Introduction
Cardiovascular diseases (CVDs) result in >19
million mortalities annually, and coronary heart disease
contributes significantly to these mortalities. Individuals with
the disease may appear healthy, yet succumb to CVD suddenly without
prior symptoms (1).
In the majority of cases, the development and
progression of CVD is characterized by the interaction of
atherosclerotic lesion and thrombus formation processes, and
platelet participation is pivotal in these processes (2,3).
Following an atheromatous plaque rupture, platelets adhere, secrete
their granule contents, aggregate and initiate thrombus formation
(3). Activation is a dynamic
process that may lead to intermittent or permanent obstruction of
blood flow, resulting in ischemic tissue injury and organ
dysfunction (4).
The inhibition of platelet function has been used
for a long time as a method to prevent ischemic complications at
late stages of the atherosclerotic process; ischemic complications
are a leading cause of cardiovascular morbidity and mortality
(5,6). Animal models are used to provide a
cost effective means for the development of techniques and/or
models that aid the study of thrombosis pathophysiology. Previous
studies of animal models have used a variety of techniques to
induce thrombosis, including the application of ferric chloride (a
compound that produces endothelial damage, causing an injury from
the adventitia to the intima that induces a loss of endothelial
integrity) (7), stasis (based on
stopping the blood flow and thereby causing damage to the blood
vessel, which leads to a hypercoagulable state) (7), ultrasound (which also induces damage
in the endothelium) (8) and laser
treatment (9–13). Laser irradiation, which causes a
directed photosensitization reaction, has been reported as a method
of forming thrombi by inducing endothelial damage in a murine model
(9). The reactive oxygen species,
which are generated by the interaction of rose bengal and the
laser, play a relevant role in the model since tissue damage
contributes to pathological environments in thrombosis formation
(14–16).
The present study describes a model of laser-induced
thrombosis that has been modified to be relatively inexpensive yet
remain effective. The main objective of the study was to develop a
low cost in vivo thrombosis model that is able to provide
results comparable with those of more sophisticated and expensive
systems.
Materials and methods
Reagents
Rose bengal, calcein acetyloxymethyl ester (AM),
2,2,2-tribromoethanol, 2-methyl-2-butanol and dimethylsulfoxide
were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animal model
Male BALB/c mice aged 8–10 weeks (weight, 25–35 g)
were bred and housed in groups of six mice per cage. The mice were
fed a pelleted basal diet (CRF-1; Oriental Yeast Co., Ltd., Tokyo,
Japan) and provided with free access to drinking water. Mice were
maintained in the animal house facilities at University of Talca
(Talca, Chile) under standard conditions of relative humidity
(50±10%), temperature (23±2°C) and light (12/12 h light/dark
cycle), according to the Institutional Animal Care Guidelines. The
experimental procedures were reviewed and approved by the Animal
Care and Use Committee at University of Talca.
In vivo murine model of thrombosis
BALB/c mice were anesthetized (0.4 ml) using a
combination of tribromoethanol (270 mg/kg) and xylazine (13 mg/kg).
Mice were placed in the supine position with the hind legs clamped
and stretched to achieve tension in the abdominal area. The
mesentery was exposed by performing a central incision in the
abdomen, permitting the visualization of thrombus development in
the mesenteric vessels.
In order to induce thrombosis in the shortest
possible time, rose bengal was administered. Intravenous injections
of various concentrations (5, 10, 25 and 50 mg/kg) of rose bengal
were administered into the tail vein, following heating of the tail
with a lamp for 3 min. Thus, thrombosis was induced in the
mesenteric arteries following illumination of the exposed
mesenteric area with a 5-mW green light laser (532 nm; O/E Land,
Inc., Montréal, QC, Canada). Images were captured every 10 min
using a zoom stereomicroscope (SMZ800; Nikon, Tokyo, Japan). Color
images were received by a camera (MF-TV; Nikon, Tokyo Japan) that
was connected to a computer system (INFINITY 1; Lumenera Corp.,
Ottawa, ON, Canada). Rectal temperatures were similar and within
the physiological range among all experimental animals throughout
the experimental period. Blood flow was monitored for 60 min and
stable occlusion was defined as a blood flow of 0 ml/min for 3 min.
Area measurement of the thrombus was performed with the software
ImageJ (National Institutes of Health, Bethesda, MD, USA).
Preparation of the human platelet
suspensions
Venous blood samples were obtained from two young
healthy volunteers who had previously provided informed consent.
