Introduction
Liver fibrosis is a common consequence of various
chronic liver diseases and the underlying pathology represents the
common response of the liver to toxicity, infection or metabolism
(1–3). Hepatic fibrosis, characterized by
excess deposition of extracellular matrix proteins, is
traditionally viewed as an irreversible pathological process
involving multiple signaling pathways (4,5).
With protracted damage, fibrosis progresses into excessive scarring
and organ damage, including liver cirrhosis. However, recent
evidence has indicated that liver fibrosis may be dynamic and
bidirectional, involving progression and regression (6), offering an opportunity of therapeutic
intervention to halt or reverse fibrosis. To date, antifibrotic
treatment represents an unconquered area for drug development, with
enormous potential but also high risks (7).
During liver fibrosis, hepatic stellate cells (HSCs)
are primarily activated by transforming growth factor-β, in
addition to other profibrotic cytokines. Upon activation, HSCs
proliferate and differentiate into myofibroblasts which secrete
several extracellular matrix constituents, including collagens
(8,9). Activated HSCs are the key cells
involved in the progression of liver fibrosis (10). Nuclear factor (NF)-κB is a
heterodimeric transcription factor that plays a central role in the
pathogenesis of a wide variety of conditions affecting the liver,
including hepatitis and fibrosis (11). Although the role of NF-κB signaling
in the liver has been extensively explored, further studies of
NF-κB signaling in liver fibrosis are required to promote
translational application in liver disease. Thus, in the present
study, the effect of an NF-κB inhibitor, BAY-11–7082 (BAY), was
investigated in carbon tetrachloride (CCl4)-induced
mouse model of liver fibrosis.
Materials and methods
Cell culture
HSC-T6 cells, an immortalized rat HSC cell line
transfected with the SV40 large T-antigen containing a Rous sarcoma
virus promoter, were purchased from the Cancer Institute and
Hospital (Chinese Academy of Medical Sciences, Beijing, China). The
Chang liver cell line (American Type Culture Collection, Manassas,
VA, USA) was used as a normal human cell line derived from normal
liver tissue (12). The cells were
cultured in Dulbecco’s modified Eagle’s medium supplemented with
10% fetal bovine serum, penicillin G and streptomycin at 37°C. Cell
passage of the cultures was performed every 3 days and the cells
were plated in culture dishes at a density of 1×106
cells. Next, the cells were treated with various concentrations
(6.25, 12.5, 25 and 50 μM) of BAY 1 h prior to stimulation with 1
μg/ml lipopolysaccharide (LPS) for 24 h. The study was approved by
the ethics committee of Xinxiang Central Hospital (Xinxiang,
China).
MTT assay
Cell viability was evaluated using an MTT assay.
HSC-T6 and Chang liver cells were independently seeded in 96-well
plates (1×104 cells per well). HSC-T6 cells were treated
with BAY (CAS19542-67-7; Cayman Chemical Co., Ann Arbor, MI, USA)
and 1 μg/ml LPS, while normal Chang liver cells were treated with
BAY. Following treatment with BAY and/or LPS for 24 h, 5 mg/ml MTT
solution was added and the cells were incubated for an additional 3
h. The results were obtained as absorbance measurements at 490 nm
using an ELISA microplate reader (3550, Bio-Rad, Hercules, CA,
USA).
Animals
C57BL/6 male mice were maintained in conditions
according to the guidelines of the National Institutes of Health
Guide for the Care and Use of Laboratory Animals (Institute of
Laboratory Animal Resources, 1996). Mice were purchased and housed
in a barrier facility. At the end of each experiment, the animals
were sacrificed with CO2 following anaesthesia. The
animals were also weighed and blood samples were collected. Whole
livers were harvested and weighed. Liver samples were harvested
from the two liver lobes to reduce sampling variability among the
experimental and control mice.
CCl4-induced mouse fibrosis
model
The fibrosis model was generated using
CCl4 (Sigma-Aldrich, St. Louis, MO, USA) dissolved at a
concentration of 20% in olive oil. Intraperitoneal injections of 1
ml pure CCl4/kg body weight (dissolved at a
concentration of 20% in olive oil) were administered twice a week
for 6 weeks (13).
Treatment protocols
The dosage of BAY was determined according to
previous studies (14,15). BAY was dissolved in 10% dimethyl
sulfoxide (DMSO)/phosphate-buffered saline (PBS). The treatment
group received intraperitoneal injections of 5 mg/kg BAY three
times a week as previously described, whereas the control groups
received the vehicle only. Mice were randomly divided into three
groups (n=12). Group 1 received 10% DMSO/PBS treatment, while group
2 received CCl4 only. Group 3 mice received 10 mg/kg BAY
and CCl4. At the end of the first week, the
CCl4-injected mice were intraperitoneally administered
10 mg/kg BAY three times a week for 6 weeks.
