml and the samples were
inoculated in a 96-well plate with six repeated wells for each
group. The celliferous nutrient solution of the negative control
group was added to 100 μl cell culture fluid (10%), whereas the
cells of the experimental groups were added to 90 μl cell culture
fluid (10%) with 10 μl MTU solution at a concentration of 10, 20,
40, 80, 160 or 320 mmol/l. A blank control well was prepared for
each group. Following inoculation, the cells were cultivated in an
incubator with 5% CO2 at 37°C for 12, 36 or 48 h.
Subsequently, 10 μl Cell Counting Kit-8 (CCK-8; Guangzhou Weijia
Bio Co., Ltd., Guangzhou, China) solution was added to each well
and further incubated for an additional 2 or 3 h at 37°C. The OD
values of each group were detected using a Danny microplate reader
at a wavelength of 450 nm. The results were recorded and the BMSC
growth curve was plotted with the MTU concentration as the x-axis
and OD as the y-axis.
Detection of BMSC apoptosis
Well-grown BMSCs in a logarithmic growth phase were
obtained for the experimental study. Cell concentration was
adjusted to 2×104 cells/ml and samples were inoculated
in a 96-well culture plate with six repeated wells for each group.
Following cultivation in an incubator with conditions of 5%
CO2 and 37°C for 24 h, the culture medium was replaced
with serum-free Dulbecco’s modified Eagle’s medium for continuous
cultivation for 12 h and the supernatant was discarded. The
negative control group (celliferous nutrient solution) was added to
2 ml cell culture fluid (10%), whereas the cells of the
experimental groups (celliferous MTU culture medium in various
concentrations) were added to 1.8 ml cell culture fluid (10%) with
0.2 ml MTU solution at a concentration of 10, 20, 40, 80, 160 or
320 mmol/l. Following cultivation in an incubator with 5%
CO2 at 37°C for 48 h, the BMSCs of each group were
digested and collected. Cells were rinsed twice with precooled PBS,
centrifuged for 5 min at 100 × g and resuspended with 500 μl
combined buffer solution. Next, ~5 μl annexin V-FITC and 10 μl
propidium iodide (PI) solution were added and incubated for 5 min
at room temperature in the dark. Annexin V-FITC presented as green
fluorescence, while PI presented as red fluorescence.
Statistical analysis
Experimental data were processed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA) and are presented as the
mean ± SD. Mean comparison among multiple samples was performed by
one-factor analysis of variance, whereas comparisons between any
two mean values of multiple samples were performed using the
homogeneity test for variance. When the population variance was
homoscedastic, Fisher’s least significance difference test was
adopted, while in other cases, Tamhane’s T2 test was selected.
