Introduction
Cerebrovascular disease is a common disease that
endangers human life and health. Ischemic stroke accounts for ~80%
of the cases of cerebrovascular disease. Post-stroke depression
(PSD) is a common complication of strokes, with an incidence of
25–80%, due to different subjects, different assessing periods
after stroke, different diagnostic criteria and psychological
testing methods, and different experiences of the researchers
(1). The pathogenesis of PSD is
complex and a number of the underlying mechanisms remain
unclear.
The brain neurotransmitters, norepinephrine (NE),
serotonin (5-HT) and dopamine (DA), are biogenic amines that
transmit information between nerve cells or neurons and effector
cells, integrating the overall coordination of human body
functions. If these neurotransmitters are defected, the normal
function of the nervous system is affected, resulting in depression
(2–4). In the pathogenesis of depression, the
function and content of these three types of monoamine
neurotransmitter may change and affect the regulation of emotions
to a varying extent.
Fibroblast growth factor (FGF) plays an important
role in the development of the neocortex and the survival and
growth of adult neurons. The main role of FGF in the brain is the
regulation of neuronal and glial cell proliferation, migration,
differentiation and survival (5).
Precursor cells of the neural plate have the capacity to
differentiate into serotonergic and dopaminergic neurons (6–8), and
are considered to be associated with neural plasticity. Abnormal
expression of FGF may lead to depression (9). FGF-2, one of the original types of
ligand in the FGF family, protects ischemic cerebral tissue by
activating the anti-apoptotic protein (10–12).
However, whether there is an association between PSD and brain
monoamine neurotransmitters and FGF remains unknown.
In the present study, the levels of NE, 5-HT, DA and
FGF-2 protein were analyzed in the brains of rats with PSD in order
to investigate the causes of PSD. The mRNA expression levels of
FGF-2 were also analyzed to identify the molecular mechanisms
underlying the change in the levels of FGF-2 protein. The aim of
the study was to provide a biological basis for the diagnosis and
treatment of PSD.
Materials and methods
Animals
White male Sprague-Dawley rats (weight, 250–300 g)
were obtained from the Experimental Animal Center at Shandong
University of Traditional Chinese Medicine (Jinan, China). A total
of 45 rats were successfully induced as stroke models with a score
of 1–3 (13). Scoring was as
follows: behavior was completely normal, 0 points; the rats were
held by their tails, the contralateral forelimb of surgery
rotation, adduction, 1 point; the rats were placed on the ground,
squeeze the sides to check their resistance, resistance decreased
in the contralateral forelimb of surgery, 2 points; the rats were
placed on the ground to observe the walk, circling around the
contralateral of surgery, 3 points; the rats were seriously injured
and unable to do independent activities, 4 points. The stroke rats
were randomly divided into PSD and stroke groups. There was no
statistically significant difference in the scores of the rats
between the two groups. The rats in the stroke group were fed
normally. In the PSD group, the animals were treated simultaneously
with unpredicted chronic mild stress (CMS). The experiments were
approved by the Animal Experimentation Ethics Board of Shandong
Univiersity, which also meet the ethical requirements of the China
National Act on the Use of Experimental Animals. All surgeries were
performed under general anesthesia, and all efforts were made to
minimize suffering. The rats were placed in individual cages and
maintained in a 12:12 h light/dark cycle at a controlled
temperature (21±2°C) and humidity (65±5%). The rats had free access
to food and tap water.
Chemicals and equipment
ABI-7500 Real-Time detector (ABI, Carlsbad, CA,
USA), AB-160 electronic analytical balance (Sartorius AG, Beijing,
China), micropipette, rabbit anti-FGF-2, horseradish
peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H+L;
Shanghai BlueGene Biotech CO, Shanghai, China), 4% chloral hydrate
(The Second Hospital Affiliated to Shandong University of
Traditional Chinese Medicine, Jinan, China), perchloric acid,
polyvinylidene difluoride (PVDF), sodium acetate, citric acid,
methanol, et al.
Modeling method
A rat stroke model was established as described
previously with minor modifications (14). At day 7 following surgery, an
unpredicted CMS procedure was performed, as described previously,
to establish the rat PSD model (15,16).
The CMS protocol consisted of the sequential application of a
variety of mild stressors: i) Fasting for 20 h; ii) water
deprivation for 17 h; iii) cage tilt (45°) for 17 h; iv) constant
illumination for 17 h; v) wet cage (100 g sawdust + 200 ml water)
for 21 h; vi) forced swimming at 4°C for 5 min; vii) horizontal
shaking for 5 min; viii) immobilization for 2 h; and ix) tail
clamping for 1 min. These stressors were randomly scheduled over a
one-week period and repeated throughout the subsequent three weeks
of the experiment. For the stroke group, the animals were left
undisturbed in their home cages, except during housekeeping
procedures such as cage cleaning.
