Introduction
Diabetic nephropathy (DN), one of the serious
complications of diabetes mellitus (DM), is the primary cause of
mortality in patients with DM. Proteinuria, a clinical symptom of
early DN, is also an important factor that increases the risk of
kidney failure. Proteinuria in DN is closely associated with
changes in molecular structure and the abnormal expression of a
variety of proteins, including nephrin and podocin, from the
fenestrations in the diaphragm of adjacent podocytes (1–3). The
fungus, Cordyceps sinensis (CS), is rich in amino acids,
polysaccharides, organic acids, trace elements, nucleosides,
peptides, steroids and other chemical components (4,5). CS
has numerous therapeutic effects, including the regulation of
immune function, intrinsic renal cell proliferation, extracellular
matrix synthesis and cytokines. CS also functions as a growth
factor, antagonizes ischemia and toxic injury to the kidneys,
improves metabolism and other multi-target, multi-link mechanisms,
reduces proteinuria and improves renal function and renal
pathological changes (6,7). Previous studies have demonstrated
that DN is closely associated with podocyte injury (8–12).
The mechanism of action of CS in protecting the kidneys via
improving lesions of glomerular podocytes in DN has not been
confirmed. Numerous studies have shown that Tripterygium
wilfordii polyglycosidium (TWP) exhibits immunosuppressive
effects and a therapeutic effect on podocyte injury. TWP is widely
used to treat various glomerular diseases (13,14).
Thus, the aim of the present study was to observe the association
between podocyte lesions and renal injury by establishing a DN rat
model and investigate the possible mechanisms of repairing DN
glomerular podocytes with CS and TWP.
Materials and methods
Animals
A total of 100 specific pathogen free-grade, male,
adult Sprague-Dawley rats, aged 18–20 weeks-old and weighing
180–200 g, were provided by the Laboratory Animal Center of the
Anhui Medical University (Hefei, China). The local legislation for
ethics of experiments on animals and the Guide for the Care and Use
of Laboratory Animals (1996) were followed in all the animal
experiments.
Materials and instruments
CS was purchased from Jimin Pharmaceutical Co.,
Ltd., (Jiangxi, China), TWP was puchased from Fudan Fuhua
Pharmaceutical Co., Ltd., (Shanghai, China), streptozotocin was
obtained from Sigma (St. Louis, MO, USA), rat urine albumin EIA
assay kit was obtained from R&D Systems (Minneapolis, MN, USA),
rabbit anti-rat nephrin antibodies, rabbit anti-rat podocin
antibodies and goat anti-rabbit antibodies were purchased from
Boster (Wuhan, China), Accu-chek Blood glucose meter was obtained
from Roche Diagnostics GmbH (Mannheim, Germany). The 7150 automatic
biochemical analyzer was purchased from Hitachi (Tokyo, Japan), the
JEOL-1230 transmission electron microscope was obtained from JEOL
(Tokyo, Japan), Vanox multifunctional microscope was purchased from
Olympus (Tokyo, Japan) and SDS-PAGE electrophoresis was obtained
from Bio-Rad (Hercules, CA, USA).
Grouping and drug administration
Animals were acclimatized to the laboratory
environment and allowed free access to food and water in
temperature- and humidity-controlled housing with natural
illumination for one week. The animals were subsequently fasted
12-h prior to the experiment. The rats were administered a single
intraperitoneal injection of 65 mg/kg streptozotocin during
fasting. Blood samples were then collected via the tail vein after
48–72 h, and the glucose (GLU) concentrations were measured based
on whole-blood GLU. The DN rat models were established to have a
random blood GLU level of ≥16.7 mmol/l (15), and were divided randomly into
groups B, C, D and E. Group A was injected with the same amount of
citrate buffer. A total of 20 rats were assigned into each group
(n=20). Group C was administered 5 g/kg/day CS by daily gavage.
Group D was administered 16 mg/kg/day TWP by daily gavage and group
E was administered 5 g/kg/day CS and 16 mg/kg/day TWP with daily
gavage. Groups A and B were administered 5 g/kg/day water by gavage
once daily in the morning. The rats were weighed weekly to adjust
the dose and continuous medication was administered for 12 weeks.
During the experiment, the rats were fed a standard diet and were
free to drink water and did not use insulin.
Specimen collection
One day prior to the end of the experiment, urine
was collected for 24 h in metal metabolic cages. The obtained
samples were then centrifuged, packed and stored in a −80°C
freezer. The rats were weighed and blood samples were collected via
the right common carotid artery, prior to the rats being sacrificed
via an intraperitoneal injection of pentobarbital. A number of the
collected samples were placed in anticoagulant tubes, while the
remaining blood samples were centrifuged at 4°C. The plasma was
stored at −20°C for biochemical tests. The kidneys were repeatedly
lavaged through the right carotid artery using 4°C saline, and
excised. Kidney sections (8 mm) were fixed in 4% paraformaldehyde
solution, embedded in paraffin and cut into 3-μm thick sections.
