Introduction
One of the most common complications following
radiotherapy for the treatment of breast tumors is
radiation-induced lung injury. Two distinct clinical stages are
recognized in radiation-induced lung disease: An early, transient
stage characterized by radiation pneumonitis and a later stage
characterized by chronic radiation fibrosis, impacting on local
tumor control rate, prognosis and quality of life following
radiotherapy (1).
The mechanism of radiation-induced lung injury has
not yet been fully elucidated. It has been suggested that the most
important pathological basis of radiation-induced lung injury is a
metabolic imbalance in the extracellular matrix (ECM) (2). Matrix metalloproteinases (MMPs) and
tissue inhibitors of metalloproteinases (TIMPs) are important in
ECM degradation and remodeling (3,4).
Numerous studies have found that MMPs have a key role in acute
respiratory distress syndrome, chronic obstructive pulmonary
disease, idiopathic pulmonary fibrosis (IPF) and lung disease
caused by fibrosis (5–8). Yang et al (9) revealed that basement membrane rupture
in radiation-induced lung injury is closely associated with high
expression of MMP-2 and MMP-9. Another member of the MMP family,
MMP-12, is produced primarily by macrophages and is capable of
degrading a broad spectrum of substrates. MMP-12 is associated with
a variety of diseases, including atherosclerosis and lung cancer
(10,11). Animal studies have shown that
MMP-12 is involved in the induction of the inflammatory response,
degradation of the ECM, airway remodeling and the regulation of
other metalloproteinases (MMP-2 and MMP-9) and cytokines (12). Matute-Bello et al (13) demonstrated that increased
expression of MMP-12 causes a progressive pulmonary fibrosis
associated with increased fibrosis gene activation during the early
stages of lung injury in mice, whilst MMP-12 gene-knockout mice do
not develop pulmonary fibrosis. Another study found that MMP-12
protein expression was significantly increased in the lung tissue
of rats following right chest irradiation (14). TIMPs, which act as major regulators
of MMPs, are capable of inhibiting matrix degradation and
maintaining homeostasis in the ECM. Animal studies have reported
that TIMP-1 and TIMP-2 are highly expressed in hepatic fibrosis
(15) and hyperoxia-induced acute
lung injury (16). Recently, a
recovery of the imbalance in MMP/TIMP levels was observed in a rat
model of lung fibrosis following treatment with Cordyceps in
preventive and therapeutic regimens (17). Thus, MMP-12 and TIMP-1 may have an
important role in the incidence of radiation-induced lung
injury.
Traditional Chinese Medicine, or herbal medicine, is
an important approach in the treatment of lung injury. An effective
treatment series, known in Chinese as Yangyinqingfei, has been
developed by physicians to decrease inflammatory mediators of the
lung. Yangyinqingfei decoction is believed to expel wind, eliminate
dampness and promote blood circulation to ameliorate pain,
invigorate the spleen and regulate qi (18). Yangyinqingfei decoction has
traditionally been used for the treatment of diphtheria (19). There are few reports on this herbal
remedy, particularly with regard to the mechanism underlying the
treatment of radiation-induced lung injury. In this study, the
therapeutic effects of Yangyinqingfei decoction were evaluated in a
rat model of radiation-induced lung injury, and the potential
mechanism underlying the effect was investigated.
Materials and methods
Yangyinqingfei decoction
Yangyinqingfei decoction was purchased from the
Traditional Chinese Medicines Pharmacy of the People’s Liberation
Army General Hospital (Beijing, China). The Yangyinqingfei
decoction prescription consisted of the following five Chinese
herbs: Sheng Di Huang (15 g), Xuan Shen (15g), Verbena (15 g),
Forsythia (10 g), and Gan Cao (6 g). The quantity of each herb in
Yangyinqingfei decoction was determined using information from the
Pharmacopoeia Commission of the People’s Republic of China
(20). Briefly, each herb was
decocted by simmering in water for 30 min, prior to being filtered
through filter paper and then concentrated into decoctions of 0.2,
0.6 and 1.8 g/ml. The extracts were stored at 4°C until use.