Following three weeks of CMS application, the rats
were anesthetized, rapidly decapitated and the brain tissue was
quickly placed in the ice plate, including the frontal lobe and
hippo-campus. The brain tissue was stored at −70°C until use.
Before total RNA was extracted, the tissue was homogenized.
Determination of the monoamine
levels
For the measurement of DA, 5-HT and NE levels, the
frozen brain tissue was thawed at room temperature and then
filtered through 0.45-μm membranes. A 2–5 μl aliquot of each sample
was injected into a high performance liquid chromatography column
and the levels were detected electrochemically. The results of the
qualitative analysis were the retention times of the sample and
standard, while the results of the quantitative analysis were the
peak areas of the sample and standard. All the results were
recorded by the N2005 chromatography data workstation.
Determination of the protein expression
levels of FGF-2
Proteins were extracted from the prefrontal cortex
and hippocampus for western blotting using a mixture of protein
extraction reagent and phosphates. The protein concentration was
determined using Coomassie Brilliant Blue dye. The denatured
proteins (50 μg) were separated by 15% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and electrotransferred
onto PVDF membranes. The membranes were incubated overnight at 4°C
with primary monoclonal rabbit anti-FGF-2 antibodies. Subsequently,
horseradish peroxidase-conjugated secondary antibodies (goat
anti-rabbit) were diluted 1:1,000 in Tris-buffered saline with
Tween 20 and were applied to the membranes. Chemiluminescent
detection was performed using enhanced chemiluminescence. For
visualization and densitometric analysis, a Chemi-Doc XRS+ imaging
system and Image Lab software were used (Bio-Rad, Hercules, CA,
USA).
Determination of mRNA expression levels
of FGF-2
Total RNA from the frontal cortex tissue of the rats
was extracted using TRIzol reagent, according to the manufacturer’s
instructions. The isolated RNA was reverse transcribed into cDNA.
The sample DNA that contained the target sequence was incubated at
95°C for 5 min. Subsequently, the temperature was lowered to 55°C
for 5 min to anneal the target to the complementary sequence. The
temperature was then raised to 72°C for 1 min to allow Taq
polymerase to attach at each priming site and extend a new DNA
strand. These steps were repeated for 40 cycles.
Aliquots of the polymerase chain reaction (PCR)
products (10 μl) were size-separated by electrophoresis on a 2%
agarose gel. Quantitative PCR was performed using SYBR Green Master
mix and ROX reference dye (Kapa SYBR Fast qPCR kit; Kapa
Biosystems), according to the manufacturer’s instructions. Briefly,
cDNA was obtained by reverse transcription of the RNA from the
brains of the rats. SYBR Green signals were detected by a Mx3000P™
Multiplex Quantitative PCR machine and the transcript levels were
quantified using the Ct value method, where the values were
normalized against the internal control, GAPDH. PCR products were
analyzed by gel electrophoresis on a 1.5% agarose gel, and the
specificity of amplification was confirmed by the melting curves.
The primers employed in the reverse transcription and quantitative
PCR analyses are listed in Table
I.
 | Table IPrimer sequences and the length of PCR
products. |
Table I
Primer sequences and the length of PCR
products.
| Gene | Direction | Primer sequences | Amplified fragment
length (bp) |
|---|
| FGF-2 | Upstream |
5′-TGCGCATCCATCCAGACGGC-3′ | 130 |
| Downstream |
5′-GCCAGGTACCGGTTCGCACA-3′ | |
| β-actin | Upstream |
5′-AGAACATCATCCCTGCATCC-3′ | 112 |
| Downstream |
5′-TGGATACATTGGGGGTAGGA-3′ | |
Statistical analysis
All the results are expressed as the mean ± standard
deviation and statistical analysis was performed using Statistical
Product and Service Solutions software, version 16.0 for Windows
(SPSS, Inc., Chicago, IL, USA). Inter-group comparisons were
performed using the Student’s t-test, assuming normal distribution,
where P<0.05 was considered to indicate a statistically
significant difference.
Results
Physiological parameters of the PSD and
stroke groups
Physiological parameters, including the mean
arterial pressure, blood pH, arterial oxygen and carbon dioxide
tensions, hematocrit and blood glucose levels, were recorded and
controlled within the normal ranges (17). No statistically significant
differences in these parameters were identified between the PSD and
stroke groups.