Animals were sacrificed 24 h following the last
injection and blood samples were collected. Serum was then
separated by centrifugation at 800 × g for 10 min at 4°C. The liver
of each mouse was removed immediately and stored at −80°C for
subsequent analysis.
Measurement of serum alanine
aminotransferase (ALT)
Mouse sera were collected and enzyme ALT levels were
measured using the serum biochemical analyzers Ektachem DTSC-II
analyzer (Eastman Kodak, Rochester, NY, USA) and Hitachi
autoanalyzer (Tokyo, Japan), according to the manufacturer’s
instructions.
Histopathological analysis
Mice were sacrificed at the end of week 6, 24 h
after the last injection). Liver samples from the left lateral and
median lobes were separated and fixed in 10% neutral buffered
formalin. The samples were then embedded in paraffin, sectioned (5
μm) and stained with Sirus red (Vector Laboratories, Inc.,
Burlingame, CA, USA) for general observations. A certified
histopathologist was blinded to the group distribution throughout
the analysis.
Western blotting
Equal amounts of protein were resolved by 12.5%
SDS-PAGE and immobilized on polyvinylidene fluoride membranes by
wet transfer. Following blocking for 30 min with 5% non-fat dry
milk in Tris-buffered saline-Tween 20, the membranes were exposed
overnight at 4°C to primary antibodies. This was followed by
incubation for 2 h at room temperature with the corresponding
horseradish peroxidase (HRP)-conjugated secondary antibodies
(Vector Laboratories, Inc.). Equal protein loading was corrected by
the immunoblotting of β-actin. Immunoreactive proteins were
visualized using a chemiluminescent HRP antibody detection reagent
(Denville Scientific, Inc., South Plainfield, NJ, USA) and exposure
to X-ray film (Eastman Kodak). Band density was analyzed using
ImageJ software. The primary antibodies anti-p-phosphatidylinositol
3-kinase (PI3K)/PI3K, anti-p-Akt/Akt, anti-collagen I, anti-α
smooth muscle actin (SMA; 1:1,000) and anti-β-actin antibody
(1:2,500), were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA).
Hydroxyproline Measurement
Liver tissue was homogenized in ice-cold distilled
water (900 μl) using a Power Gen homogenizer (Fisher).
Subsequently, 125 μl of 50% (wt/vol) trichloroacetic acid was
added, and the homogenates were incubated further on ice for 20