Animal conditions and treatments
Seventy-five male Wistar rats, aged 6–8 weeks and
with body weights of 200±20 g, were obtained from the Experimental
Animal Center of the Academy of Military Medical Sciences (Beijing,
China). All animal experiments were approved by the Veterinary
Institute of the Academy of Military Medical Sciences Animal Ethics
Committee. The rats were housed at 23±2°C and 55±5% humidity with a
standard 12-h light/dark cycle. The rats had free access to water
and were fed a normal diet. After three days of adaptation, the
rats were randomly divided into five groups (n=15/group): Control
rats with sham irradiation and without drug administration (group
A); irradiated rats without drug administration (group B/model
group); irradiated rats with low-dose drug administration (group
C); irradiated rats with intermediate-dose drug administration
(group D) and irradiated rats with high-dose drug administration
(group E). All the rats were irradiated with a single dose of 25 Gy
to their right hemi-thoraxes by a 60Co γ-ray, with the
exception of rats in group A, which underwent sham irradiation. One
week prior to irradiation, the rats in group A and B were
administered saline, while rats in groups C, D and E were
simultaneously administered Yangyinqingfei decoction at a dosage of
2, 6 and 18 g/kg body weight/day, respectively. All drugs were
administered by intragastric administration once a day until the
rats were sacrificed.
Histopathological examination
At one, two and four weeks post-irradiation, five
animals in each group were sacrificed and the lung tissues were
harvested. The right lung tissues were paraffin-embedded and
sectioned at a thickness of 5 μm prior to being stained with
hematoxylin and eosin (H&E) and observed under a light
microscope [Olympus (BH-2), Nagano, Japan].
Western blot analysis
To assess protein levels of MMP-12 and TIMP-1 in
lung tissues, western blot analysis was performed. Lung tissues
were adequately homogenized with non-denaturing lysis buffer and
centrifuged at 12,000 × g for 15 min at 4°C. The protein
concentration of the supernatant was determined, prior to
denaturation of the protein using a protein loading buffer. A total
of 30 μg protein was then loaded onto a 12% SDS polyacrylamide gel,
blotted onto a polyvinylidene difluoride membrane and blocked for 1
h with 5% skimmed milk in Tris-buffered saline with 0.05% Tween 20.
Membranes were incubated with primary antibodies against MMP-12,
TIMP-1 and β-actin (1:1,000; Takara Bio, Inc., Shiga, Japan)
overnight at 4°C followed by a 2-h incubation with a horseradish
peroxidase-conjugated secondary antibody (1:3,000; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The membranes were
washed with TBS-T buffer three times and then visualized using
enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ, USA)
detection.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from lung tissues with
TRIzol reagent (Invitrogen Life Technologies, Karlsruhe, Germany).
Total RNA (1 μg) was reverse-transcribed to cDNA, which was then
used to determine the levels of MMP-12 and TIMP-1 mRNA using PCR
with Taq DNA polymerase (Fermentas, Pittsburgh, PA, USA). PCR was
performed under the following conditions: Initial denaturing at
94°C for 30 sec, annealing at 58°C for 30 sec and extension at 72°C
for 1 min, followed by 30 cycles of amplification for MMP-12 and
TIMP-1 and 35 cycles for β-actin. The primer sequences for MMP-12,
TIMP-1 and β-actin were designed with Primer Premier 5.0 software
(Premier Biosoft, Palo Alto, CA, USA) and were as follows: MMP-12
forward 5′-AGGTCAAGATGGATGAAGCGG-3′, reverse
5′-GAAGTAATGTTGGTGGCTGGACTC-3′; TIMP-1 forward
5′-ACAGCTTTCTGCAACTCG-3′, reverse 5′-CTATAGGTCTTTACGAAGGCC-3′;
β-actin forward 5′-TGGCCTCACTGTCCACCTTC-3′, reverse
5′-CGAATGGCTGACCATTCAGA -3′. All processes were performed according
to the manufacturer’s instructions. Samples were then analyzed
using gel electrophoresis (2% agarose), DNA bands were examined and
the level of DNA was measured semiquantitatively using a Gel
Documentation System (Bio-Rad Model Gel Doc 2000; Bio-Rad,
Hercules, CA, USA).