The sections were treated with polylysine for immunofluorescence
studies. The remaining kidney tissues were cut into 1-mm sections
and immersed in 4°C ethyl alcohol for at least 4 h. The sections
were prepared and observed under a transmission electron
microscope.
24-h urine protein determination
The 24-h urinary protein concentration was
determined using a kit, according to manufacturer’s
instructions.
Blood biochemical parameters
Serum creatinine (SCR), blood urea nitrogen (BUN),
aspartate aminotransferase (AST) and alanine aminotransferase (ALT)
were detected in the serum using a 7150 automatic biochemical
analyzer. Whole-blood GLU was measured using a blood GLU meter.
Light microscopy examination of renal
tissue
The left kidney was cut coronally through the renal
hilum at a thickness of 2 mm. Sections were fixed in 10% neutral
formaldehyde, embedded in paraffin and cut into 2-μm thick
sections. The tissues were stained with hematoxylin and eosin (HE),
and observed with light microscopy for pathological changes.
Ultrastructural changes under electron
microscopy
Tissue specimens were cut into sections and washed
in pH 7.6 phosphate buffer. Glutaraldehyde fixation solution was
used to post-fix the tissues, which were then dehydrated with
graded acetone concentrations. The tissues were embedded in
Araldite, cut into ultrathin sections, stained with uranyl acetate
and aluminium citrate for examination under a JEOL-1230
transmission electron microscope.
Distribution of nephrin and podocin
Sections (2 μm) were deparaffinized and incubated
overnight with rabbit anti-rat nephrin (1:400) and rabbit anti-rat
podocin (1:400) antibodies at 4°C. The slices were rinsed three
times with phosphate-buffered saline (0.1 M) for 3 min and were
reacted for 50 min with fluorescein isothiocyanate-labeled goat
anti-rabbit antibodies at room temperature. The results were
observed and photographed under immunofluorescence microscopy.
Statistical analysis
Normally distributed data are presented as the mean
± standard deviation. The 24-h urinary protein results did not
follow a normal distribution and were therefore subjected to
logarithmic conversion. These data are presented as geometric mean
x/÷ tolerance factor. The data were statistically analyzed using
the SPSS 11.0 software package (SPSS, Inc., Chicago, IL, USA).
Parametric data were analyzed using one-way analysis of variance
and nonparametric data were analyzed with the Kruskal-Wallis test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Treatment for proteinuria in rats with
DN
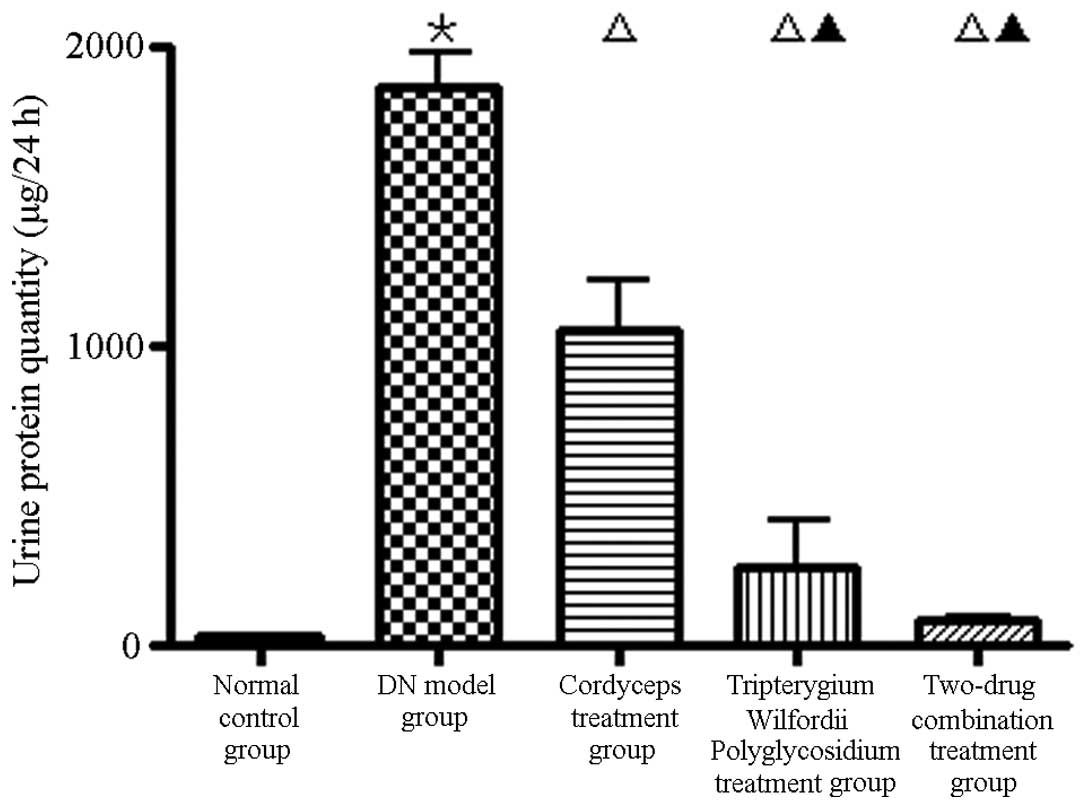
The 24-h proteinuria in group B was significantly
higher than in groups A, C, D and E (Fig. 1; P<0.01).