min. Precipitated pellets were hydrolyzed for 18 h at 110°C in 6 N
HCL. After hydrolysis, the samples were filtered and neutralized
with 10 N NaOH, and the hydrolysates were oxidized with
Chloramine-T (Sigma) for 25 min at room temperature. The reaction
mixture then was incubated in Ehrlich’s perchloric acid solution at
65°C for 20 min and cooled to room temperature. Sample absorbance
was measured at 560 nm in duplicate. Purified hydroxyproline
(Sigma) was used to set a standard. Hydroxyproline content was
expressed as microgram of hydroxyproline per g liver.
Statistical analysis
Data are expressed as mean ± SD. Animal experiments
were performed with 12 animals in each treatment and control group.
All in vitro data is reported as the result of three
independent experiments, including three replicates per experiment.
Statistical analysis was performed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA) and statistical differences between the
groups were analyzed using the Student’s T test or one-way analysis
of variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
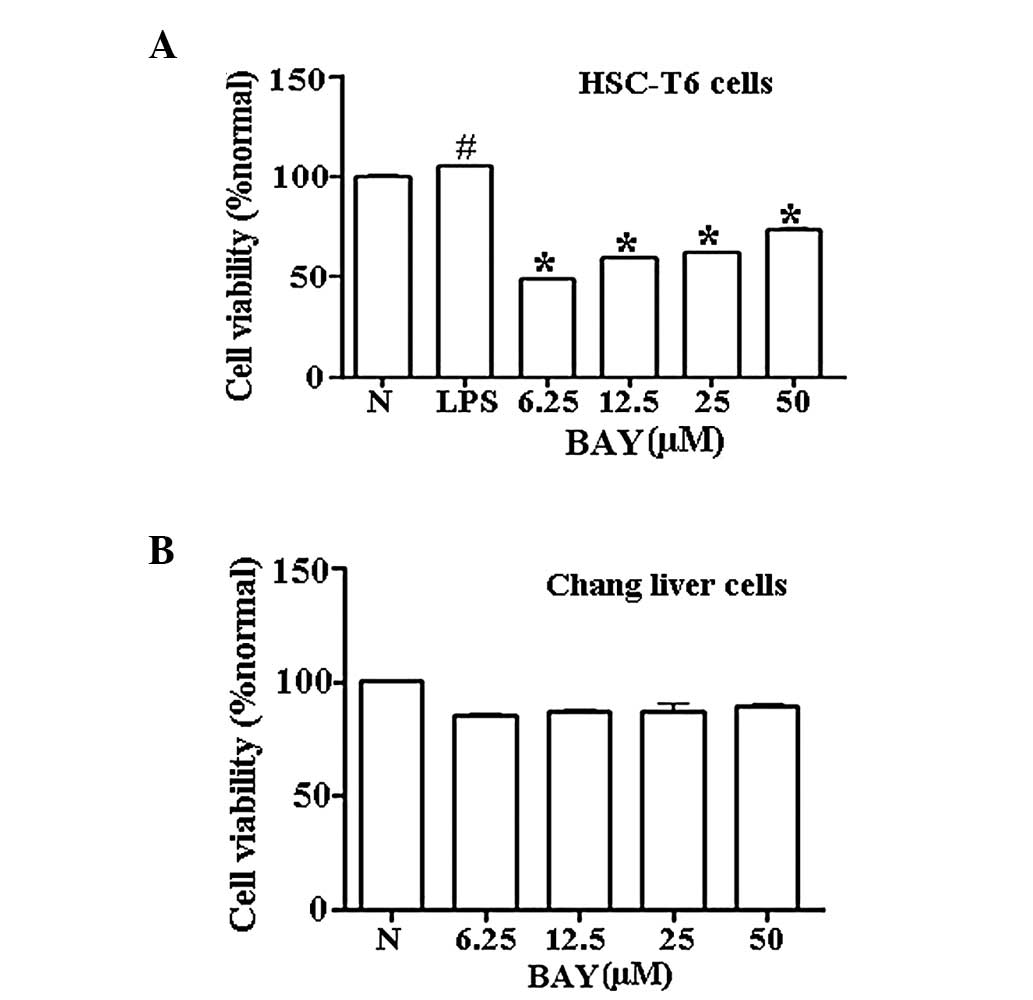
Effect of BAY on cell viability
Various concentrations of BAY (6.25–50 μM)
significantly reduced the cell viability of HSC-T6 cells in a
dose-dependent manner within 24 h following LPS stimulation
(Fig. 1A). To determine whether
BAY was cytotoxic to normal hepatocytes, normal human Chang liver
cells were selected as a normal control to test the cell viability
in the presence of various concentrations of BAY. At concentrations
between 6.25 and 50 μM, BAY exhibited insignificant toxicity in
normal Chang liver cells (Fig.
1B).
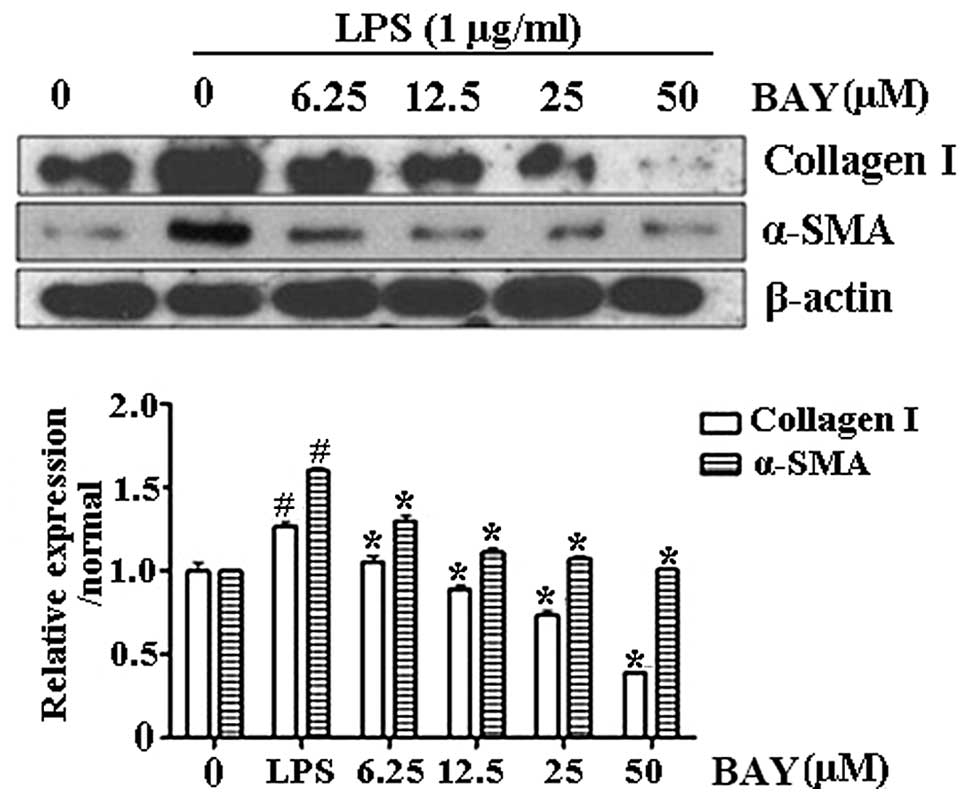
Effect of BAY on the protein expression
of collagen I and α-SMA
Activation of HSCs plays a central role in liver
fibrosis and α-SMA is an established indicator of HSC activation
(3). Collagen I is the principal
collagen responsible for fibrosis and is generated by activated
HSCs. The levels of α-SMA and collagen I were upregulated in
LPS-activated HSC-T6 cells, indicating that HSCs were activated
upon LPS administration. By contrast, BAY decreased the protein
levels of α-SMA and collagen I in the LPS-treated cells (Fig. 2). These results demonstrated that
BAY reduced HSC activation.