Statistical analysis
Data are presented as mean ± standard deviation.
Statistical evaluation of the results was performed using analysis
of variance with a Tukey post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of Yangyinqingfei decoction on
clinical signs in rats with radiation-induced lung injury
Throughout the experiment, rats in the control group
(group A) were active, exhibited good spirits, had normal food
intake and showed no obvious abnormalities in the stools. However,
rats in group B presented with anepithymia, inactivity, ruffled
fur, redness of the nose and dry stools, as well as skin erosion
and ulcers. In comparison with group B, rats in groups C-E
exhibited alleviated symptoms, and had no skin erosion or ulcers.
Among groups C-E, rats in group D exhibited the mildest symptoms,
and rats in group E had diarrhea.
The increase of body weight of rats in groups B-E
was markedly slower than that of rats in group A. One week after
irradiation, the body weights of rats in group D were significantly
increased compared with the model group (group B), while no
significant differences were observed among the body weights of
rats in groups B, C and E. Two weeks after radiation, the body
weights of rats in group C and D were significantly higher than
those of rats in group B. However, no significant difference was
identified between the body weights of rats in group E and those of
rats in group B (Table I). These
results suggest that Yangyinqingfei decoction may prevent weight
loss in irradiated rats.
 | Table IBody weight of rats following
irradiation with or without Yangyinqingfei decoction
administration. |
Table I
Body weight of rats following
irradiation with or without Yangyinqingfei decoction
administration.
| | Body weight (g) |
|---|
| |
|
|---|
| Time | n | A | B | C | D | E |
|---|
| 1 week | 5 | 312.8±1.8 | 261.2±2.7a | 273.1±9.3a | 284.6±9.6a,c | 253.1±16.8a |
| 2 weeks | 5 | 347.9±6.6 | 287.5±5.8a | 302.7±6.4a,b | 311.3±4.8a,b | 292.8±12.3a |
| 4 weeks | 5 | 377.2±7.4 | 315.0±8.5a | 333.5±12.3a,c | 344.9±12.3a,b | 324.0±14.3a |
Histopathological effects of
Yangyinqingfei decoction on rats with radiation-induced lung
injury
Gross observations revealed that the right lungs of
group A rats were smooth, pink, shiny and elastic throughout the
duration of the study. In group B, the surfaces of the right lungs
exhibited congestion without other obvious changes in morphology at
one week post-irradiation, while at two weeks post-irradiation more
serious swelling was apparent in the right lungs than in the left
lungs. Four weeks after irradiation, the surfaces of the right
lungs of group B rats exhibited petechiae, visible white
nodule-like structures and pleural effusion. However, fewer lesions
of the right lungs were observed in groups C-E compared with group
B (data not shown).