P<0.05 was considered to indicate a statistically significant
difference.
Results
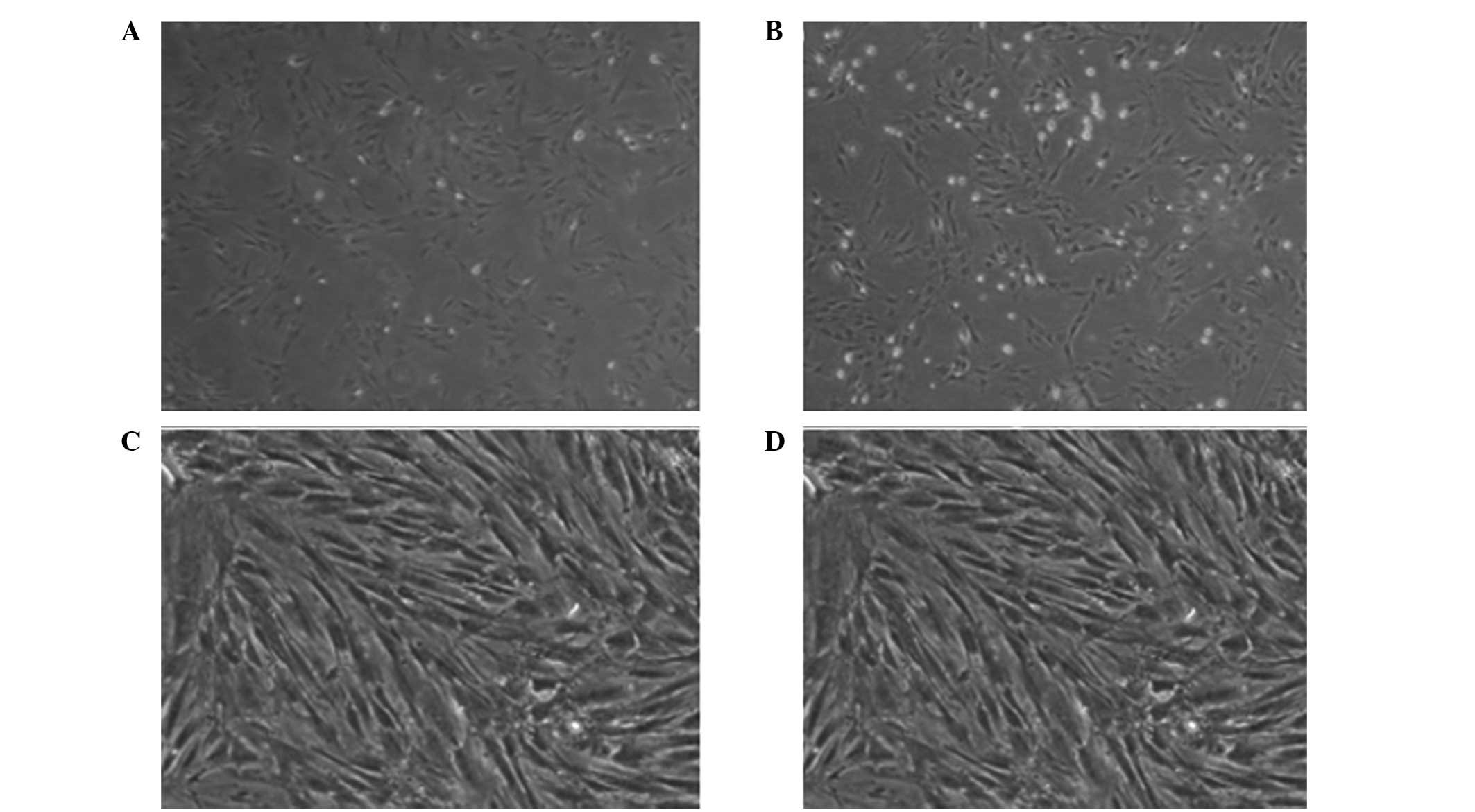
Morphological observations of BMSCs
The extracorporeal whole bone marrow adherent method
is an effective and practical method for isolating and cultivating
BMSCs. The method is based on various morphological observational
studies on BMSCs under an inverted phase contrast microscope
(14,15). The inoculated BMSCs were spherical
and had various volumes. The cells had transparent somas, but
exhibited the appearance of cell growth process and were suspended
in a culture flask with peripheral hemocyte series, including
erythrocytes. BMSCs partially adhered to the flask wall after 12 h
of cultivation. In the initial stage, the BMSCs had a short
rod-like shape, which became short and fusiform and gradually
presented with a fibroblast appearance. Rapid cell growth was
observed between day 5 and 7 with typical colony formation. These
cells were colony forming unit-fibroblasts. Cells in the center of
the colony steadily increased between day 8 and 10, with close
permutation and homogeneous morphology of spindle and polygon
shapes. Between day 12 and 14, with the increase in the number of
cell colonies, cells closely attached with each other and arranged
in a direction along the long axis of the cell body, presenting
with reticulate or vorticose shapes. The cells were due for passage
when they fused to the monolayer gradually until they spread over
the bottom of the flask. The cells began to adhere to the flask
wall following cell passage for 1–2 h, with cell morphology ranging
between round and spindle shapes. The cells spread over the bottom
of the flask between day 3 and 4 and exhibited more homogeneous
morphology. The next passage was then processed, at which the
suspended erythrocytes and other hemocyte series were removed along
with fluid exchange, consequently achieving cell purification.
The BMSCs were partially adhered to the flask wall
after 12 h of cultivation. In the initial stage, the BMSCs had a
short rod-like shape, which became short and fusiform, and
gradually presented with a fibroblast appearance. Rapid cell growth
was observed between day 5 and 7 with typical colony formation.
These cells were colony forming unit-fibroblasts, in the center of
the colony steadily increased between day 8 and 10, with close
permutation and homogeneous morphology of spindle and polygon
shapes. Between day 12 and 14, with the increase in the number of
cell colonies, cells closely attached with each other and arranged
in a direction along the long axis of the cell body, presenting
with reticulate or vorticose shapes. The cells were due for passage
when they fused to the monolayer gradually until they spread over
the bottom of the flask (Fig.