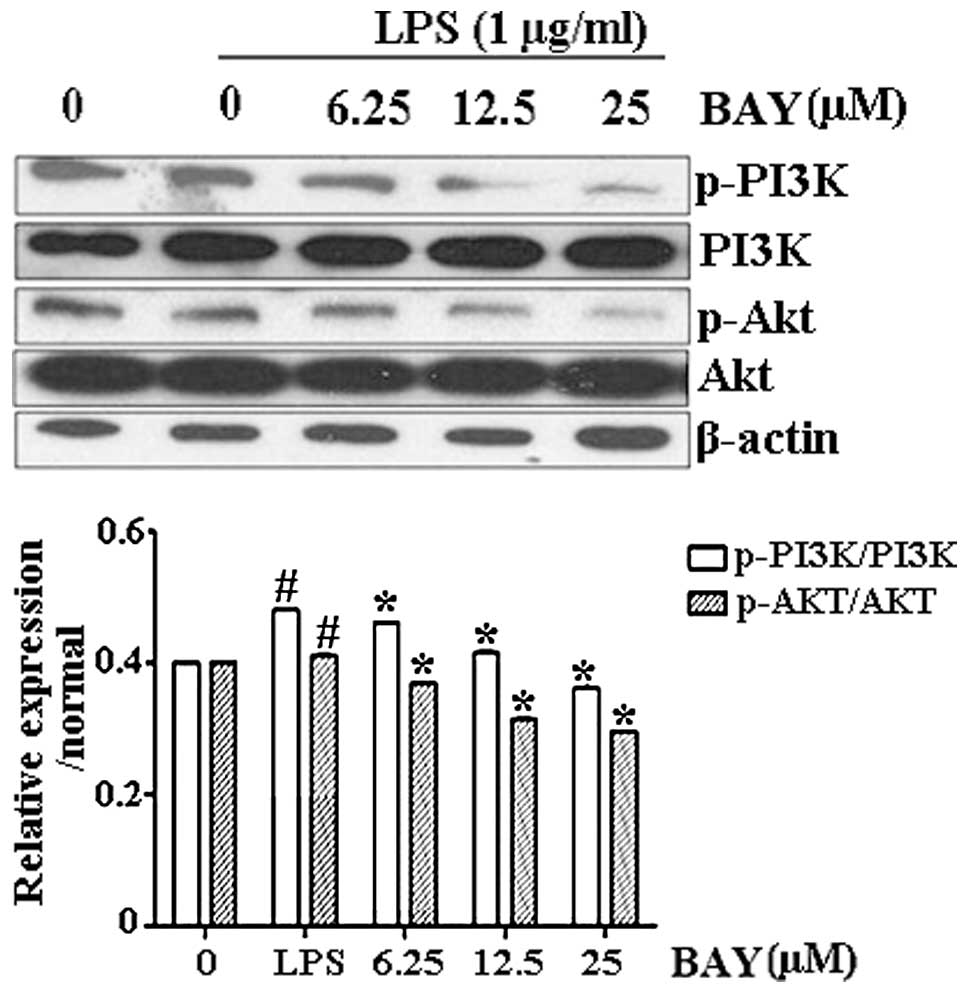
Effect of BAY on LPS-induced
phosphorylation of PI3K/Akt
To investigate the antifibrotic mechanism of BAY and
the possible association with the PI3K/Akt signaling pathway,
PI3K/Akt expression was observed in activated HSC-T6 cells. PI3K
and Akt phosphorylation was upregulated following LPS stimulation;
however, the phosphorylation levels of PI3K/Akt were significantly
reduced by BAY treatment in a dose-dependent manner (Fig. 3).