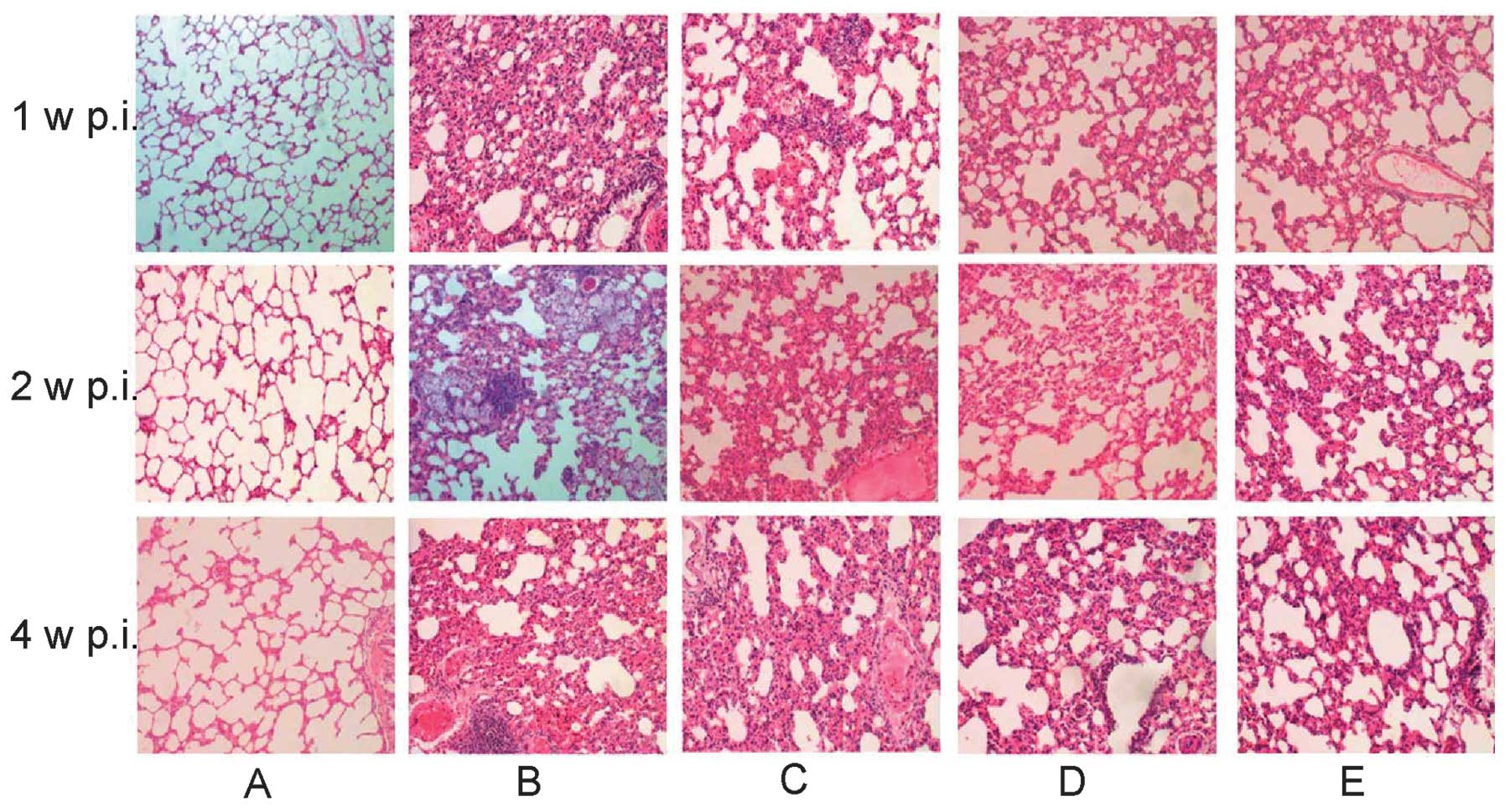
Photomicrographs of lung specimens stained with
H&E are shown in Fig. 1.
Photomicrographs of Group A lungs show normal alveolar structure,
comprising of a thin alveolar wall without exudate or effusion. In
group B rats, at one week post-irradiation the right lung showed
mild edema, capillary congestion, a small amount of exudate and
inflammatory infiltration, while at two weeks post-irradiation,
part of the alveolar structure had disappeared. In addition,
significantly thicker alveolar septa and more notable inflammatory
invasion were observed. Four weeks after irradiation, severe
perivascular inflammatory infiltration, interstitial congestion,
macrophage and lymphocyte accumulation and alveolar wall thickening
were observed. Part of the alveolar space had also collapsed or
disappeared, while part of the alveolar space had expanded
compensatorily. The formation of emphysema, visible fiber cell
hyperplasia and focal fibrosis were also observed in group B.