The samples were collected in 3.2% citrate tubes (9:1 v/v) by
phlebotomy with a vacuum tube system (Becton Dickinson, Franklin
Lakes, NJ, USA). The experimental procedures were authorized by the
Ethics Committee of the University of Talca and were conducted in
accordance with the Declaration of Helsinki (approved by the 18th
World Medical Assembly in Helsinki, Finland, 1964). Tubes were
centrifuged (DCS-16 Centrifugal Presvac RV; Presvac, Buenos Aires,
Argentina) at 240 × g for 10 min to obtain platelet-rich plasma
(PRP). The PRP was adjusted to 2×1011 platelets/l with
platelet-poor plasma obtained by centrifugation of the original
tubes at 650 × g for 10 min. Washed platelets, at a concentration
of 2×1011 platelets/l, were prepared in Tyrode-HEPES
buffer containing 50 ng/ml prostaglandin E1 (PGE1) and 1
mmol/l ethylenediamine-N,N,N′,N′-tetraacetic acid (EDTA; pH 7.4).
To avoid platelet activation, 50 ng/ml PGE1 was added to
the PRP prior to centrifugation at 750 × g for 10 min. Platelet
counts were performed in a hematologic counter (Advia 60 Hematology
System; Bayer Corporation & Diagnostics, Tarrytown, NY,
USA).
Dynamics of platelet thrombus
formation
Washed platelets (2×1011 platelets/l)
were labeled with 4 μmol/l calcein AM for 1 h in the dark. Then,
0.2 ml platelets were intravenously injected into the tail vein of
the mice. Next, it was necessary to exteriorize and isolate the
mesentery, securing a stable platform to achieve maximum tension.
An epifluorescence microscope (Thermo Fisher Scientific, Inc., New
York, NY, USA) was used to identify the blood vessels. Then, using
a magnification of ×63, the injury induction process was initiated.