1).
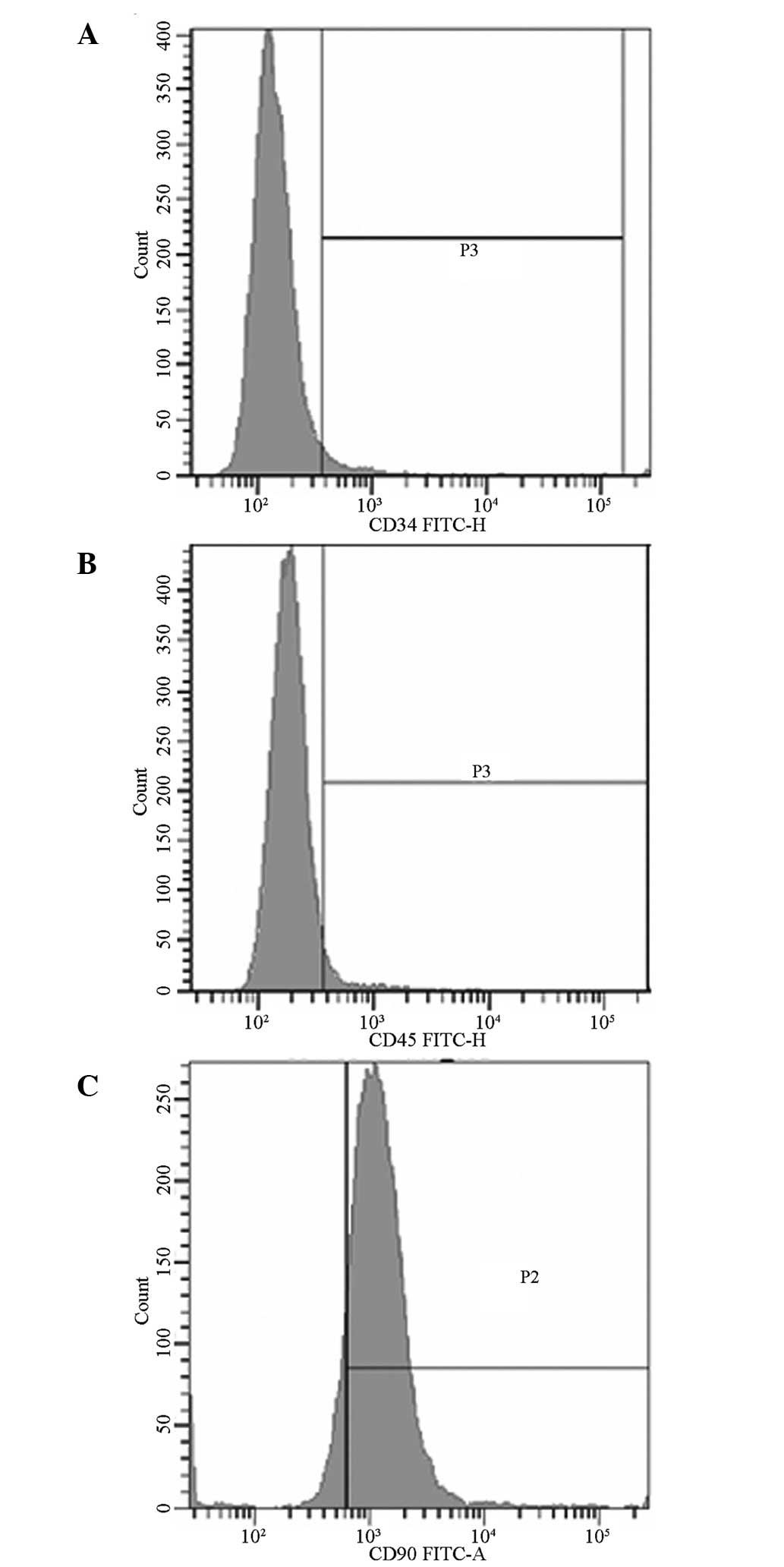
Detection of cell surface antigens by
FCM
FCM results are shown in Fig. 2. The positive rates of CD34, CD45
and CD90 were 3.9, 5.00 and 95.60%, respectively.