Monoamine transmitter levels in the
brains of the rats
Compared with the control group, the reduction in
the levels of NE, 5-HT and DA in the frontal lobe of the rats in
the PSD group was statistically significant (P<0.01; Student’s
t-test; Table II).
 | Table IILevels of NE, 5-HT and DA in the
frontal lobe (n=8 per group; ng/ml). |
Table II
Levels of NE, 5-HT and DA in the
frontal lobe (n=8 per group; ng/ml).
| Group | NE | 5-HT | DA |
|---|
| PSD | 173.9±13.2 | 349.9±21.9 | 233.9±12.1 |
| Stroke | 285.1±19.5 | 722.1±13.2 | 284.3±14.0 |
| F-value | 0.671 | 1.163 | 1.050 |
| P-value | <0.01 | <0.01 | <0.01 |
The effect of PSD on the brain was further evaluated
in terms of the levels of NE, 5-HT and DA in the hippocampus. When
compared with the control stroke group, the reduction in the levels
of NE, 5-HT and DA in the hippocampus of the rats in the PSD group
was statistically significant (P<0.01; Student’s t-test;
Table III)
 | Table IIILevels of NE, 5-HT and DA in the
hippocampus (n=8 per group; ng/ml). |
Table III
Levels of NE, 5-HT and DA in the
hippocampus (n=8 per group; ng/ml).
| Group | NE | 5-HT | DA |
|---|
| PSD | 157.5±15.4 | 327.9±27.9 | 230.8±13.1 |
| Stroke | 271.2±18.4 | 725.9±15.4 | 285.4±15.6 |
| F-value | 0.962 | 2.175 | 0.485 |
| P-value | <0.01 | <0.01 | <0.01 |
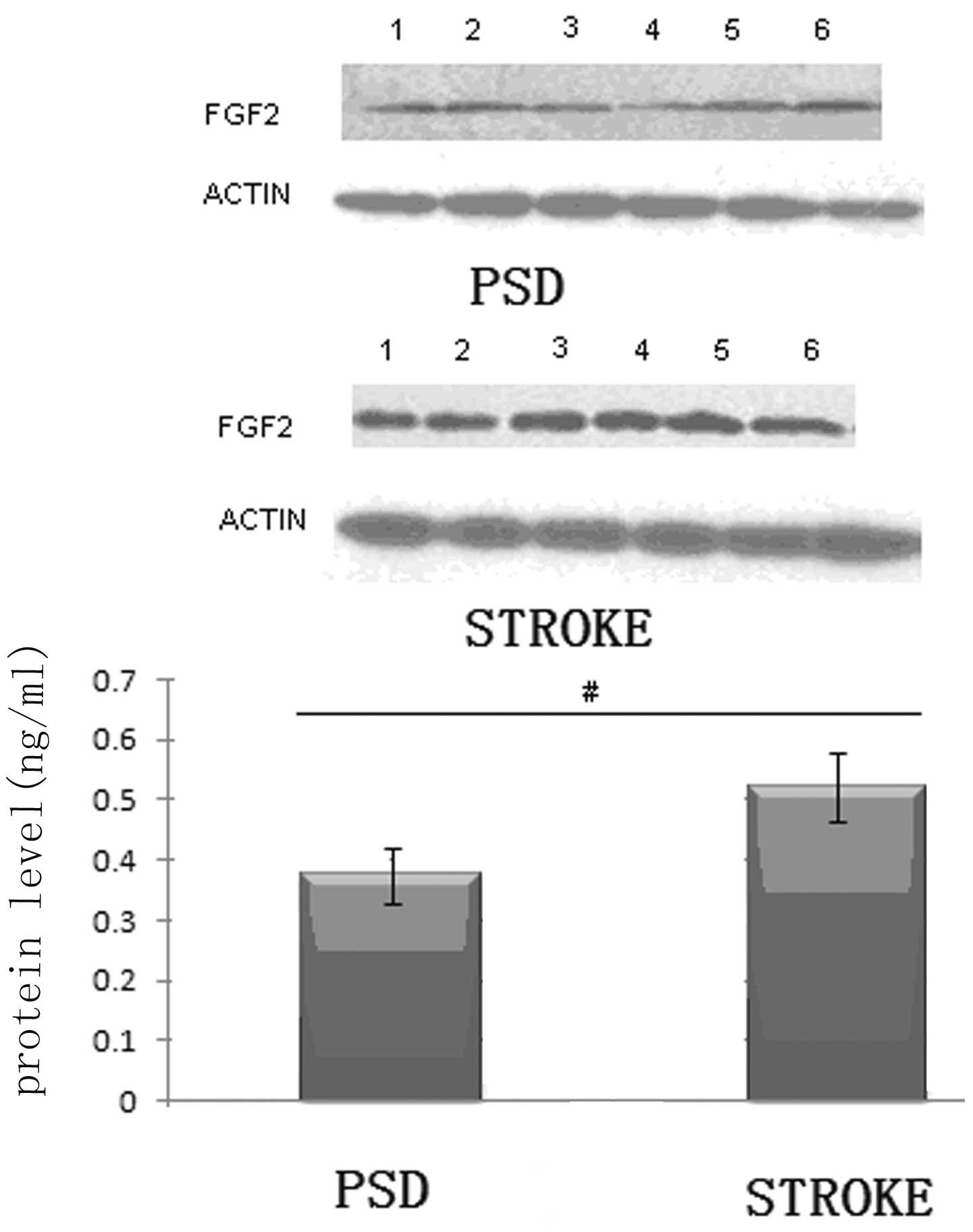
Protein expression levels of FGF-2 in the
frontal lobe and hippocampus of the rats
It is well established that the addition of FGF-2 is
critical to the palingenesis of nerves (18–21).