Comparatively, the pathological changes in the lungs of group C-E
rats were milder, among which the mildest changes were observed in
group E. These findings indicate that Yangyinqingfei decoction may
alleviate lung injury induced by radiation.
Effects of Yangyinqingfei decoction on
expression of MMP-12 and TIMP-1
To investigate the effects of Yangyinqingfei
decoction on mRNA and protein expression of MMP-12 and TIMP-1 in
rats with radiation-induced lung injury, RT-PCR and western blot
analysis were performed, respectively. As shown in Fig. 2A, the level of MMP-12 mRNA in lung
tissues increased following irradiation and peaked at two weeks
post-irradiation; however, at four weeks post-irradiation the level
of mRNA was reduced markedly. By contrast, the level of TIMP-1 mRNA
in lung tissues increased following irradiation and was highest
four weeks post-irradiation. In comparison with group B, the levels
of MMP-12 and TIMP-1 mRNA in groups C-E were reduced. These results
indicate that Yangyinqingfei decoction had an inhibitory effect on
mRNA expression of MMP-12 and TIMP-1, particularly at the higher
dose.
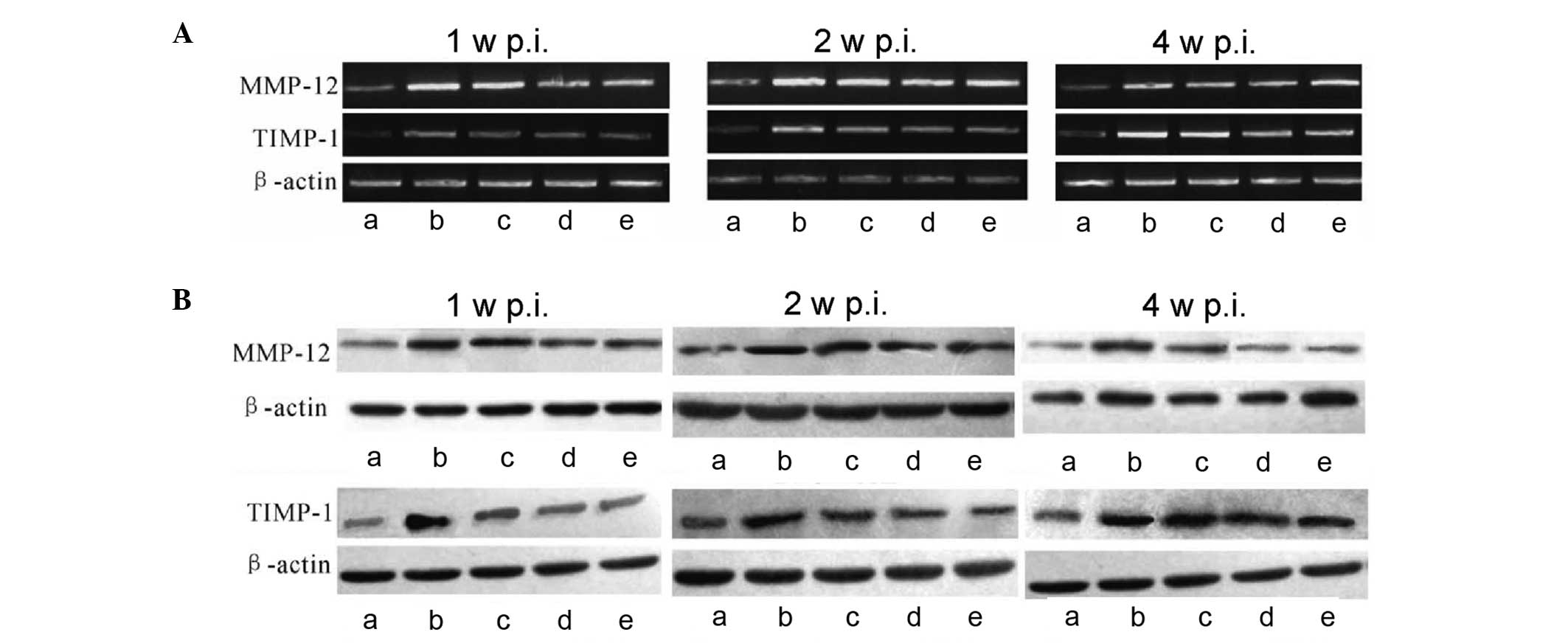 | Figure 2Effect of Yangyinqingfei decoction on
MMP-12 and TIMP-1 expression. At different time-points following
irradiation, total RNA was extracted from lung tissues and reverse
transcription-polymerase chain reaction and western blot analysis
were performed. (A) mRNA expression of MMP-12 and TIMP-1 one, two
and four weeks after irradiation; (B) protein expression of MMP-12
and TIMP-1 one, two and four weeks after irradiation. β-actin was
used as an internal control. a, Control group; b, model group
(irradiated rats without drug administration); c, low-dose
treatment group (2 g/kg/day); d, intermediate-dose treatment group
(6 g/kg/day); e, high-dose treatment group (18 g/kg/day); MMP-12,
matrix metalloproteinase-12; TIMP-1, tissue inhibitors of matrix
metalloproteinase-1; p.i., post-irradiation. |
The protein levels of MMP-12 and TIMP-1 were
observed in lung tissue using western blotting. As shown in
Fig. 2B, MMP-12 and TIMP-1
proteins were positively expressed in each group. The protein
expression levels of MMP-12 and TIMP-1 were significantly increased
in group B following irradiation compared with those of group A.
Compared with group B, the protein expression of MMP-12 and TIMP-1
was effectively downregulated in groups C-E, particularly in group