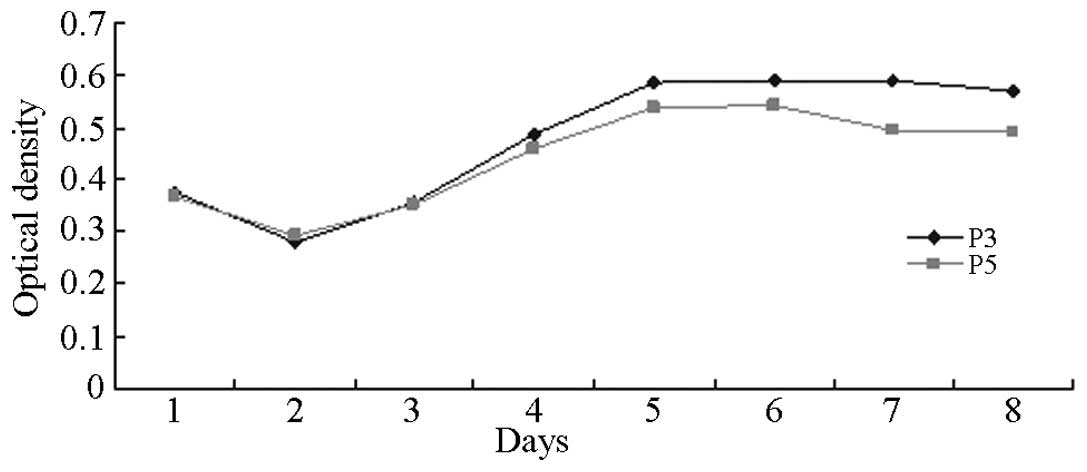
Growth curve of BMSCs
The proliferative growth rates of the P3 and P5 BMSC
generations were observed and the results were plotted as growth
curves using the MMT method. The results are shown in Fig. 3. Common features with similar
sigmoid growth curves were identified. Fig. 3 demonstrates that the OD values
varied slightly between day 1 and 2 following inoculation.
Proliferation was not evident, whereas the OD values varied
markedly between day 3 and 5, indicating that cell proliferation
was more intensive than during the logarithmic growth phase. OD
values started to increase from day 6 and gradually entered the
platform phase. Cells in a logarithmic growth phase were selected
for further experimental study.
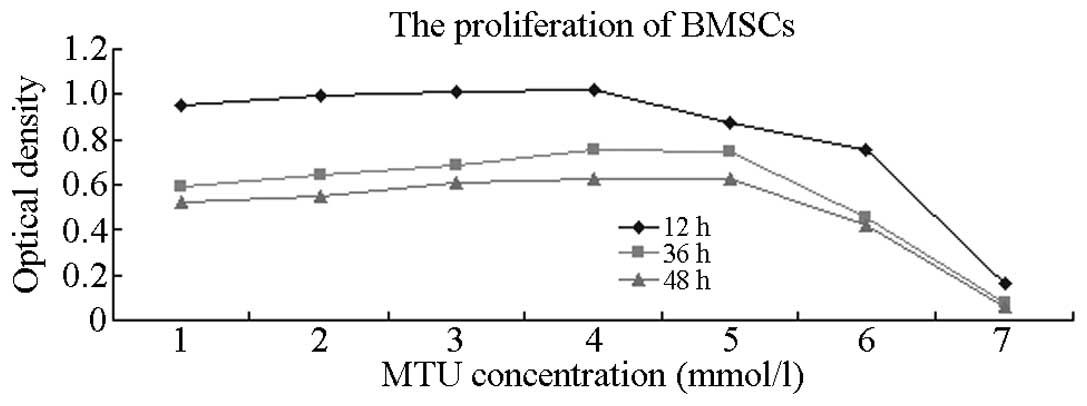
Effect of MTU on BMSC proliferation
MTU solutions of various concentrations (10, 20, 40,
80, 160 and 320 mmol/l) were incubated with BMSCs for 12, 36 and 48
h. The effects on proliferation were detected using the CCK-8
method (Table I and Fig. 4) and the results indicated that MTU
inhibited BMSC proliferation. At an MTU concentration of 40 mmol/l,
the cell proliferation-inhibition rate steadily increased with
increasing drug concentrations. Significant differences were
identified between the control and the various treatment groups
(P<0.05). The proliferation-inhibition effect was most
significant when the action time was 48 h.
 | Table IEffect of various concentrations of
MTU (optical density) on the proliferation of BMSCs over 48 h (mean
± SD; n=6). |
Table I
Effect of various concentrations of
MTU (optical density) on the proliferation of BMSCs over 48 h (mean
± SD; n=6).