Thus, the reduction in monoamine transmitters levels observed in
the brains of the rats may be due to changes in the levels of FGF-2
expression in the frontal lobe and hippocampus. To further test
this hypothesis, western blotting was used to analyze the protein
expression levels of FGF-2 in the frontal lobe and hippocampus
tissues of the rats.
In the frontal lobe tissue, the FGF-2 protein
expression levels in the PSD group were significantly lower than
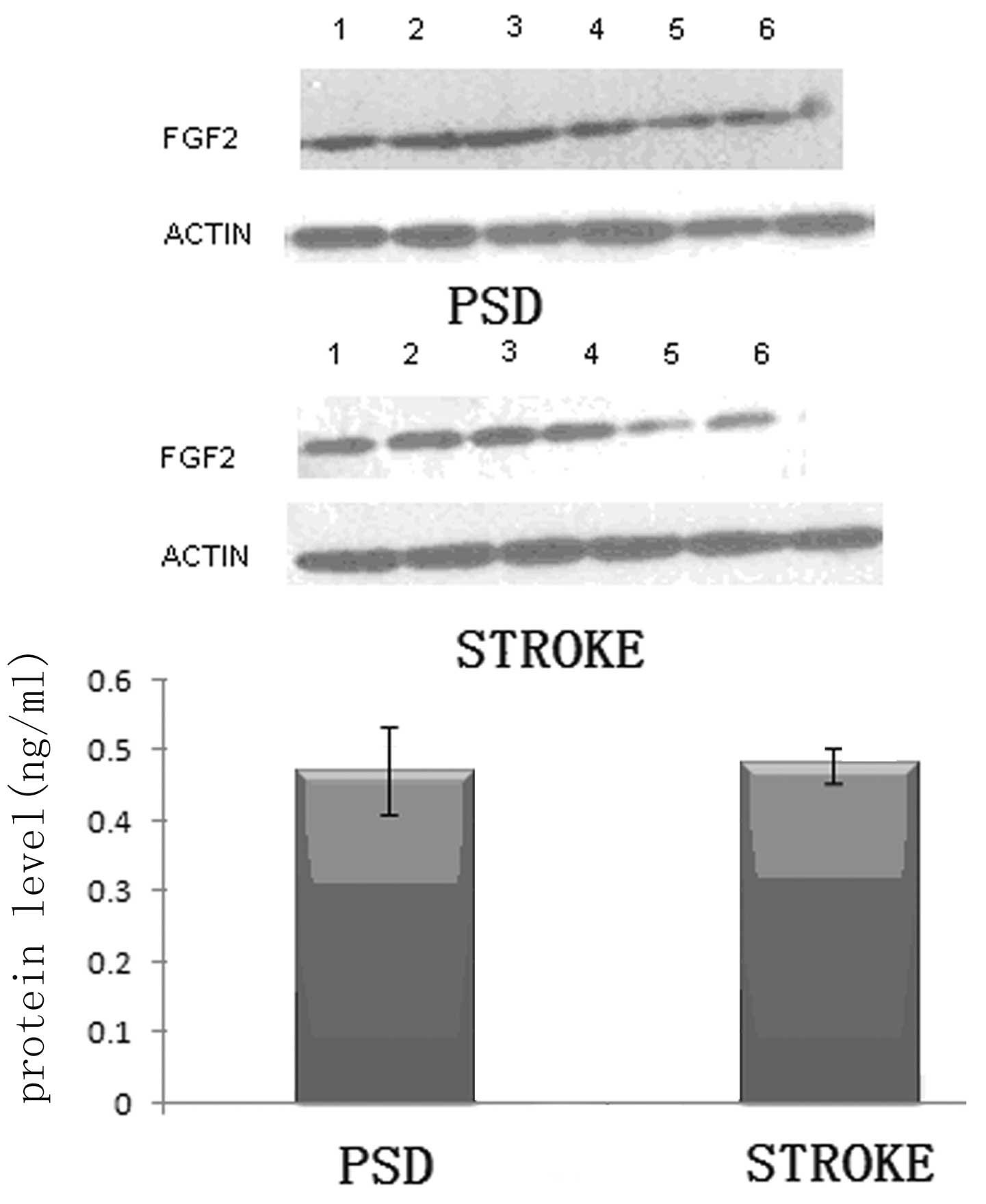
those in the stroke group (P<0.01; Student’s t-test; Fig. 1). Subsequently, the same analysis
was performed on the hippocampal tissue. Although the expression
levels of FGF-2 appeared to be reduced in the hippocampus of the
rats in the PSD group as compared with those in the stroke group,
the decrease in the relative protein expression levels was not
statistically significant (P>0.05; Student’s t-test; Fig. 2). The results demonstrate that in
the frontal lobe, a correlation exists between the level of
monoamine transmitters and FGF-2 protein expression. To further
analyze this hypothesis, the mRNA expression levels of FGF-2 in the
frontal lobe were determined.