Effect of BAY on CCl4-induced
hepatic fibrosis
Mouse hepatic fibrosis was determined using Sirius
red staining. As expected, marked bridging fibrosis was observed in
the mice treated with vehicle (Fig.
4B). BAY significantly attenuated the CCl4-induced
liver fibrosis (Fig. 4D). Further
analysis demonstrated that the area of hepatic fibrosis was
significantly reduced in BAY and CCl4-treated mice
compared with that in the mice treated with CCl4 alone
(Fig. 4E). The effect of BAY on
hepatic hydroxyproline, which is indicative of hepatic fibrosis,
was then studied. CCl4 administration significantly
increased the hepatic hydroxyproline content in the mice, while BAY
administration significantly reduced this CCl4-induced
increase in hepatic hydroxyproline content (Fig. 4F).
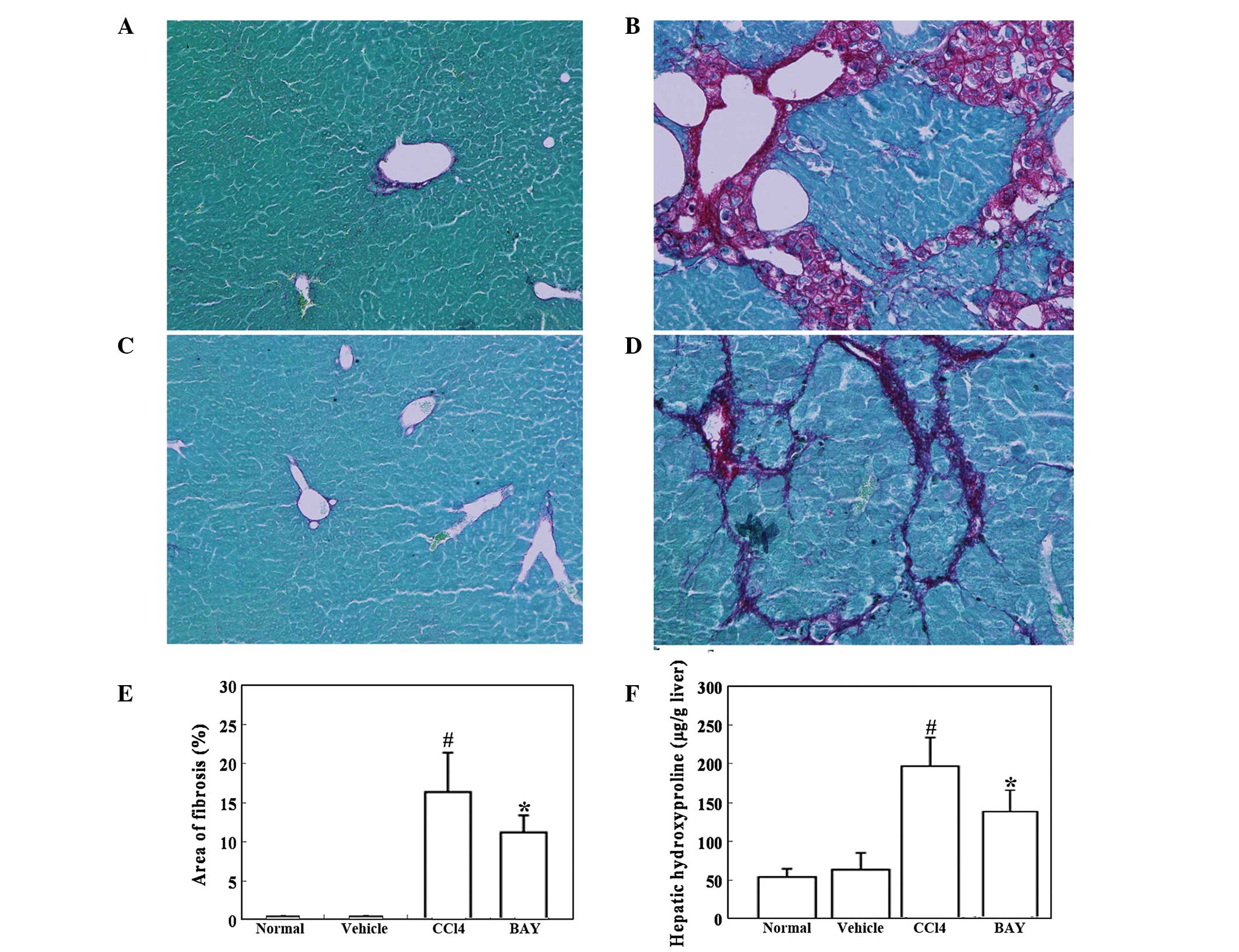 | Figure 4Effect of BAY on
CCl4-induced liver fibrosis. Mice were intraperitoneally
injected with 1 ml/kg CCl4 twice a week in combination
with 5 mg/kg BAY three times a week for 6 weeks. (A–D) Liver
fibrosis was detected by Sirius red staining. Representative
micrographs of histology from (A) normal, (B) vehicle (10%
DMSO/PBS), (C) CCl4 and (D) CCl4 + BAY
treated mice (magnification, ×100). (E) Morphometrical analysis for
evaluating the percentages of α-SMA-positive areas in 12 random
fields. (F) Hepatic hydroxyproline was detected. All data are
expressed as the mean ± SD of 12 mice. #P<0.01, vs.