Effect of kidney weight/body weight
(KW/BW) on DN
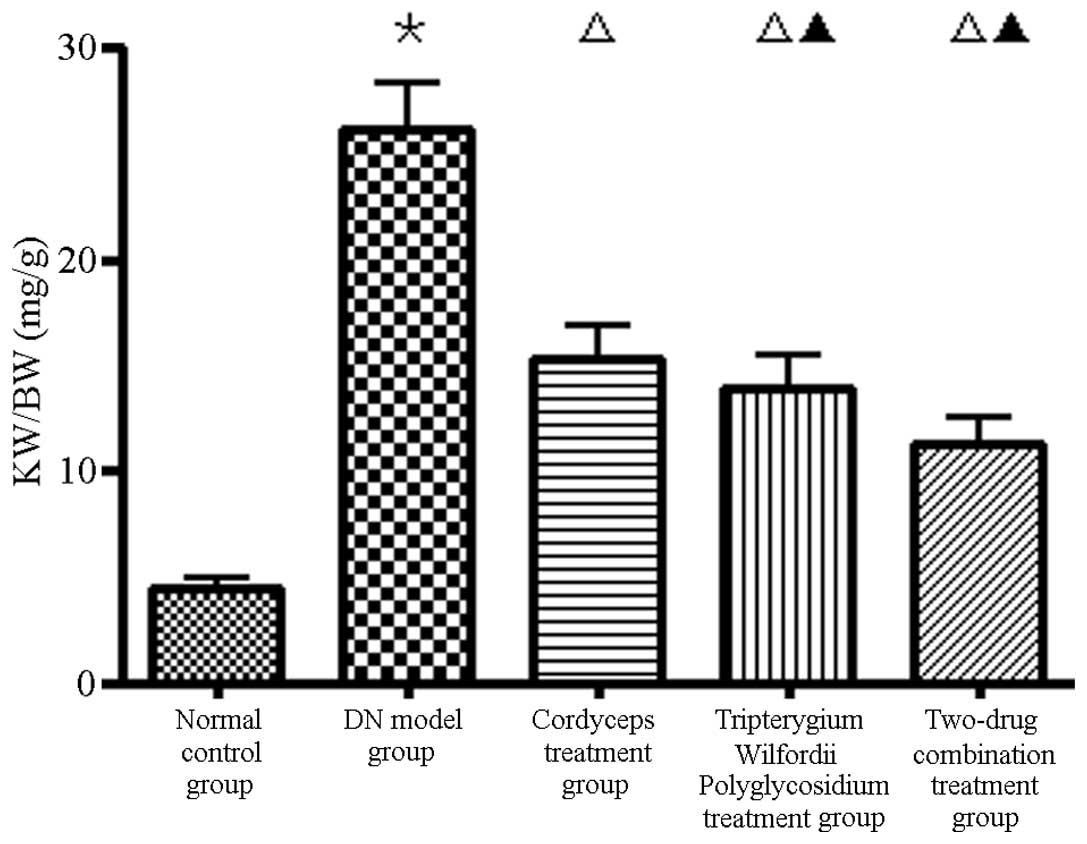
Compared with group B, the KW/BW ratios in group A
were higher (P<0.01) and those in group E were significantly
lower (Fig. 2).
Effect of serum biochemistry parameters
of DN rats
Compared with group A, the levels of liver enzymes
and peripheral white blood cells (WBCs) were not significantly
different from those in group B. However, the SCR and BUN levels
were significantly higher (P<0.05), as well as the blood GLU
level (P<0.01). Compared with group B, the levels of blood GLU,
SCR, BUN, liver enzymes and peripheral WBCs were not significantly
different from those in groups C and E, however, the level of liver
enzymes was higher and the peripheral WBC count was lower in group
D (3/20, 15%; P<0.05). No significant difference was observed in
group E, compared with all other groups (Table I).
 | Table IComparison of serum biochemical
parameters in DN rats of each group. |
Table I
Comparison of serum biochemical
parameters in DN rats of each group.