Thrombus formation was monitored over a 60-min period as
aforementioned.
Statistical analysis
Data were analyzed using SPSS version 17.0 software
(SPSS, Inc., Chicago, IL, USA) and expressed as mean ± standard
error of the mean. Three or more independent experiments were
performed for the various assays. Differences among groups were
analyzed with the unpaired t-test and by one-way analysis of
variance using Tukey’s post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of rose bengal on laser-induced
thrombosis
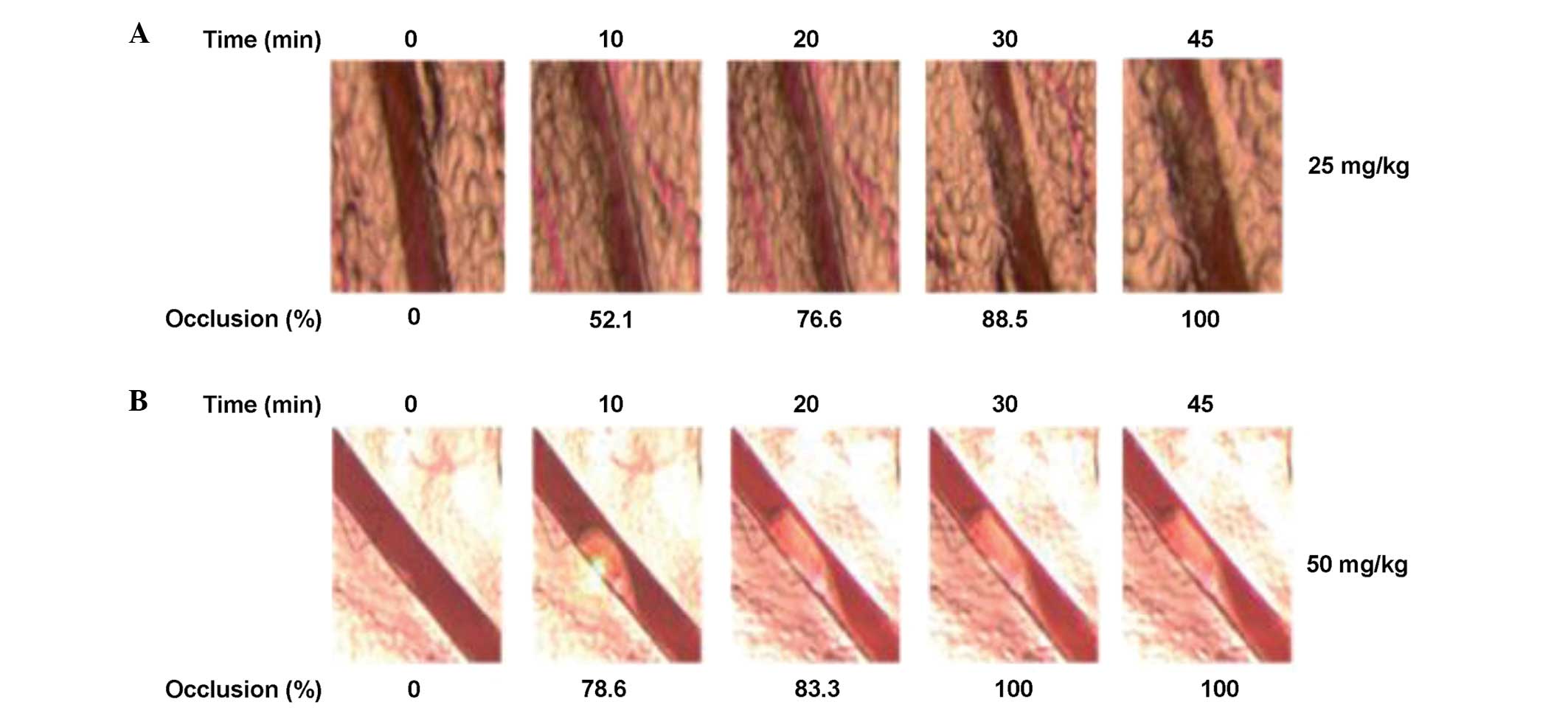
The effects of various concentrations of rose bengal
on the stimulation of thrombosis formation are shown in Table I. Laser irradiation with 5 and 10
mg/kg concentrations of rose bengal was ineffective in initiating
thrombosis formation in the mesenteric arteries. By contrast, at
concentrations of 25 and 50 mg/kg, thrombus formation (total
occlusion) occurred at ~45 and ~30 min, respectively (Fig. 1). Additionally was observed that
the presence of the laser and rose bengal together was required for
the induction of thrombosis, and that either alone did not induce
thrombosis (data not shown).
 | Table IPhotochemically induced thrombosis
with various concentrations of rose bengal in mice. |
Table I
Photochemically induced thrombosis
with various concentrations of rose bengal in mice.
| Rose bengal
(mg/kg) | Laser fluence
(mW/mm2) | Mice (n) | Outcome |
|---|
| 5 | 255 | 3 | No thrombosis |
| 10 | 255 | 3 | No thrombosis |
| 25 | 255 | 3 | Total occlusion |
| 50 | 255 | 3 | Total occlusion |
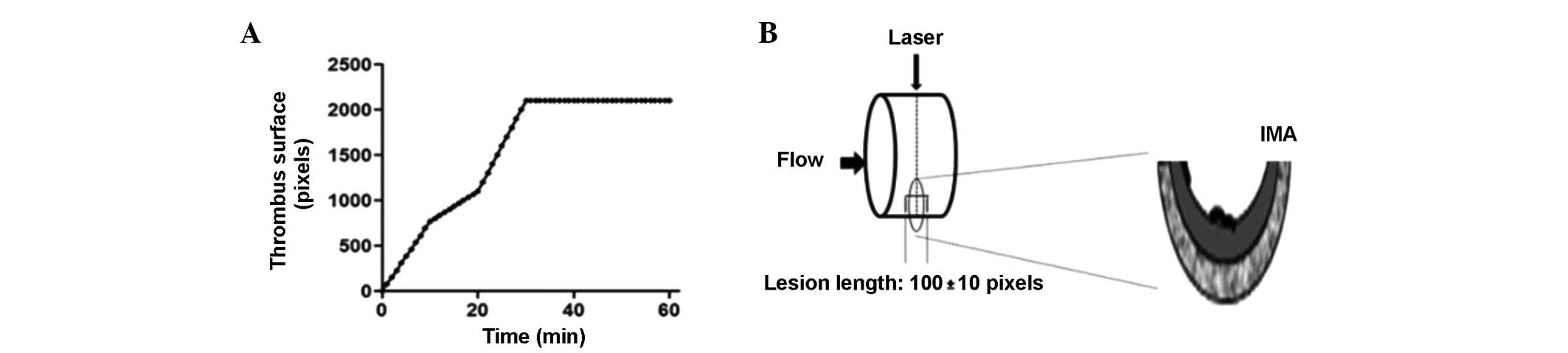
Fig. 2A shows
thrombus formation over time at a concentration of 50 mg/kg.