| MTU, mmol/l | 12 h | 36 h | 48 h |
|---|
| Control | 0.948±0.100a | 0.593±0.072b | 0.526±0.041b |
| 10 | 0.993±0.070a | 0.645±0.052ab | 0.552±0.038ab |
| 20 | 1.014±0.047a | 0.689±0.043ab | 0.611±0.025ab |
| 40 | 1.018±0.115c | 0.757±0.067abc | 0.625±0.041abc |
| 80 | 0.877±0.052c | 0.744±0.117abc | 0.623±0.073abc |
| 160 | 0.757±0.047c | 0.458±0.061c | 0.424±0.073c |
| 320 | 0.166±0.040c | 0.077±0.024c | 0.058±0.032c |
Effect of MTU on BMSC apoptosis
Following the incubation of BMSCs with MTU solutions
of various concentrations (10, 20, 40, 80, 160 and 320 mmol/l) for
48 h, the apoptosis effect was detected by FCM (Table II and Fig. 5). The results indicated that at an
MTU concentration of 40 mmol/l, the cell apoptosis rate steadily
increased with increasing drug concentrations. Significant
differences were identified between the control and the various
treatment groups (P<0.05).
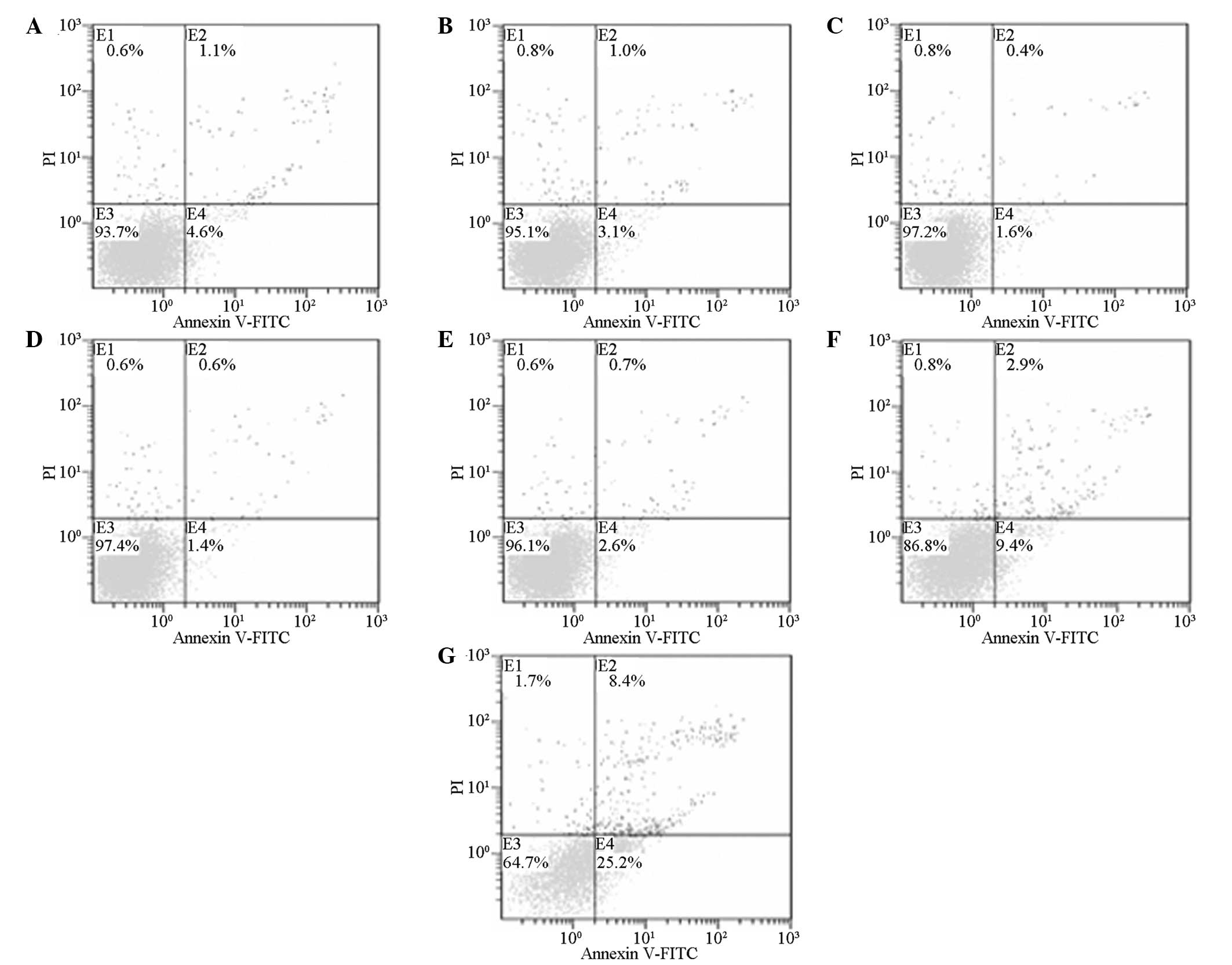 | Figure 5Effect of MTU on the apoptosis of
BMSCs in the (A) control, (B) 10, (C) 20, (D) 40, (E) 80, (F) 160
and (G) 320 mmol/l treatment groups. E1, left superior quadrant;
E2, right superior quadrant representing dead cells and apoptotic
cells in an advanced stage; E3, left inferior quadrant representing
normal cells; E4, lower right quadrant representing apoptotic cells
at an early stage; MTU, methylthiouracil; BMSCs, bone marrow
stromal cells. |
 | Table IIEffect of various concentrations of
MTU on the apoptosis of BMSCs over 48 h (mean ± SD; n=3). |
Table II
Effect of various concentrations of
MTU on the apoptosis of BMSCs over 48 h (mean ± SD; n=3).
| Parameter | Apoptosis ratio,
% |
|---|
| Control group | 4.13±0.45 |
| MTU, mmol/l |
| 10 | 2.33±0.23 |
| 20 | 1.67±0.12 |
| 40 | 1.10±0.26a |
| 80 | 2.27±0.32a |
| 160 | 9.50±0.66ab |
| 320 | 24.2±1.40ab |
| F-value | 513.489 |
| P-value | 0.000 |
Discussion
The present study adopted a whole bone marrow
adherent method to conduct in vitro separation and
cultivation of BMSCs in SD rats. As normative surgery, the method
was able to complete the separation, extraction and cultivation
within a short time, thus, promoting rapid cell proliferation and
passage stabilization. In addition, the method was convenient,
economical, less invasive and obtained various types of matrix cell
groups (16). In the present
study, when the BMSCs adherently grew, the suspended HSCs and other
cells were gradually eliminated with the solution replacement.