| Group | Glu (mmol/l) | BUN (mmol/l) | Cr (μmol/l) | AST (U/l) | ALT (U/l) | WBC
(×109/l) |
|---|
| A | 4.91±0.78 | 6.83±1.12 | 67.83±5.56 | 57.80±9.98 | 52.16±8.63 | 4.82±1.26 |
| B | 24.92±4.42b | 12.2±2.63a | 94.57±12.6a | 69.61±9.95 | 55.42±1.25 | 4.51±1.21 |
| C | 24.76±3.12 | 11.17±2.02 | 92.63±12.19 | 64.83±9.93 | 54.62±4.58 | 4.35±1.22 |
| D | 24.54±2.07 | 10.41±1.98 | 98.78±12.81 | 85.39±19.34c | 78.07±18.34c | 3.84±0.69c |
| E | 23.76±2.48 | 11.20±2.03 | 88.51±10.96 | 64.86±9.88 | 57.85±9.05 | 4.23±1.14 |
Changes of renal tissue pathology of
DN
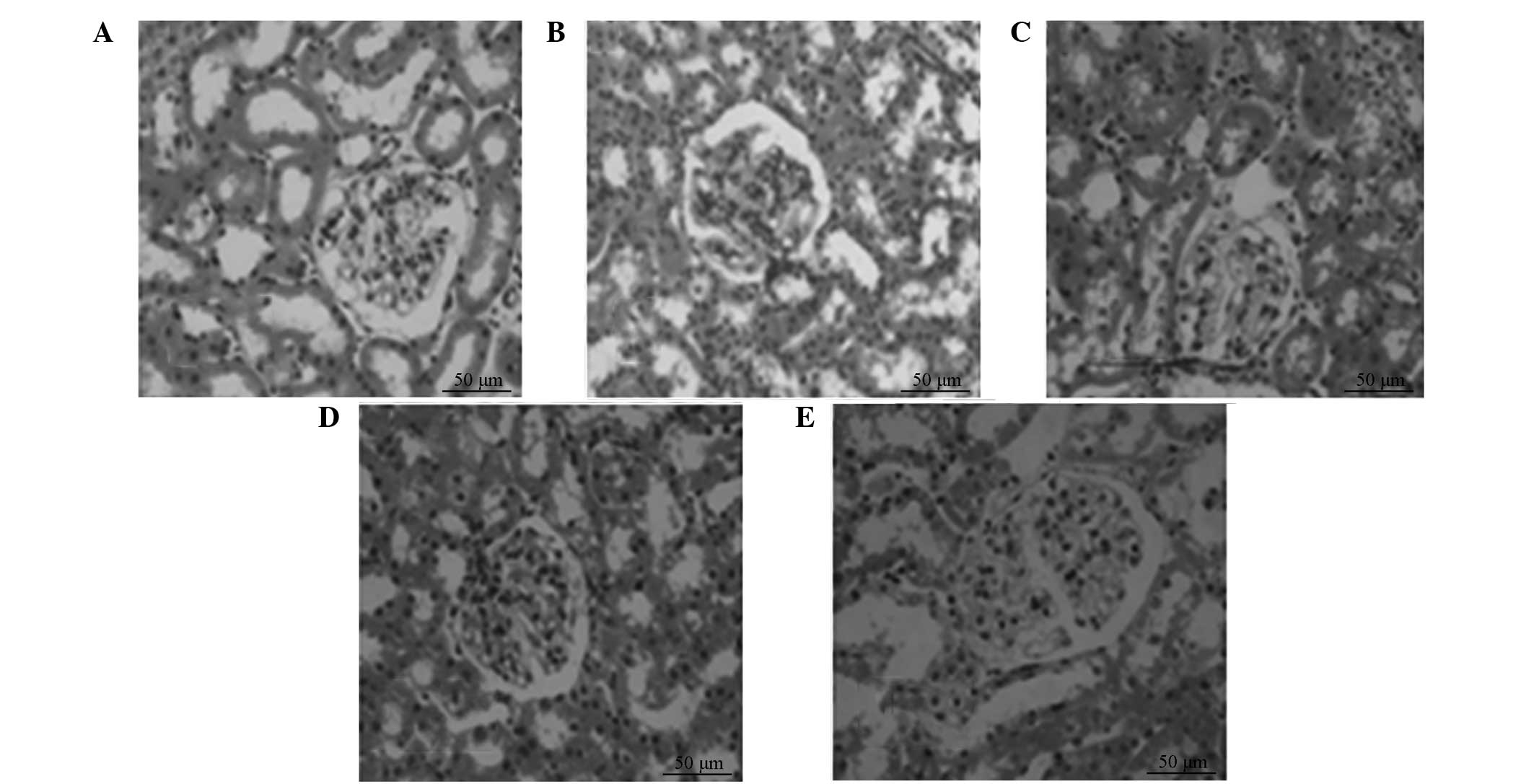
HE-stained renal biopsy samples exhibited no
pathological changes in the kidney tissue of group A. The tubular
deformation and glomerular hypertrophy observed in group B, as well
as other pathological changes, were not markedly reduced in
treatment groups C, D and E, but the most significant change
occurred in group E (Fig. 3).