D; however, expression levels remained higher than those in group
A. This result further demonstrates the inhibitory effect of
Yangyinqingfei decoction on the expression of MMP-12 and
TIMP-1.
Discussion
In the present study it was demonstrated that
Yangyinqingfei decoction exerted a protective effect on rats with
radiation-induced lung injury. The mechanism underlying this
protective effect may involve the downregulation of MMP-12 and
TIMP-1 expression.
Pathological changes in radiation-induced lung
injury include interstitial pulmonary congestion and edema,
inflammatory cell infiltration, interstitial lung fiber hyperplasia
and alveolar atrophy (21). At
present, radiation-induced lung injury is a well-established model
in rats that has been used in numerous studies (22,23).
In the present study, Wistar rats were irradiated to induce lung
injury. One week after irradiation, alveolar wall edema and
capillary congestion were observed. Two weeks post-irradiation,
macrophage infiltration was observed. Alveolar septal thickening
and alveolar structure disappeared four weeks after irradiation.
Lesions were dominated by signs of chronic inflammation, visible
fibroblast hyperplasia and alveolar septa thickening, as well as
the shrinking or compensatory expansion of alveolar space. Thus,
the rat model for radiation-induced lung injury was successfully
established.
In this study, the results suggested that early
intervention with Yangyinqingfei decoction alleviated pulmonary
edema, congestion, inflammation and alveolar septal thickening
compared with the model group (group B). This indicates that early
application of Yangyinqingfei decoction may be effective for the
control and the mitigation of radiation-induced lung injury. The
analysis of histopathological changes in the lung showed that rats
administered a high dose of Yangyinqingfei decoction exhibited
reduced interstitial lung congestion, mild edema and less alveolar
structural damage compared with the groups administered lower
doses. However, rats treated with a high dose of Yangyinqingfei
decoction exhibited side effects, including diarrhea. These results
indicate that the dosage of Yangyinqingfei decoction has an impact
on the efficacy of the treatment. However, despite the side
effects, high-dose Yangyinqingfei decoction showed a greater
protective effect against radiation-induced lung injury than the
doses used in the other groups.