Through cell passage, purified and amplified fibroblast-like cells
were finally obtained with adherent growth and rapid proliferation
(Fig. 1), according to the
observed morphological features. FCM results indicated that the
obtained cells showed positive expression for CD90, whereas
negative expression was observed for CD34 and CD45. This result was
in accordance with previous studies (17,18)
and indicated that cultivated cells conform with the immunological
marker features of BMSCs (19).
The MTT method was used in the current study
(20) to detect the proliferation
of P3 and P5 BMSC generations. The corresponding growth curves are
shown in Fig. 3. Proliferation was
solely observed during the incubation period, whereas multiple
proliferations occurred during the exponential phase. This phase is
the most vital period and was also the optimum phase for the
current study. As the cells approached inhibition, they entered a
plateau phase at which cell density saturation was reached. Cell
proliferation stopped with the gradual depletion of nutrient
sources in the culture solution and the accumulation of
metabolites. Therefore, cells in an exponential phase were selected
for empirical study.
BMSCs are the other type of stem cell present in the
bone marrow, aside from HSCs. BMSCs provide indispensable support
for the survival and functionalization of HSCs. Currently, various
methods are available for the isolation of BMSCs, which can then be
used for further study (16).
However, different methods result in varying effects (18,21).
The present study demonstrated that a delay in bone marrow recovery
may be relevant to the injury of bone marrow stroma. A leukemic
mouse model previously demonstrated that a leukemic
microenvironment can inhibit the growth of normal hematopoietic
progenitor cells, thus, improving and maintaining the proliferation
and long-term survival of leukemic cells (22). By inhibiting the immunosuppressive
function of cyclin D2, BMSCs can block leukemic cells at the G0/G1
phase of the cell cycle (23).
A CCK-8 kit was used in the current study to detect
the effects of various concentrations of MTU on the
proliferation-inhibition rate of BMSCs following incubation for 12,
36 and 48 h. MTU may be dissolved in ammonia solution or hydroxide
alkaline solution. In the present study, 1 mol/l sodium hydroxide
solution was selected to prepare the MTU solution and 10% fresh
cell culture solution was diluted to the required concentration.