Effect of podocyte disease on DN
rats
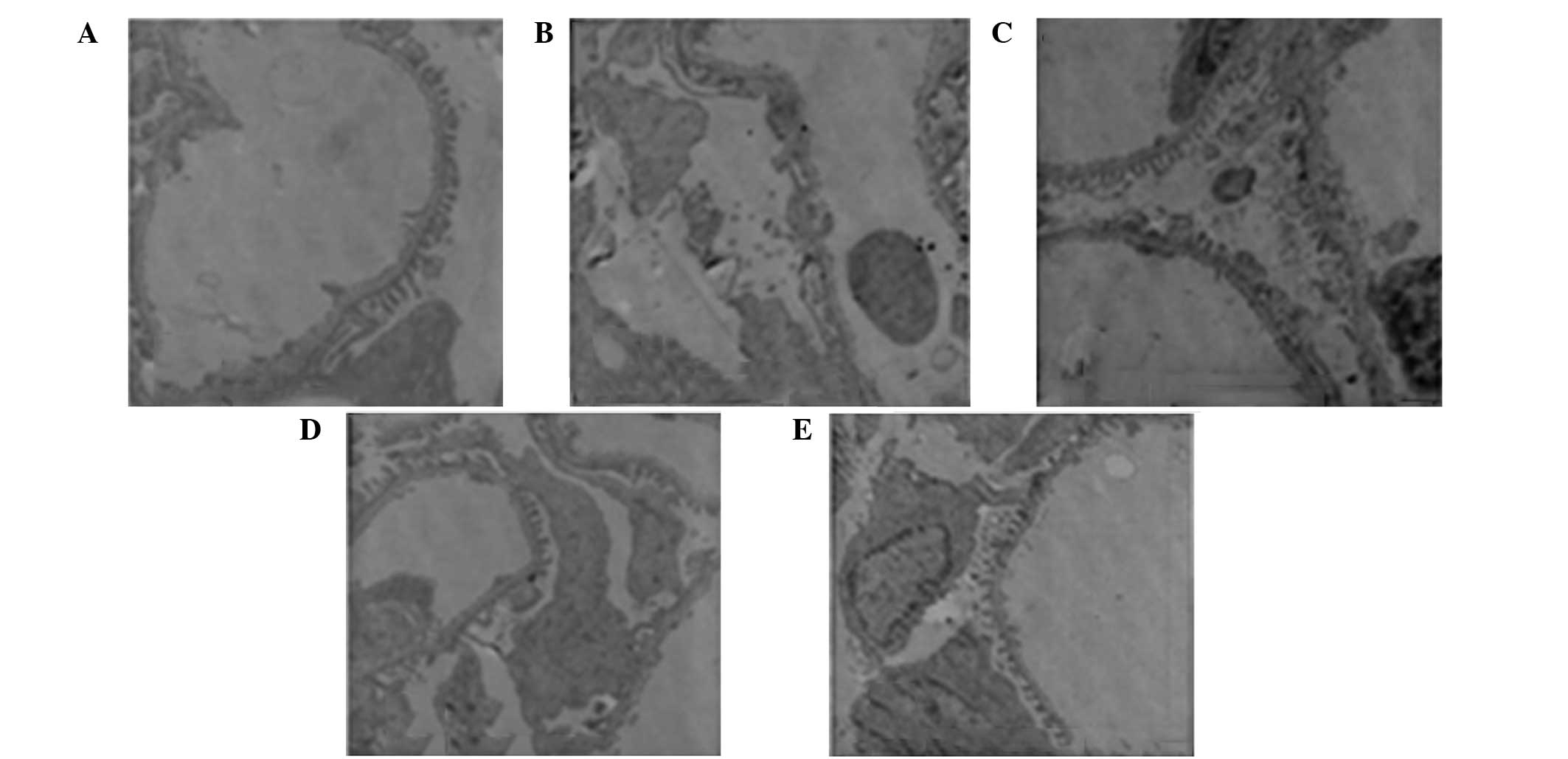
No pathological changes were observed in the
podocytes of group A. The foot processes of the podocytes fused and
the number of foot processes decreased. By contrast, the
fenestrated membrane disappeared in group B and podocyte morphology
returned to normal in groups C, D and E. The podocyte injury in
group E (combined treatment group) was significantly reduced
compared with groups C and D, with the podocytes in group D less
injured than in group C (Fig.
4).