Radiation causes alveolar injury, leading to
alveolar interstitial inflammation, cell proliferation and
apoptosis. The pathological repair process in the lung tissue
includes interstitial lung cell proliferation, ECM deposition and
lung tissue remodeling (24).
Excessive deposition of ECM is a key factor leading to pulmonary
fibrosis. ECM is degraded by proteases, of which MMPs are the most
important and are capable of degrading almost all the components of
the ECM. Studies have shown that MMPs are involved in a wide range
of biological activities, including cell migration, angiogenesis
and atherosclerosis. MMPs have also been reported to be involved in
the invasion and metastasis of malignant tumors, as well as other
pathological processes, in a variety of fibrotic diseases (25–28).
In the present study, the mRNA and protein expression of MMP-12 and
TIMP-1 increased in the model group following radiation. This may
have been the result of the radiation damage to the endothelial
cells, which led to the accumulation of a series of inflammatory
cells, including macrophages, neutrophils and lymphocytes, in the
alveolar cavity. These cells release a series of cytokines, growth
factors and inflammatory mediators to promote the synthesis and
activation of MMPs. Wright et al (29) reported that MMP-12 expression
significantly increased in mice exposed to cigarette smoke;
however, in mice lacking tumor necrosis factor-α (TNF-α) receptors,
expression of MMP-12 did not change, indicating that TNF-α can
activate the expression of MMP-12. Another study indicated that
MMP-12 is a pro-inflammatory factor, which is capable of inducing
expression of other MMPs, including MMP-2 and MMP-9, and activating
the MMP-12 hydrolysis cascade, leading to the degradation of other
ECM components and causing the dissolution of the basement membrane
in lung tissue (30). Destruction
of the integrity of the basement membrane, as well as alveolar and
interstitial injuries caused by various factors, leads to acute
radiation-induced lung injury (31). In addition, the destruction of the
basement membrane, which regulates lung epithelial permeability,
may promote migration of fibroblasts, as well as deposition of the
alveolar cavity collagen fiber, and cause pulmonary fibrosis. TIMPs
are endogenous inhibitors of MMPs, and it has been demonstrated
that the MMP/TIMP and ECM metabolic imbalance is involved in the
development and progression of pulmonary fibrosis (32). Selman et al (33) showed that in patients with IPF,
dominant active TIMP expression resulted in filamentous collagen
degradation, which caused the occurrence of pulmonary fibrosis.
Using a macrophage-dependent immunoglobulin A immune complex mouse
model, Gibbs et al (34)
demonstrated that lung damage in this model may be inhibited by
TIMP-2. These studies not only suggest that macrophages may be the
source of MMPs in the early stages of lung damage, but also suggest
that TIMPs may have a role in the prevention of injury during the
early stages of lung damage (35).
In the present study, increased expression of MMP-12 and TIMP-1
mRNA was observed in lung tissue during the first four weeks
post-irradiation. However, at four weeks post-irradiation, MMP-12
expression was reduced while expression of TIMP-1 remained
elevated, which may have led to an MMP-12/TIMP-1 imbalance and the
accumulation of ECM fibrosis. Compared with the irradiation group
without drug treatment (group B), mRNA and protein expression of
MMP-12 and TIMP-1 in the lungs of rats in the Yangyinqingfei
decoction intervention groups were lower at each time-point, thus
maintaining the MMP-12/TIMP-1 balance. Accordingly, this led to the
balance of synthesis and degradation of the ECM, alleviating the
occurrence of pulmonary fibrosis.
In conclusion, this study showed that Yangyinqingfei
decoction has a preventative and therapeutic effect on the early
phase of radiation-induced lung injury, the mechanism of which may
involve the suppression of MMP-12 and TIMP-1. However, more
detailed studies are required in order to further evaluate the
mechanisms underlying the observed effects.
References
|
1
|
Inoue A, Kunitoh H, Sekine I, Sumi M,
Tokuuye K and Saijo N: Radiation pneumonitis in lung cancer
patients: a retrospective study of risk factors and the long-term
prognosis. Int J Radiat Oncol Biol Phys. 49:649–655. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Todd NW, Luzina IG and Atamas SP:
Molecular and cellular mechanisms of pulmonary fibrosis.