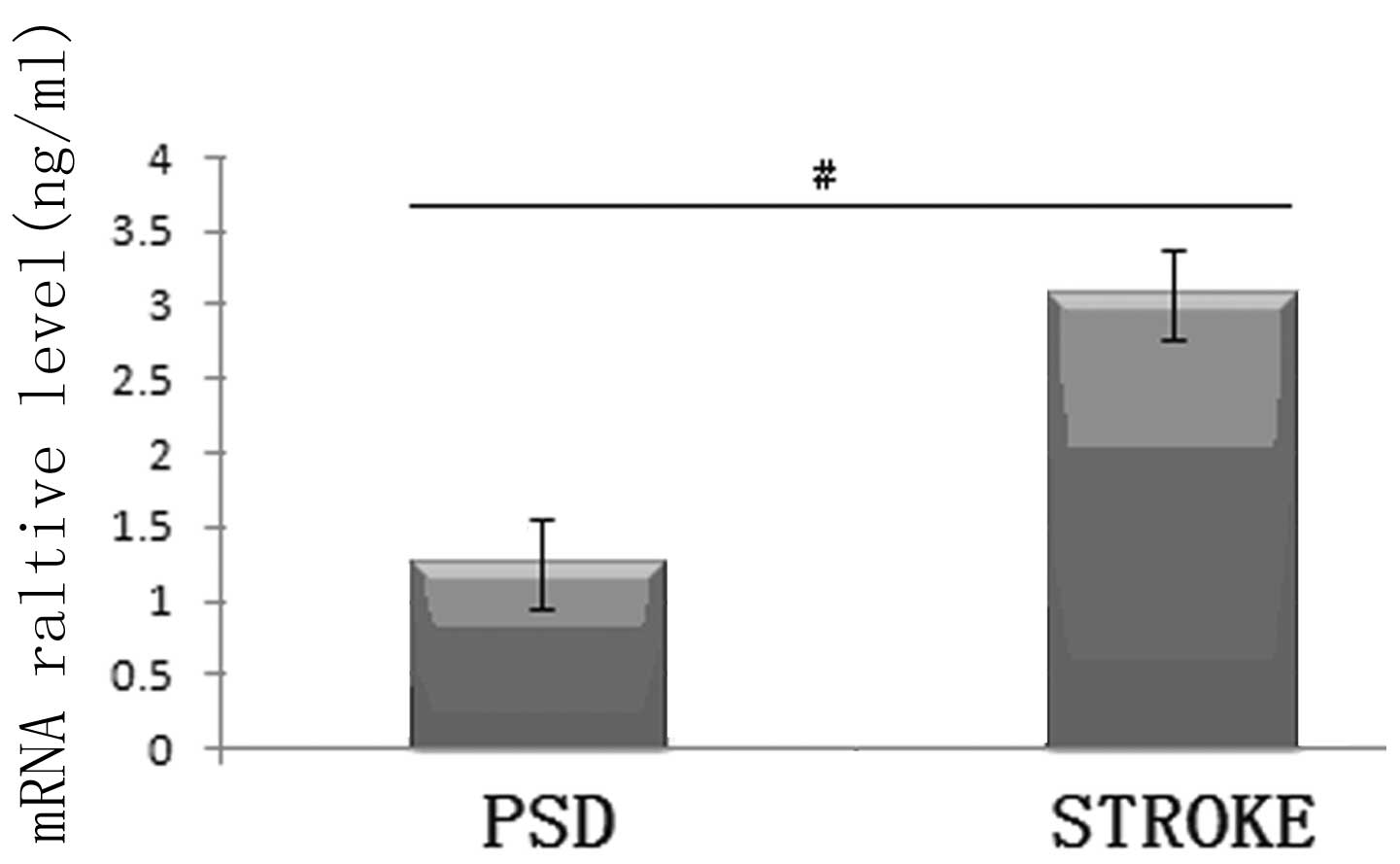
mRNA expression levels of FGF-2 in the
frontal lobe of the rats
Compared with the stroke group, the mRNA expression
levels of FGF-2 in the frontal cortex tissue of the PSD rats were
significantly decreased (P<0.01; Student’s t-test; Fig. 3). This marked reduction in the
frontal lobe of the rats indicated that the decreased FGF-2 protein
expression levels, as observed in the previous experiment, were
caused by the downregulation of FGF-2 mRNA expression.