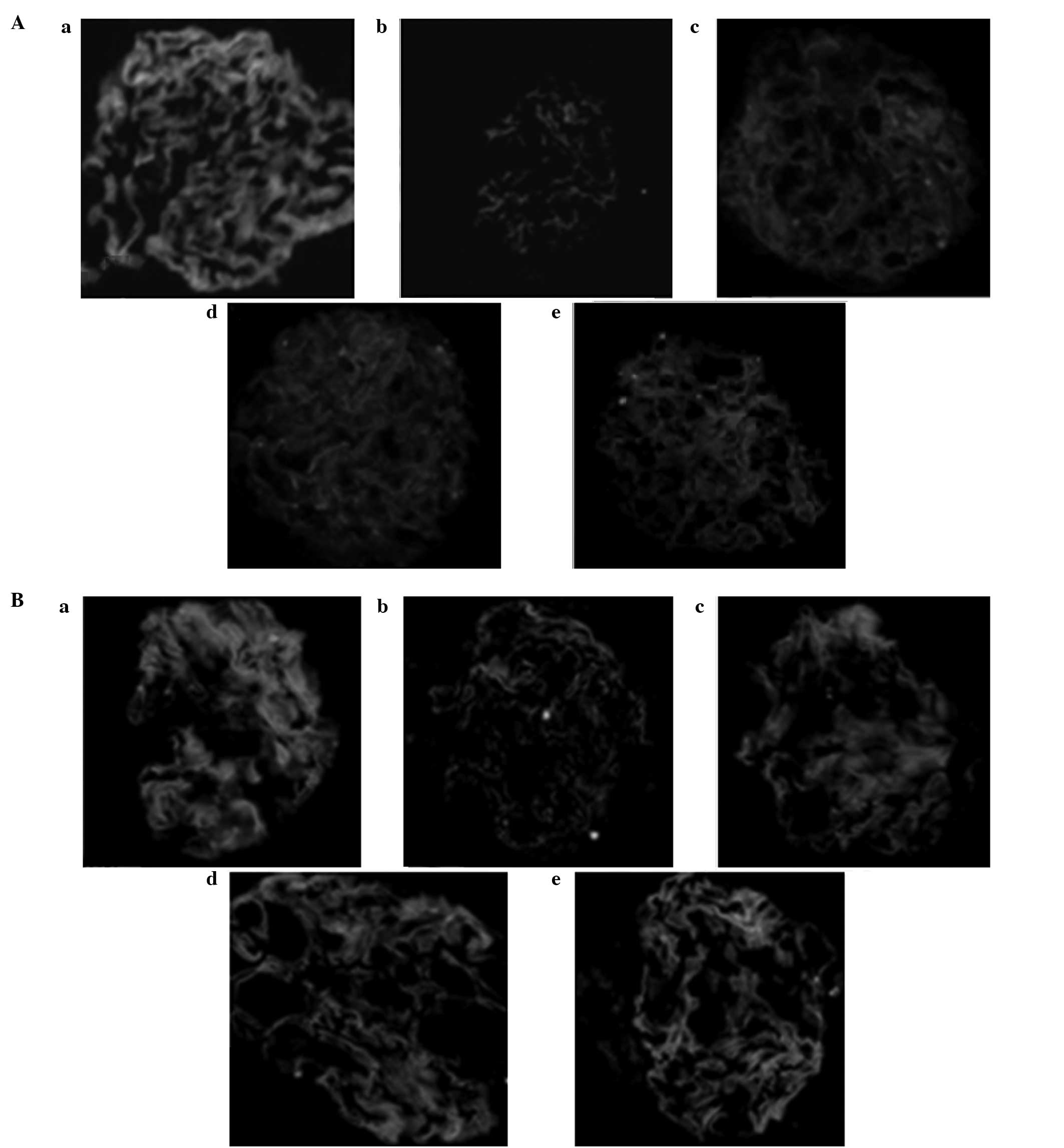
Nephrin and podocin protein
expression
Immunofluorescence images showed that nephrin and
podocin expression levels in the podocyte protein slit in the
normal glomerular capillary loops were uniform along the continuous
linear distribution. Compared with the treatment groups, nephrin
and podocin protein expression levels were significantly decreased
in group B (P<0.05) and the continuous linear distribution had
changed to diffuse granular distribution (Fig. 5).
Discussion
With advances in DN research, the influence of
massive proteinuria on the prognosis was found to be an important
factor. Urinary protein that accumulates in the mesangial cells
damages the mesangium, disrupting mesangial proliferation and
matrix synthesis, which promotes glomerular sclerosis (16,17).
Tubulointerstitial proteins can cause hypoxia and increased
lysosomal activity. This also leads to tubular cell damage,
inflammation and scar formation (18). In addition, urinary proteins
directly regulate tubular cell function, which changes the growth
characteristics of cytokines and matrix proteins, as well as their
phenotypic expression and induction of fibrosis (19). In the current study, proteinuria
was found to be one of the major clinical manifestations in
diabetic rats. CS and TWP significantly reduced the proteinuria in
the DN rats and the combination of the two drugs significantly
increased the effect.
Previous studies have hypothesized that the critical
lesions of DN, caused by the glomerular basement membrane, change
the extracellular matrix composition. However, previous studies
have shown that the change in podocyte ultrastructure and the
expression of associated molecules play an important role in the
production and development of DN proteinuria (20,21).