control; *P<0.01, vs. CCl4. BAY,
BAY-11–7082; SMA, smooth muscle actin; CCl4, carbon
tetrachloride; DMSO, dimethyl sulfoxide; PBS, phosphate-buffered
saline. |
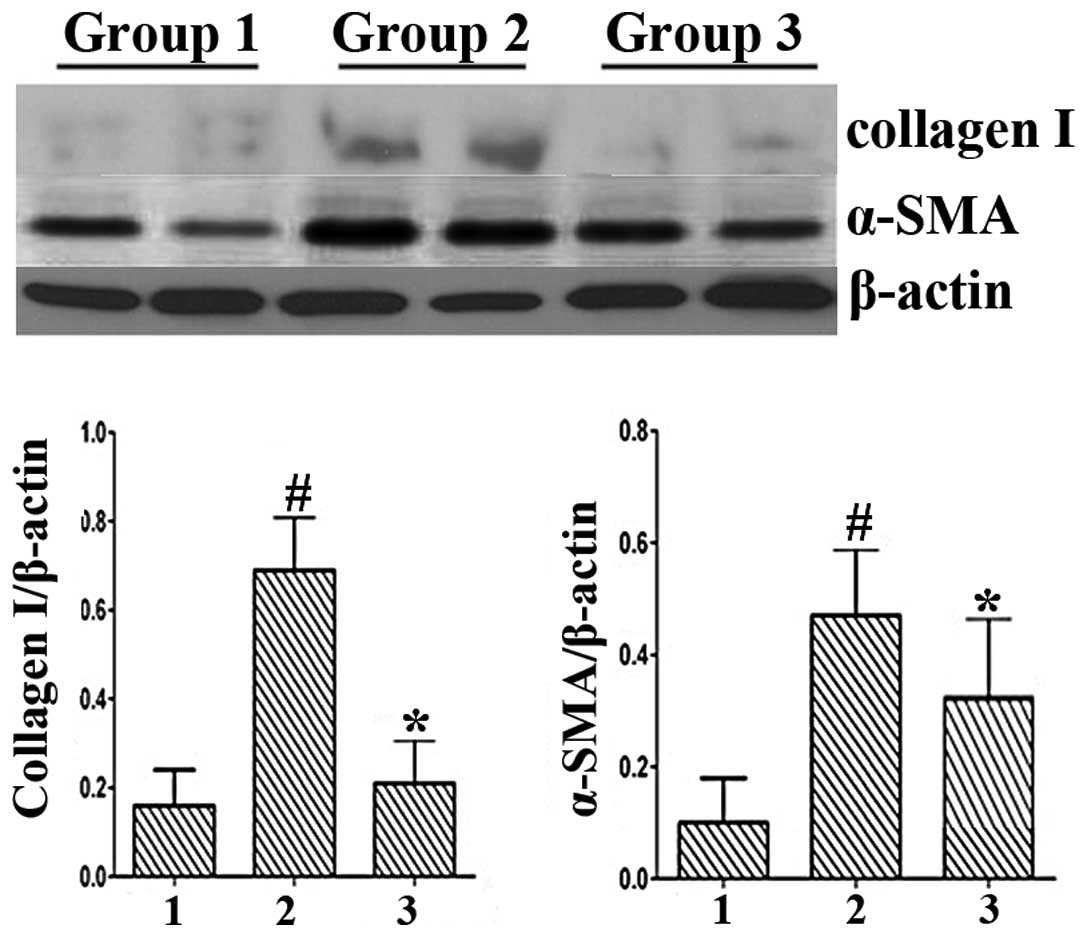
Effect of BAY on serum ALT levels and
expression of collagen I and α-SMA in CCl4-induced mouse
liver injury
Serum ALT levels were determined as an indicator of
liver function and the ability of BAY to reduce serum ALT levels in
CCl4-induced liver injury was investigated. ALT levels
were significantly elevated in the CCl4 group (Group 1
vs. Group 2, 38.96±5.88 vs. 448.45±78.40 U/l; P<0.001). However,
BAY treatment significantly attenuated the CCl4-induced
increase in ALT levels (Group 2 vs. Group 3, 448.45±78.40 vs.