Several concentrations of MTU were used at relatively large
gradients since no previous studies on the application of MTU for
in vitro cell experiments were identified. The CCK-8 method
was performed to observe the proliferation status and to narrow the
selection for a suitable medical concentration, according to the
observational results. When 640 mmol/l MTU solution was added to
the culture solution containing the cells, a large number of
floating BMSCs were observed under an inverted phase contrast
microscope. Therefore, this concentration was disregarded. The
detection results of the CCK-8 kit are shown in Table I. At a final concentration of 10 or
20 mmol/l, the OD values gradually increased, but there were no
statistically significant differences when compared with the
control group (P>0.05). This result indicates that low
concentrations of MTU have no effect on cell proliferation. At an
MTU concentration of 40 mmol/l, the OD values decreased with
increasing drug concentrations and this difference was
statistically significant when compared with the control group
(P<0.05). Comparisons among the groups that had been subjected
to various action times also revealed statistically significant
differences (P<0.05), indicating that cell proliferation
inhibition with MTU was time-dependent. The 48 h group exhibited
the lowest OD value and showed statistically significant
differences (P<0.05) when compared with the 12 and 36 h groups.
This result may be relevant to the chronergy of drug metabolism.
Therefore, an action time of 48 h can be selected for follow-up
empirical study of cell apoptosis.
An annexin V-FITC/PI double-color apoptosis kit was
used to detect changes in cell apoptosis following the incubation
of BMSCs with various concentrations of MTU for 48 h. As shown in
Fig. 2, when compared with the
control group, no statistically significant differences were
observed at MTU concentrations of 10 and 20 mmol/l (P>0.05),
indicating that these concentrations had no effect on the early
apoptotic rate of the cells. At an MTU concentration of 40 mmol/l,
the apoptotic rate increased with increasing drug concentrations;
the differences were statistically significant when compared with
the control group (P<0.05). Differences identified through
comparisons among the various groups were statistically
significant, indicating that MTU solutions at a certain
concentration can dose-dependently promote the apoptosis of
BMSCs.
In conclusion, MTU inhibits BMSC proliferation and
promotes BMSC apoptosis. BMSCs are an important element in the BMM,
indicating that MTU may cause changes in the medullary
hematopoiesis microenvironment. This effect can influence the
proliferation, differentiation and maturation of stem cells and
their peripheral blood release or apoptosis (22,23).
The promotion effect of MTU on BMSC proliferation and apoptosis
also indicates that MTU may exert certain toxic effects on BMSCs
(24). This observation indicates
that a side effect of bone marrow suppression may result from the
treatment of hyperthyroidism with MTU. However, the toxicity of MTU
has provided novel ideas in the search for cytotoxic drugs.
References
|
1
|
Ferrer RA, Wobus M, List C, et al:
Mesenchymal stromal cells from patients with myelodyplastic
syndrome display distinct functional alterations that are modulated
by lenalidomide. Haematologica. 98:1677–1685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arndt K, Grinenko T, Mende N, et al: CD133
is a modifier of hematopoietic progenitor frequencies but is
dispensable for the maintenance of mouse hematopoietic stem cells.
Proc Natl Acad Sci USA. 110:5582–5587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bai H, Xie YL, Gao YX, Cheng T and Wang
ZZ: The balance of positive and negative effects of TGF-β signaling
regulates the development of hematopoietic and endothelial
progenitors in human pluripotent stem cells. Stem Cells Dev.
22:2765–2776. 2013.
|
|
4
|
Itkin T, Kaufmann KB, Gur-Cohen S, Ludin A
and Lapidot T: Fibroblast growth factor signaling promotes
physiological bone remodeling and stem cell self-renewal. Curr Opin
Hematol. 20:237–244. 2013.PubMed/NCBI
|
|
5
|
Mizer JC, Ichim TE, Alexandrescu DT, et
al: Exogenous endothelial cells as accelerators of hematopoietic
reconstitution. J Transl Med. 10:2312012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao W, Wang Y, Wang D, et al: TGF-beta
expression by allogeneic bone marrow stromal cells ameliorates
diabetes in NOD mice through modulating the distribution of CD4+ T
cell subsets. Cell Immunol. 253:23–30. 2008.PubMed/NCBI
|
|
7
|
Boroujeni MB, Salehnia M, Valojerdi MR,
Mowla SJ, Forouzandeh M and Hajizadeh E: Comparison of gene
expression profiles in erythroid-like cells derived from mouse
embryonic stem cells differentiated in simple and co-culture
systems. Am J Hematol. 83:109–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Angelopoulou M, Novelli E, Grove JE, et
al: Cotransplantation of human mesenchymal stem cells enhances
human myelopoiesis and megakaryocytopoiesis in NOD/SCID mice. Exp
Hematol. 31:413–420. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu H, Fujimori Y, Kai S, Fujibayashi Y,
Nishioka K and Hara H: Establishment of mouse embryonic fibroblast
cell lines that promote ex vivo expanssion of human cord blood
CD34+ hematopoietic progenitors. J Hematother Stem Cell Res.