Glomerular volume was also found to increase in the early stage of
DM (22). Although no significant
change in podocyte number was observed during this stage, the cells
and their nuclei increased in size and decreased in density. This
change was prolonged in patients with DM, although urinary albumin
excretion was observed in normal patients. With the emergence of
microalbuminuria in DN, the number of podocytes begins to decrease.
The remaining podocytes undergo compensatory hypertrophy to cover
the area of the increased basement membrane and broadened foot
process. This leads to increased permeability of the glomerular
filtration barrier, which produces abundant proteinuria and
subsequently increases podocyte injury. A series of phenotypic
changes are observed following podocyte injury. Podocytes that
detach from the basement membrane expose the basement membrane
region, damaging the fenestrated membrane from which a large number
of proteins are filtered and forming a glomerulus with high
filtration, perfusion and transmembrane pressures; this ultimately
leads to glomerular sclerosis and progressive loss of renal
function (23).
Podocytes are attached to the basement membrane
through sparse foot processes. The cracks between the adjacent foot
processes are connected by a slit diaphragm (SD). The SD is the
main barrier which filters protein macromolecules and is composed
of neph-1, nephrin, podocin and FAT1, among others (24). Since the first SD protein, nephrin,
was identified by Karl in 1998 (25), the mechanism of selective
permeability of the glomerular filtration barrier and proteinuria
has been further understood (26).
In animal experiments, researchers have found that DM worsens
kidney damage in rats. Moreover, nephrin expression is
significantly reduced and albuminuria is increased in urine
(27). The results by Langham
et al revealed that decreased expression and redistribution
of nephrin preceded glomerular tissue damage and is an early event
in DN, with nephrin expression negatively correlating with
proteinuria levels. In the DN model, changes in podocin were
associated with protein and mRNA expression levels of nephrin
(28).
Glomerular hypertrophy and tubular deformation were
significantly reduced in the DN rats treated with CS and TWP after
12 weeks. In addition, other lesions, including fusion of the
podocyte foot processes, disappearance of membrane slits and
reduced number of slits, markedly improved. Group B rats
demonstrated that, under normal conditions, the distribution of
glomerular nephrin and podocin changes from a continuous
distribution into a scattered granular distribution. Moreover, the
expression level visibly decreased, whereas the expression level of
nephrin and podocin in groups C, D and E increased significantly.
The combination therapy group, in particular, showed recovery of a
clear continuous linear distribution.
TWP which has immunosuppressive action is widely
used in the treatment of autoimmune diseases, but toxicity of TWP
was a key factor in limiting its clinical application and a
positive correlation may be observed with the dose and treatment.
The incidence of liver dysfunction and leukopenia was shown to be
15% with high-dose treatment (29). In the present study, three cases of
liver dysfunction and leukopenia were observed in group D, while no
evident abnormalities were identified in the liver enzymes and
peripheral blood WBC count following the administration of CS.
Therefore, the results of the present study demonstrate that CS
combined with TWP treatment increases the efficacy and reduces the
adverse effects of TWP, including liver damage and bone marrow
suppression.
Acknowledgements
The study was supported by a grant from the Natural
Science Foundation of Anhui Province, China (no. 070413066).
References
|
1
|
Luimula P, Ahola H, Wang SX, et al:
Nephrin in experimental glomerular disease. Kidney Int.
58:1461–1468. 2000. View Article : Google Scholar
|
|
2
|
Furness PN, Hall LL, Shaw JA and Pringle
JH: Glomerular expression of nephrin is decreased in acquired human
nephritic syndrome. Nephrol Dial Transplant. 14:1234–1237. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White KE and Bilous RW; Diabiopsies Study
Group. Structural alterations to the podocyte are related to
proteinuria in type 2 diabetic patients. Nephrol Dial Transplant.
19:1437–1440. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Z, Li HP, Ye MQ, Yu HD and Zou GL:
Investigation advance in pharmacological activity of Cordyceps
sinensis. Anjisuan He Shengwu Ziyuan. 25:20–23. 2003.(In
Chinese).
|
|
5
|
Zhang XH, Shi LF and Hu JH: Research
progress of Cordyceps chemical constituents and pharmacological
functions. Zhong Yao Cai. 23:722–724. 2000.(In Chinese).
|
|
6
|
Lin RQ, Cheng H and Zhan YP: The mechanism
and application of Cordyceps agents and the application in kidney
disease (I). Zhongguo Zhong Xi Yi Jie He Shen Bing Za Zhi.
10:924–926. 2009.(In Chinese).
|
|
7
|
Lin RQ, Cheng H and Zuo Z: The mechanism
and application of Cordyceps agents and the application in kidney
disease (II). Zhongguo Zhong Xi Yi Jie He Shen Bing Za Zhi.