Following laser irradiation, the thrombus formed within 30 min,
causing total occlusion of the vessels. During the 60 min of
monitoring, the formation of a stable thrombus and blood vessel
occlusion was observed. The thrombus was 4,878.3 pixels in size and
occluded the blood vessel by 100% at 30 min (n=6). Fig. 2B schematically shows the incidence
of the laser beam causing localized damage to the endothelium.
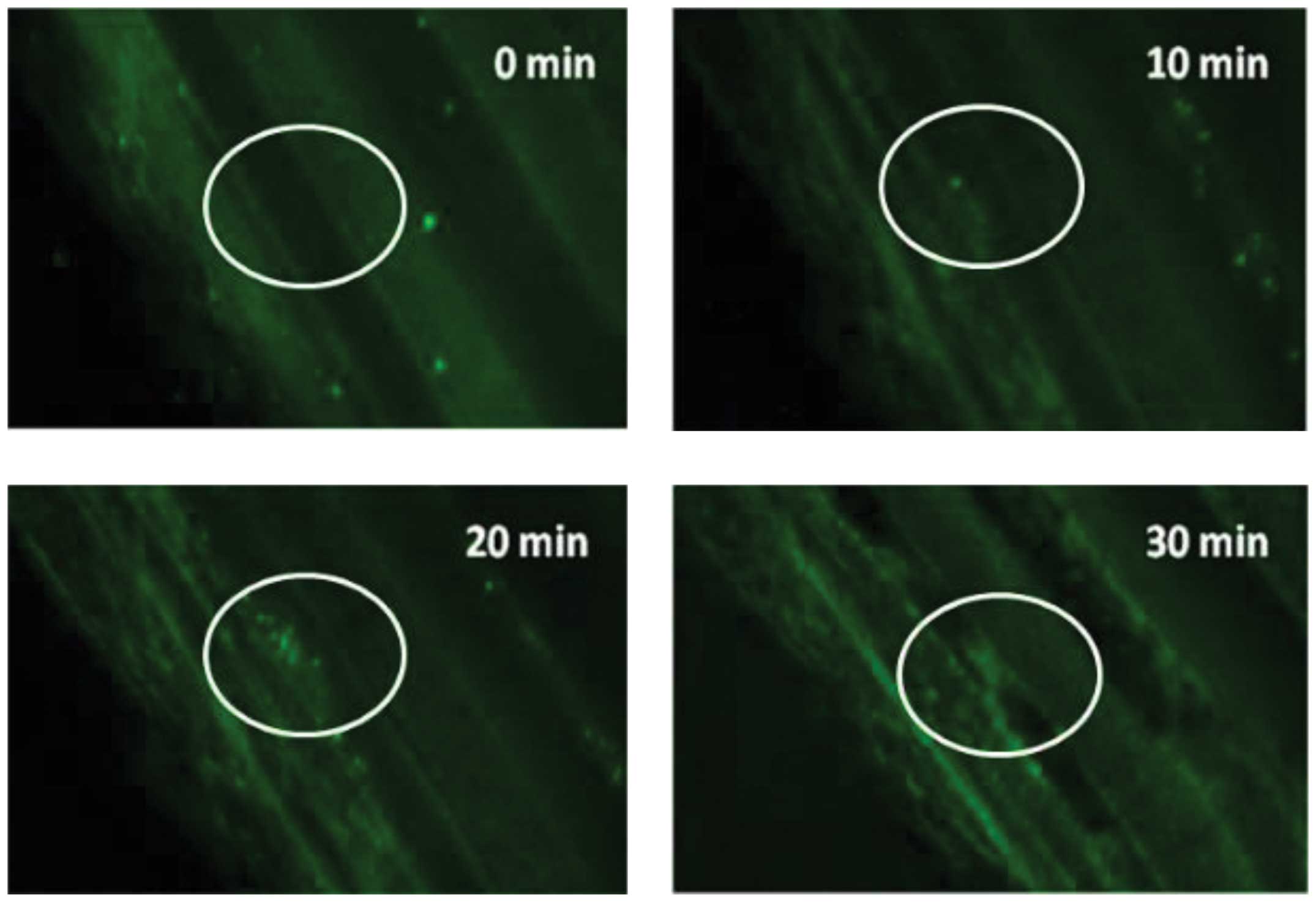
Fig. 3 shows in vivo
thrombus formation following irradiation with 50 mg/kg rose bengal
and fluorescence detection using calcein-labeled platelets.
Discussion
The present study establishes a simple system using
a rose bengal concentration of 50 mg/kg and a laser power of 5 mW
that is effective for use in studies of thrombosis. The results
obtained also indicate that increasing the concentration of rose
bengal, whilst maintaining the same laser power (5 mW), decreases
the time taken for thrombus formation in the mesenteric arteries.