Discussion
As a secondary disease, PSD directly affects the
quality of life and functional rehabilitation of patients,
significantly reducing the desire for rehabilitation and delaying
the functional recovery of nervous and cognitive aspects. PSD not
only increases the mortality rate and rate of suicide, but also
increases the burden on society and the family of the patient
(22–29). The main theory underlying the
pathogenesis of PSD is a series of abnormalities regarding
neurotransmitters and endocrine and neurotropic factors following a
stroke (30). These abnormalities
cause a variety of psychological stresses. The interaction of
physiological, psychological and social factors causes an imbalance
of NE and 5-HT neurons in the feedback loop system formed by the
frontotemporal lobe, basal ganglia, ventral brainstem and its
pathway disruption. All these factors promote the occurrence of PSD
(31,32). However, the cause of the reduction
in the levels of monoamine transmitters may be complex and is yet
to be completely understood.
In the present study, a stroke model was established
in rats by adopting the method of occlusion of the left middle
cerebral artery. The cerebrovascular pathological changes in the
brains of the rats following this procedure are similar to clinical
stroke pathology. Following the successful establishment of the rat
stroke model, the rats underwent unpredictable CMS, which simulated
the pathological state of patients with PSD. A significant
reduction in the levels of 5-HT, NE and DA was observed in the
frontal cortex and hippocampus of the rats with PSD, as compared
with the rats in the control group. These results may be associated
with the direct disruption of the pathways involving 5-HT and NE
following a stroke. Neuronal cell bodies are located in the
brainstem and the axons of these neuronal cell bodies reach the
frontal cortex and hippocampus through the thalamus and basal
ganglia, which play an important role in mood and emotion
regulation. When these pathways involving 5-HT and NE are invaded
by a lesion, reduced levels of 5-HT and NE lead to the occurrence
of PSD.
It is well established that FGF-2 is necessary for
progenitor cell proliferation in the developing brain in
vivo (33–36), and FGF-2 stimulates the
proliferation of neural stem cells and progenitors isolated from
the embryonic brain (33,37–44).
To further investigate the cellular mechanism underlying the
significant reduction in the levels of monoamine transmitters in
the frontal lobe and hippocampus of the rats, the protein
expression levels of FGF-2 in the two parts of the brains were
determined by western blot analysis. Through western blotting, the
protein expression levels of FGF-2 in the frontal lobe were found
to be significantly lower than those in the stroke group
(P<0.01; Student’s t-test). By contrast, the reduction in the
protein expression levels of FGF-2 in the hippocampus of the PSD
group was not statistically significant when compared with the
stroke group, although a slight decrease was observed (P>0.05;
Student’s t-test). The results indicate that the FGF-2 signaling
pathway may be the cause of the reduction in monoamine levels and
the occurrence of PSD. Additional experiments, which detected the
FGF-2 mRNA expression levels in the frontal lobe by quantitative
PCR, revealed that the decreased protein expression levels of FGF-2
in the PSD group were caused by the decreased mRNA expression
levels of FGF-2. The FGF signaling pathway consists of multiple
ligands and receptors. The major role of FGF in the brain is the
regulation of differentiation, proliferation, migration and
survival of vascular endothelial cells, neurons and glial cells.
The FGF signaling pathway also has the capacity to induce the
differentiation of neural plate precursor cells into dopaminergic
and serotonergic neurons, which plays an important role in brain
development (11). A previous
study (45) demonstrated that the
dysfunction of the FGF system in patients with major depression is
not induced by antidepressants. The results of the present study
demonstrated that the decreased protein and mRNA expression levels
of FGF-2 in the frontal lobe may be associated with the occurrence
of PSD and the decreased levels of 5-HT, NE and DA. Accordingly,
the observations of the present study may aid the understanding of
the underlying mechanisms that lead to PSD under physiological and
pathological conditions.
In conclusion, using a variety of assays, the
present study has demonstrated that a reduction in FGF-2 mRNA
expression levels is key to the reduced levels of monoamine
neurotransmitters and the occurrence of PSD.
References
|
1
|
Yang LL, Zhang ZJ and Sun DM: Incidence
and its risk factors of post-stroke depression in cerebral stroke
patients at acute stage. Lin Chuang Shen Jing Bing Xue Za Zhi.
23:185–187. 2010.(In Chinese).
|
|
2
|
Berg C, Backström T, Winberg S, et al:
Developmental exposure to fluoxetine modulates the serotonin system
in hypothalamus. PLoS One. 8:e550532013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sullivan GM, Ogden RT, Huang YY, et al:
Higher in vivo serotonin-1a binding in posttraumatic stress
disorder: a PET study with [11C]WAY-100635. Depress Anxiety.