361.37±82.51 U/l; P<0.001). In addition, CCl4-induced
liver injury revealed high expression levels of collagen I and
α-SMA by western blotting (Fig. 5)
and BAY was shown to decrease the protein expression levels of
collagen I and α-SMA in the liver injury model. These in
vivo results were consistent with the in vitro
results.
Discussion
Chronic inflammation and the associated regenerative
wound-healing response are strongly associated with the development
of fibrosis and cirrhosis (16).
In the past decade, numerous inflammatory mediators have been shown
to contribute to the progression of chronic liver disease, a number
of which are targets or activators of NF-κB (17–20).
Studies targeting this molecule as an appropriate therapeutic agent
in various diseases are ongoing. However, it is necessary to
demonstrate whether NF-κB antagonism effectively treats
pre-existing hepatic fibrosis and the potential mechanism of
action. In the present study, the NF-κB inhibitor, BAY, effectively
suppressed HSC-T6 activation by downregulating the expression of
collagen I and α-SMA. BAY also inhibited PI3K and Akt
phosphorylation in activated HSC-T6 cells.
Kupffer cells contribute to HSC activation and liver
fibrosis (21). Inhibition of
NF-κB in Kupffer cells results in decreased liver fibrosis;
however, the underlying mechanisms remain largely elusive (22). While the role of NF-κB activation
in hepatocytes and Kupffer cells leading to liver fibrosis is not
completely understood, there is growing evidence that NF-κB
functions as a key mediator of fibrosis. A wide range of
proinflammatory mediators activate NF-κB in HSCs, including LPS,
tumor necrosis factor and interleukin-1β (23–26).
In addition, HSCs activate NF-κB during culture activation
(27) and in human and mouse
models of liver fibrosis, as demonstrated by the presence of Ser
536-phosphorylated p65 (25).
Notably, NF-κB activation is almost exclusively observed in HSCs,
indicating that these cells are an important site of inflammation
in a chronically injured and fibrotic liver (28). Notably, the results of the present
study demonstrate that the administration of BAY attenuates liver
fibrosis induced in mice by the administration of CCl4.
BAY also significantly decreased the levels of serum ALT in the
model mice.
PI3K is a key signaling molecule that controls
numerous cellular functions (29).
In the liver, PI3K activation promotes cytokine production and
subsequent hepatocyte proliferation following partial hepatectomy
(30). Hepatocyte-associated PI3K
regulates hepatocyte growth by a process involving Akt activation
(30). In the present study,
fibrogenesis, which may be promoted by PI3K, was inhibited in
association with reduced α-SMA expression and collagen production.
PI3K/Akt signaling activation was also inhibited by BAY in
LPS-treated HSCs and the fibrotic mouse liver. Inhibition of
PI3K/Akt signaling may be strongly associated with the
antifibrogenic effect.
In summary, the present study demonstrates that
NF-κB signaling is activated in the pathogenesis of
CCl4-induced hepatic fibrosis. BAY, an NF-κB inhibitor,
inhibits CCl4-induced hepatic PI3K/Akt signaling
activation. In addition, BAY attenuates CCl4-induced HSC
activation and effectively alleviates CCl4-induced
hepatic fibrosis in mice. Thus, NF-κB inhibition may have potential
therapeutic value against hepatic fibrosis.
References
|
1
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiao J, Friedman SL and Aloman C: Hepatic
fibrosis. Curr Opin Gastroenterol. 25:223–229. 2009. View Article : Google Scholar
|
|
3
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View
Article : Google Scholar
|
|
4
|
Friedman SL: Reversibility of hepatic
fibrosis and cirrhosis - is it all hype? Nat Clin Pract
Gastroenterol Hepatol. 4:236–237. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tangkijvanich P and Yee HF Jr: Cirrhosis -
can we reverse hepatic fibrosis? Eur J Surg Suppl. 587:100–112.
2002.PubMed/NCBI
|
|
6
|
Povero D, Busletta C, Novo E, et al: Liver
fibrosis: a dynamic and potentially reversible process. Histol
Histopathol. 25:1075–1091. 2010.PubMed/NCBI
|
|
7
|
Schuppan D and Kim YO: Evolving therapies
for liver fibrosis. J Clin Invest. 123:1887–1901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gressner AM and Weiskirchen R: Modern
pathogenetic concepts of liver fibrosis suggest stellate cells and
TGF-beta as major players and therapeutic targets. J Cell Mol Med.
10:76–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gressner AM, Weiskirchen R, Breitkopf K
and Dooley S: Roles of TGF-beta in hepatic fibrosis. Front Biosci.