Previous studies have described techniques for imaging thrombi
formed by photochemical action on arteries and/or arterioles in a
murine model (17–19). Thrombus formation following laser
irradiation in the mesenteric arteries has been evaluated in
vivo in our laboratory using an adaptation of the system used
by Sigler et al (10).
Experimental models of thrombosis are essential for
the study of the complex factors involved in thrombus formation;
however, each model has advantages and limitations (20). The majority of models are based on
the initiation of thrombosis by injury to the endothelium, which
exposes subendothelial components and subsequently leads to the
development of thrombosis (21).
Fukuoka et al (9), showed that thrombus formation using a
laser (9.8 mW; 532 nm) directed at the pial artery in C57BL/6 mice
induced thrombosis. In the present study, an adaptation of the
method was used to induce thrombosis formation in BALB/c mice using
a laser with a power of 5 mW and a wavelength of 532 nm. Rose
bengal was administered to the mice, which were then irradiated
with green light from the laser. This process generates singlet
oxygen and superoxide anions in the irradiated site, leading to
endothelial cell damage at the site of injury (16). During the induction of thrombus
formation using rose bengal, there is no extravasation damage to
the vasculature; therefore, damage occurs only at the site of
irradiation (16,22).
Przyklenk et al (20) demonstrated that the use of 20, 25
and 50 mg/kg rose bengal and a laser power of 0.34 mW did not
result in thrombus formation. However, thrombus formation did occur
when the laser power was increased to 0.84 mW with 25 mg/kg rose
bengal (22). In the present
study, it was shown that a specific laser power of 5 mW and
concentrations of 5 and 10 mg/kg rose bengal did not result in
thrombus formation in the mesenteric arteries. However, increasing
the concentration of rose bengal to 25 and 50 mg/kg did result in
thrombus formation. In this context, a major difference is that the
laser in the present study had a greater power, producing greater
endothelial damage, thereby enhancing the induction of thrombus
formation. The results demonstrate that laser power, the site of
induction and the concentration of rose bengal are important
factors in thrombus formation. The images captured allow the
extraction of substantial and important information, which improves
the description of the results. The key to this technique is the
use of fluorophores that are activated at longer wavelengths,
allowing thrombus detection (17).
This type of technique allows the study of thrombi formation in
real time, yielding excellent results. However, the costs are
extremely high, as expensive microscopes and accessories are
required. Therefore, these techniques are used only in laboratories
that are well-equipped and well-funded (17,23,24).
In the present study, the technique was modified to make it less
expensive and remained highly effective for the analysis of thrombi
formed in real time. Visualization of the clot was performed using
platelets previously labeled with calcein AM and an epifluorescence
microscope was used for thrombus imaging. Therefore, the
modification of this technique allows the study of a wide range of
functions, including the study of thrombosis and antithrombotic
agents in vivo.
In conclusion, thrombosis was successfully induced
using the modified mouse model of in vivo thrombosis
described in the present study. The effect of a given concentration
of rose bengal, a specific laser power and the integration of
labeled platelets allowed the visualization and evaluation of
thrombi production in a mouse model. Therefore, this in vivo
system of thrombosis may be useful in future studies due to its low
cost and high reproducibility. In addition, modification of the
technique is likely to enable its use in various types of in
vivo studies, including studies that demonstrate the
antithrombotic effects of any compound and/or molecule. Therefore,
this modified mouse model may be a critical tool for the study of
CVD.
Acknowledgements
This study was funded by the CONICYT Regional and
the Interdisciplinary Excellence Research Program on Healthy Aging
(PIEI-ES). It was supported by a grant from Fondecyt (no.
1130216).
References
|
1
|
Naghavi M, Libby P, Falk E, Casscells SW,
et al: From vulnerable plaque to vulnerable patient: a call for new
definitions and risk assessment strategies: Part I. Circulation.
108:1664–1672. 2003. View Article : Google Scholar
|
|
2
|
Palomo I, Toro C and Alarcón M: The role
of platelets in the pathophysiology of atherosclerosis (Review).