30:197–206. 2013.PubMed/NCBI
|
|
4
|
Spasojevic N, Jovanovic P and Dronjak S:
Chronic fluoxetine treatment affects gene expression of
catecholamine enzymes in the heart of depression model rats. Indian
J Exp Biol. 50:771–775. 2012.PubMed/NCBI
|
|
5
|
Vergaño-Vera E, Méndez-Gómez HR,
Hurtado-Chong A, et al: Fibroblast growth factor-2 increases the
expression of neurogenic genes and promotes the migration and
differentiation of neurons derived from transplanted neural
stem/progenitor cells. Neuroscience. 162:39–54. 2009.PubMed/NCBI
|
|
6
|
Reynolds BA and Weiss S: Generation of
neurons and astrocytes from isolated cells of the adult mammalian
central nervous system. Science. 255:1707–1710. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vicario-Abejón C, Yusta-Boyo MJ,
Fernández-Moreno C and de Pablo F: Locally born olfactory bulb stem
cells proliferate in response to insulin-related factors and
require endogenous insulin-like growth factor-I for differentiation
into neurons and glia. J Neurosci. 23:895–906. 2003.PubMed/NCBI
|
|
8
|
Gritti A, Parati EA, Cova L, Frolichsthal
P, et al: Multipotential stem cells from the adult mouse brain
proliferate and self-renew in response to basic fibroblast growth
factor. J Neurosci. 16:1091–1100. 1996.PubMed/NCBI
|
|
9
|
Turner CA, Watson SJ and Akil H: The
fibroblast growth factor family: neuromodulation of affective
behavior. Neuron. 76:160–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fukuchi-Shimogori T and Grove EA:
Neocortex patterning by the secreted signaling molecule FGF8.
Science. 294:1071–1074. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin DM, Korada S, Raballo R, et al: Loss
of glutamatergic pyramidal neurons in frontal and temporal cortex
resulting from attenuation of FGFR1 signaling is associated with
spontaneous hyperactivity in mice. J Neurosci. 24:2247–2258. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guillemot F and Zimmer C: From cradle to
grave: the multiple roles of fibroblast growth factors in neural
development. Neuron. 71:574–588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaccarino FM, Schwartz ML, Raballo R, et
al: Changes in cerebral cortex size are governed by fibroblast
growth factor during embryogenesis. Nat Neurosci. 2:246–253. 1999.
View Article : Google Scholar
|
|
14
|
Raballo R, Rhee J, Lyn-Cook R, et al:
Basic fibroblast growth factor (Fgf2) is necessary for cell
proliferation and neurogenesis in the developing cerebral cortex. J
Neurosci. 20:5012–5023
|
|
15
|
Mason I: Initiation to end point: the
multiple roles of fibroblast growth factors in neural development.
Nat Rev Neurosci. 8:583–596. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ciccolini F and Svendsen CN: Fibroblast
growth factor 2 (FGF-2) promotes acquisition of epidermal growth
factor (EGF) responsiveness in mouse striatal precursor cells:
identification of neural precursors responding to both EGF and
FGF-2. J Neurosci. 18:7869–7880. 1998.
|
|
17
|
Ito Y, Tsurushima H, Sato M, et al:
Angiogenesis therapy for brain infarction using a slow-releasing
drug delivery system for fibroblast growth factor 2. Biochem
Biophys Res Commun. 432:182–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maric D, Fiorio Pla A, Chang YH and Barker
JL: Self-renewing and differentiating properties of cortical neural
stem cells are selectively regulated by basic fibroblast growth
factor (FGF) signaling via specific FGF receptors. J Neurosci.
27:1836–1852. 2007. View Article : Google Scholar
|
|
19
|
Sun Y, Hu J, Zhou L, et al: Interplay
between FGF2 and BMP controls the self-renewal, dormancy and
differentiation of rat neural stem cells. J Cell Sci.
124:1867–1877. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vicario-Abejón C, Johe KK, Hazel TG, et
al: Functions of basic fibroblast growth factor and neurotrophins
in the differentiation of hippocampal neurons. Neuron. 15:105–114.
1995.PubMed/NCBI
|
|
21
|
Cattaneo E and McKay R: Proliferation and
differentiation of neuronal stem cells regulated by nerve growth
factor. Nature. 347:762–765. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gillen R, Tennen H, McKee TE, et al:
Depressive symptoms and history of depression predict
rehabilitation efficiency in stroke patients. Arch Phys Med
Rehabil. 82:1645–1649. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh A, Black SE, Herrmann N, et al:
Functional and neuroanatomic correlations in poststroke depression:
the Sunnybrook Stroke Study. Stroke. 31:637–644. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vataja R, Pohjasvaara T, Mäntylä R, et al:
Depression-executive dysfunction syndrome in stroke patients. Am J
Geriatr Psychiatry. 13:99–107. 2005.PubMed/NCBI
|
|
25
|
Kishi Y, Kosier T and Robinson RG:
Suicidal plans in patients with acute stroke. J Nerv Ment Dis.