7:d793–d807. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gressner OA and Gressner AM: Connective
tissue growth factor: a fibrogenic master switch in fibrotic liver
diseases. Liver Int. 28:1065–1079. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robinson SM and Mann DA: Role of nuclear
factor kappaB in liver health and disease. Clin Sci (Lond).
118:691–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Q, Wang XY, Zhou J and Fan J: Cell
line misidentification: the case of the Chang liver cell line.
Hepatology. 54:1894–1895. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao ZM, Cai M, Lv YF, Huang YH and Li HH:
Oral administration of recombinant adeno-associated virus-mediated
bone morphogenetic protein-7 suppresses CCl(4)-induced hepatic
fibrosis in mice. Mol Ther. 20:2043–2051. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kar S, Ukil A and Das PK: Cystatin cures
visceral leishmaniasis by NF-κB-mediated proinflammatory response
through co-ordination of TLR/MyD88 signaling with p105-TpI2-ERK
pathway. Eur J Immunol. 41:116–127. 2011.PubMed/NCBI
|
|
15
|
Zhao J, Zhang H, Huang Y, et al:
Bay11–7082 attenuates murine lupus nephritis via inhibiting NLRP3
inflammasome and NF-κB activation. Int Immunopharmacol. 17:116–122.
2013.
|
|
16
|
Luedde T and Schwabe RF: NF-κB in the
liver - linking injury, fibrosis and hepatocellular carcinoma. Nat
Rev Gastroenterol Hepatol. 8:108–118. 2011.
|
|
17
|
Bonacchi A, Petrai I, Defranco RM, et al:
The chemokine CCL21 modulates lymphocyte recruitment and fibrosis
in chronic hepatitis C. Gastroenterology. 125:1060–1076.
2003.PubMed/NCBI
|
|
18
|
Seki E, De Minicis S, Osterreicher CH, et
al: TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med.
13:1324–1332. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seki E, De Minicis S, Gwak GY, et al: CCR1
and CCR5 promote hepatic fibrosis in mice. J Clin Invest.
119:1858–1870. 2009.PubMed/NCBI
|
|
20
|
Miura K, Kodama Y, Inokuchi S, et al:
Toll-like receptor 9 promotes steatohepatitis by induction of
interleukin-1beta in mice. Gastroenterology. 139:323–334. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duffield JS, Forbes SJ, Constandinou CM,
et al: Selective depletion of macrophages reveals distinct,
opposing roles during liver injury and repair. J Clin Invest.
115:56–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Son G, Iimuro Y, Seki E, Hirano T, Kaneda
Y and Fujimoto J: Selective inactivation of NF-kappaB in the liver
using NF-kappaB decoy suppresses CCl4-induced liver
injury and fibrosis. Am J Physiol Gastrointest Liver Physiol.
293:G631–G639. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paik YH, Schwabe RF, Bataller R, Russo MP,
Jobin C and Brenner DA: Toll-like receptor 4 mediates inflammatory
signaling by bacterial lipopolysaccharide in human hepatic stellate
cells. Hepatology. 37:1043–1055. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hellerbrand C, Jobin C, Iimuro Y, Licato
L, Sartor RB and Brenner DA: Inhibition of NFkappaB in activated
rat hepatic stellate cells by proteasome inhibitors and an IkappaB
super-repressor. Hepatology. 27:1285–1295. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oakley F, Teoh V, Ching-A-Sue G, et al:
Angiotensin II activates I kappaB kinase phosphorylation of RelA at
Ser 536 to promote myofibroblast survival and liver fibrosis.
Gastroenterology. 136:2334–2344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schwabe RF, Schnabl B, Kweon YO and
Brenner DA: CD40 activates NF-kappa B and c-Jun N-terminal kinase
and enhances chemokine secretion on activated human hepatic
stellate cells. J Immunol. 166:6812–6819. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Elsharkawy AM, Wright MC, Hay RT, et al:
Persistent activation of nuclear factor-kappaB in cultured rat
hepatic stellate cells involves the induction of potentially novel
Rel-like factors and prolonged changes in the expression of IkappaB
family proteins. Hepatology. 30:761–769. 1999. View Article : Google Scholar
|
|
28
|
Kluwe J, Pradere JP, Gwak GY, et al:
Modulation of hepatic fibrosis by c-Jun-N-terminal kinase
inhibition. Gastroenterology. 138:347–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cushing TD, Metz DP, Whittington DA and
McGee LR: PI3Kδ and PI3Kγ as targets for autoimmune and
inflammatory diseases. J Med Chem. 55:8559–8581. 2012.
|
|
30
|
Jackson LN, Larson SD, Silva SR, et al:
PI3K/Akt activation is critical for early hepatic regeneration
after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol.
294:G1401–G1410. 2008. View Article : Google Scholar : PubMed/NCBI
|