Mol Med Rep. 1:179–184. 2008.PubMed/NCBI
|
|
3
|
Fuentes QE, Fuentes QF, Andrés V, Pello
OM, et al: Role of platelets as mediators that link inflammation
and thrombosis in atherosclerosis. Platelets. 24:255–262. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruggeri ZM: Mechanisms initiating platelet
thrombus formation. Thromb Haemost. 78:611–616. 1997.PubMed/NCBI
|
|
5
|
Zoungas S, McGrath BP, Branley P, Kerr PG,
et al: Cardiovascular morbidity and mortality in the
Atherosclerosis and Folic Acid Supplementation Trial (ASFAST) in
chronic renal failure: a multicenter, randomized, controlled trial.
J Am Coll Cardiol. 47:1108–1116. 2006. View Article : Google Scholar
|
|
6
|
Fuentes E, Castro R, Astudillo L, Carrasco
G, et al: Bioassay-guided isolation and HPLC determination of
bioactive compound that relate to the antiplatelet activity
(adhesion, secretion, and aggregation) from Solanum
lycopersicum. Evid Based Complement Alternat Med.
2012:1470312012.PubMed/NCBI
|
|
7
|
Aghourian MN, Lemarié CA and Blostein MD:
In vivo monitoring of venous thrombosis in mice. J Thromb Haemost.
10:447–452. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hechler B and Gachet C: Comparison of two
murine models of thrombosis induced by atherosclerotic plaque
injury. Thromb Haemost. 105(Suppl 1): S3–S12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukuoka T, Hattori K, Maruyama H, Hirayama
M and Tanahashi N: Laser-induced thrombus formation in mouse brain
microvasculature: effect of clopidogrel. J Thromb Thrombolysis.
34:193–198. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sigler A, Goroshkov A and Murphy TH:
Hardware and methodology for targeting single brain arterioles for
photothrombotic stroke on an upright microscope. J Neurosci
Methods. 170:35–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosen ED, Raymond S, Zollman A, Noria F,
et al: Laser-induced noninvasive vascular injury models in mice
generate platelet- and coagulation-dependent thrombi. Am J Pathol.
158:1613–1622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eitzman DT, Westrick RJ, Nabel EG and
Ginsburg D: Plasminogen activator inhibitor-1 and vitronectin
promote vascular thrombosis in mice. Blood. 95:577–580.
2000.PubMed/NCBI
|
|
13
|
Arad A, Proulle V, Furie RA, Furie BC and
Furie B: β2-Glycoprotein-1 autoantibodies from patients
with antiphospholipid syndrome are sufficient to potentiate
arterial thrombus formation in a mouse model. Blood. 117:3453–3459.
2011.
|
|
14
|
Salvemini D and Botting R: Modulation of
platelet function by free radicals and free-radical scavengers.
Trends Pharmacol Sci. 14:36–42. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watson BD, Dietrich WD, Busto R, Wachtel
MS and Ginsberg MD: Induction of reproducible brain infarction by
photochemically initiated thrombosis. Ann Neurol. 17:497–504. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inamo J, Belougne E and Doutremepuich C:
Importance of photo activation of rose bengal for platelet
activation in experimental models of photochemically induced
thrombosis. Thromb Res. 83:229–235. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cooley BC: In vivo fluorescence imaging of
large-vessel thrombosis in mice. Arterioscler Thromb Vasc Biol.
31:1351–1356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gross A, Tilly P, Hentsch D, Vonesch JL
and Fabre JE: Vascular wall-produced prostaglandin E2 exacerbates
arterial thrombosis and atherothrombosis through platelet EP3
receptors. J Exp Med. 204:311–20. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosen ED, Raymond S, Zollman A, et al:
Laser-Induced Noninvasive Vascular Injury Models in Mice Generate
Platelet- and Coagulation-Dependent Thrombi. Am J Pathol.
158:1613–1622. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Westrick RJ, Winn ME and Eitzman DT:
Murine models of vascular thrombosis (Eitzman series). Arterioscler
Thromb Vasc Biol. 27:2079–2093. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Massberg S, Brand K, Grüner S, Page S, et
al: A critical role of platelet adhesion in the initiation of
atherosclerotic lesion formation. J Exp Med. 196:887–896. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Przyklenk K and Whittaker P: Adaptation of
a photochemical method to initiate recurrent platelet-mediated
thrombosis in small animals. Lasers Med Sci. 22:42–45. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niesner RA and Hauser AE: Recent advances
in dynamic intravital multi-photon microscopy. Cytometry A.
79:789–798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iba T, Aihara K, Kawasaki S, Yanagawa Y,
et al: Formation of the venous thrombus after venous occlusion in
the experimental mouse model of metabolic syndrome. Thromb Res.
129:e246–e250. 2012. View Article : Google Scholar : PubMed/NCBI
|