12:39–46. 2003.PubMed/NCBI
|
|
10
|
Campana D, Coustan-Smith E, Kumagai MA and
Manabe A: Growth requirements of normal and leukemic human B cell
progenitors. Leuk Lymphoma. 13:359–371. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fischer D, Poncin J, Dunet R, Dry J and
Thomas M: Evaluation of the results of treatment of Graves’ disease
by synthetic antithyroid drugs. Sem Hop. 53:1843–1849. 1977.(In
French).
|
|
12
|
Junik R, Lacka K, Sowiński J,
Horst-Sikorska W and Gembicki M: Anti-thyroid antibodies and
thyroglobulin in the serum of patients with hyperthyroidism treated
with methylthiouracil. Pol Tyg Lek. 42:1120–1123. 1987.(In
Polish).
|
|
13
|
Léger J, Gelwane G, Kaguelidou F, Benmerad
M and Alberti C; French Childhood Graves’ Disease Study Group.
Positive impact of long-term antithyroid drug treatment on the
outcome of children with Graves’ disease: national long-term cohort
study. J Clin Endocrinol Metab. 97:110–119. 2012.
|
|
14
|
Oliveira PH, Boura JS, Abecasis MM, Gimble
JM, da Silva CL and Cabral JM: Impact of hypoxia and long-term
cultivation on the genomic stability and mitochondrial performance
of ex vivo expanded human stem/stromal cells. Stem Cell Res.
9:225–236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Havlas V, Kos P, Jendelová P, Lesný P, Trč
T and Syková E: Comparison of chondrogenic differentiation of
adipose tissue-derived mesenchymal stem cells with cultured
chondrocytes and bone marrow mesenchymal stem cells. Acta Chir
Orthop Traumatol Cech. 78:138–144. 2011.(In Czech).
|
|
16
|
Colter DC, Sekiya I and Prockop DJ:
Identification of a subpopulation of rapidly self-renewing and
multipotential adult stem cells in colonies of human marrow stromal
cells. Proc Natl Acad Sci USA. 98:7841–7845. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo YC, Yeh CF and Yang JT:
Differentiation of bone marrow stromal cells in
poly(lactide-co-glycolide)/chitosan scaffolds. Biomaterials.
30:6604–6613. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dezawa M, Kanno H, Hoshino M, et al:
Specific induction of neuronal cells from bone marrow stromal cells
and application for autologous transplantation. J Clin Invest.
113:1701–1710. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung YC, Ma MC, Huang BY, Chiang HS and
Chou SH: Protection of bone marrow-derived CD45+/CD34−/lin-stromal
cells with immunosuppressant activity against ischemia/reperfusion
injury in rats. Chin J Physiol. 54:169–182. 2011.
|
|
20
|
Mosmann T: Rapid colorimetric asay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fromigué O, Kheddoumi N, Lomri A, Marie PJ
and Body JJ: Breast cancer cells release factors that induced
apoptosis in human bone morrow stromal cells. J Bone Miner Res.
16:1600–1610. 2001.PubMed/NCBI
|
|
22
|
Basak P, Chatterjee S, Das P, et al:
Leukemic stromal hematopoietic microenvironment negatively
regulates the normal hematopoiesis in mouse model of leukemia. Chin
J Cancer. 29:969–979. 2010. View Article : Google Scholar
|
|
23
|
Glennie S, Soeiro I, Dyson PJ, Lam EW and
Dazzi F: Bone marrow mesenchymal stem cells induce division arrest
anergy of activated T cells. Blood. 105:2821–2827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palii CG, Pasha R and Brand M:
Lentiviral-mediated knockdown during ex vivo erythropoiesis of
human hematopoietic stem cells. J Vis Exp. 16:28132011.PubMed/NCBI
|