184:274–280. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
House A, Knapp E, Bamford J and Vail A:
Mortality at 12 and 24 months after stroke may be associated with
depressive symptoms at 1 month. Stroke. 32:696–701. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Everson SA, Roberts RE, Goldberg DE and
Kaplan GA: Depressive symptoms and increased risk of stroke
mortality over a 29-year period. Arch Intern Med. 158:1133–1138.
1998.PubMed/NCBI
|
|
28
|
Robinson RG, Bolduc PL and Price TR:
Two-year longitudinal study of poststroke mood disorders:diagnosis
and outcome at one and two years. Stroke. 18:837–843.
1987.PubMed/NCBI
|
|
29
|
Simonsick EM, Wallace RB, Blazer DG and
Berkman LF: Depressive symptomatology and hypertension-associated
morbidity and mortality in older adults. Psychosom Med. 57:427–435.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vescovi AL, Reynolds BA, Fraser DD and
Weiss S: bFGF regulates the proliferative fate of unipotent
(neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS
progenitor cells. Neuron. 11:951–966. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lukaszewicz A, Savatier P, Cortay V, et
al: Contrasting effects of basic fibroblast growth factor and
neurotrophin 3 on cell cycle kinetics of mouse cortical stem cells.
J Neurosci. 22:6610–6622. 2002.PubMed/NCBI
|
|
32
|
Lillien L and Raphael H: BMP and FGF
regulate the development of EGF-responsive neural progenitor cells.
Development. 127:4993–5005. 2000.PubMed/NCBI
|
|
33
|
Ikeda N, Nonoguchi N, Zhao MZ, et al: Bone
marrow stromal cells that enhanced fibroblast growth factor-2
secretion by herpes simplex virus vector improve neurological
outcome after transient focal cerebral ischemia in rats. Stroke.
36:2725–2730. 2005. View Article : Google Scholar
|
|
34
|
Yi ZH, Fang YR and Yu SY: Research
progress of gene differential expression of depression brain
tissue. Zhongguo Shen Jing Jing Shen Ji Bing Za Zhi. 36:56–59.
2010.(In Chinese).
|
|
35
|
Ay I, Sugimori H and Finkelstein SP:
Intravenous basic fibroblast growth factor (bFGF) decreases DNA
fragmentation and prevents downregulation of Bcl-2 expression in
the ischemic brain following middle cerebral artery occlusion in
rats. Brain Res Mol Brain Res. 87:71–80. 2001. View Article : Google Scholar
|
|
36
|
Rosenblatt S, Irikura K, Caday CG, et al:
Basic fibroblast growth factor dilates rat pial arterioles. J Cereb
Blood Flow Metab. 14:70–74. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Koizumi J, Yoshida Y, Nakazawa T and
Ooneda G: Experimental studies of ischemic brain edema: A new
experimental model of cerebral embolism in rats in which
recirculation can be introduced in the ischemic area. Jpn J Stroke.
16:1–8. 1986.(In Japanese).
|
|
39
|
Willner P, Towell A, Sampson D, et al:
Reduction of sucrose preference by chronic mild unpredictable
stress, and its restoration by a tricyclic antidepressant.
Psychopharmacology (Berl). 93:358–364. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rasul A, El-Nour H, Blakely RD, et al:
Effect of chronic mild stress on serotonergic markers in the skin
and brain of the NC/Nga atopic-like mouse strain. Arch Dermatol
Res. 303:625–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ji YH and Song JG: Relevance among
cognition function, autonomic nerve function and the serum levels
of monoamine neurotransmitter in patients with post-stroke
depression. Lin Chuang Shen Jing Bing Xue Za Zhi. 20:426–428.
2007.(In Chinese).
|
|
42
|
Loubinoux I, Kronenberg G, Endres M, et
al: Post-stroke depression: mechanisms, translation and therapy. J
Cell Mol Med. 16:1961–1969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Haneda E, Higuchi M, Maeda J, et al: In
vivo mapping of substance P receptors in brains of laboratory
animals by high-resolution imaging systems. Synapse. 61:205–215.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chatterjee K, Fall S and Barer D: Mood
after stroke: a case control study of biochemical, neuro-imaging
and socio-economic risk factors for major depression in stroke
survivors. BMC Neurol. 10:1252010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Evans SJ, Choudary PV, Neal CR, et al:
Dysregulation of the fibroblast growth factor system in major
depression. Proc Natl Acad Sci USA. 101:15506–15511. 2004.
View Article : Google Scholar : PubMed/NCBI
|