10:1016–1018. 2009.(In Chinese).
|
|
8
|
Camussi G, Mariano F, Biancone L,
Montrucchio G and Vercellone A: Effect of cytokines on the
cytoskeleton of resident glomerular cells. Kidney Int Suppl.
39:S32–S36. 1993.PubMed/NCBI
|
|
9
|
Garg P, Verma R and Holzman LB: Slit
diaphragm junctional complex and regulation of the cytoskeleton.
Nephron Exp Nephrol. 106:e67–e72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ichimura K, Kurihara H and Sakai T: Actin
filament organization of foot processes in vertebrate glomerular
podocytes. Cell Tissue Res. 329:541–557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schlondorf J: Nephrin AKTs on actin: The
slit diaphragm-actin cytoskeleton signaling network expands. Kidney
Int. 73:524–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doublier S, Ruotsalainen V, Salvidio G, et
al: Nephrin redistribution on podocytes is a potential mechanism
for proteinuria in patients with primay acquired nephrotic
syndrome. Am J Pathol. 158:1723–1731. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen ZH, Liu ZH, Hong YM, et al:
Triptolide ameliorates podocyte injury induced by the terminal
complement factor C5b-9 in vitro. Shen Zang Bing Yu Tou Xi Shen Yi
Zhi Za Zhi. 18:310–317. 2009.(In Chinese).
|
|
14
|
Qin WS and Liu ZH: The therapeutic
mechanism of Triptolide. Shen Zang Bing Yu Tou Xi Shen Yi Zhi Za
Zhi. 16:158–161. 2007.(In Chinese).
|
|
15
|
Huang S: Studies in animal models of
diabetes status and progress. Guangxi Medical Journal. 24:46–48.
2002.(In Chinese).
|
|
16
|
Benigni A, Coma D, Zoja C, et al: Targeted
deletion of angiotensin II type 1A receptor does not protect mice
from progressive nephropathy of overload proteinuria. J Am Soc
Nephrol. 15:2666–2674. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morigi M, Buelli S, Angioletti S, et al:
In response to protein load podocytes reorganize cytoskeleton and
modulate endothelin-1 gene: implication for permselective
dysfunction of chronic nephropathies. Am J Pathol. 166:1309–1320.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Christensen EI and Neilson S: Structural
and functional features of protein handling in the kidney proximal
tubule. Semin Nephrol. 11:414–439. 1991.PubMed/NCBI
|
|
19
|
Zou Z, Chung B, Nguyen T, et al: Linking
receptor-mediated endocytosis and cell signaling: evidence for
regulated intramembrane proteolysis of megalin in proximal tubule.
J Biol Chem. 279:343102–34310. 2004.PubMed/NCBI
|
|
20
|
Koop K, Eikmans M, Baelde HJ, et al:
Expression of podocyte-associated molecules in acquired human
kidney diseases. J Am Soc Nephrol. 14:2063–2071. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toyoda M, Najafian B, Kim Y, Caramori ML
and Mauer M: Podocyte detachment and reduced glomerular capillary
endothelial fenestration in human type 1 diabetic nephropathy.
Diabetes. 56:2155–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saleem MA, Ni L, Witherden I, Tryggvason
K, Ruotsalainen V, Mundel P and Mathieson PW: Co-localization of
nephrin, podocin, and the action cytoskeleton. Am J Pathol.
161:1459–1466. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu ZH, Li SJ, Chen C, Zeng C, Zhang B,
Zhou H and Li L: Glomerular podocyte lesion in patients with
diabetic nephropathy. Shen Zang Bing Yu Tou Xi Shen Yi Zhi Za Zhi.
12:144–148. 2003.
|
|
24
|
Barisoni L and Koppb BJ: Update in
podocyte biology: putting one’s best foot forward. Curr Opin
Nephrol Hypertens. 12:251–258. 2003.
|
|
25
|
Tryggvason K: Unraveling the mechanisms of
glomerular ultrafiltration: nephrin, a key component of the slit
diaphragm. J Am Soc Nephrol. 10:2440–2445. 1999.PubMed/NCBI
|
|
26
|
Shen XG and Shen HC: Research advancement
of diabetic nephropathy and podocytes injury. Urology and
nephrology FMS. 25:680–683. 2005.(In Chinese).
|
|
27
|
Benzing T: Signaling at the slit
diaphragm. J Am Soc Nephrol. 15:1382–1391. 2004. View Article : Google Scholar
|
|
28
|
Langham RG, Kelly DJ, Cox AJ, et al:
Proteinuria and the expression of the podocyte slit diaphragm
protein, nephrin, in diabetic nephropathy: effects of angiotensin
converting enzyme inhibition. Diabetologia. 45:1572–1576. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng Y, Hao Li, Pan MS and Ding N: Effect
of Triperygium wilfordii polyglucoside on the podocytes of
diabetic nephropathy rats. Zhonghua Shen Zang Bing Za Zhi.
27:288–292. 2011.(In Chinese).
|