Fibrogenesis Tissue Repair. 5:112012. View Article : Google Scholar
|
|
3
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
structure, function, and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kong MY, Li Y, Oster R, Gaggar A and
Clancy JP: Early elevation of matrix metalloproteinase-8 and -9 in
pediatric ARDS is associated with an increased risk of prolonged
mechanical ventilation. PLoS One. 6:e225962011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Cui D, Tong X, et al: The role of
matrix metalloproteinases in extracellular matrix remodelling in
chronic obstructive pulmonary disease rat models. Zhonghua Nei Ke
Za Zhi. 41:393–398. 2002.(In Chinese).
|
|
7
|
Yu G, Kovkarova-Naumovski E, Jara P, et
al: Matrix metalloproteinase-19 is a key regulator of lung fibrosis
in mice and humans. Am J Respir Crit Care Med. 186:752–762. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Willems S, Verleden SE, Vanaudenaerde BM,
et al: Multiplex protein profiling of bronchoalveolar lavage in
idiopathic pulmonary fibrosis and hypersensitivity pneumonitis. Ann
Thorac Med. 8:38–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang K, Palm J, König J, et al:
Matrix-Metallo-Proteinases and their tissue inhibitors in
radiation-induced lung injury. Int J Radiat Biol. 83:665–676. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamada S, Wang KY, Tanimoto A, et al:
Matrix metalloproteinase 12 accelerates the initiation of
atherosclerosis and stimulates the progression of fatty streaks to
fibrous plaques in transgenic rabbits. Am J Pathol. 172:1419–1429.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu P, Yan C and Du H: Matrix
metalloproteinase 12 overexpression in myeloid lineage cells plays
a key role in modulating myelopoiesis, immune suppression, and lung
tumorigenesis. Blood. 117:4476–4489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Madala SK, Pesce JT, Ramalingam TR, et al:
Matrix metalloproteinase 12-deficiency augments extracellular
matrix degrading metalloproteinases and attenuates IL-13-dependent
fibrosis. J Immunol. 184:3955–3963. 2010. View Article : Google Scholar
|
|
13
|
Matute-Bello G, Wurfel MM, Lee JS, et al:
Essential role of MMP-12 in Fas-induced lung fibrosis. Am J Respir
Cell Mol Biol. 37:210–221. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwakawa M, Noda S, Ohta T, et al: Strain
dependent differences in a histological study of CD44 and collagen
fibers with an expression analysis of inflammatory response-related
genes in irradiated murine lung. J Radiat Res. 45:423–433. 2004.
View Article : Google Scholar
|
|
15
|
Nie QH, Duan GR, Luo XD, et al: Expression
of TIMP-1 and TIMP-2 in rats with hepatic fibrosis. World J
Gastroenterol. 10:86–90. 2004.PubMed/NCBI
|
|
16
|
Zhang XF, Zhu GF, Liu S and Foda HD: The
role of disequilibrium expression of matrix matelloproteinase-2/9
and their tissue inhibitors in pathogenesis of hyperoxia-induced
acute lung injury in mice. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue.
20:597–600. 2008.(In Chinese).
|
|
17
|
Chen M, Cheung FW, Chan MH, et al:
Protective roles of Cordyceps on lung fibrosis in cellular and rat
models. J Ethnopharmacol. 143:448–454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang S, Du XY, Hou W, et al: The effect of
of Yangyinqingfei decoction on pathology and serous TGF-β1 of
radiation induced lung injury in rats. Beijing Journal of
Traditional Chinese Medicine. 31:858–860. 2012.(In Chinese).
|
|
19
|
Yu YY: A brief history of prevention and
treatment of diphtheria in modern TCM. Zhonghua Yi Shi Za Zhi.
34:79–82. 2004.(In Chinese).
|
|
20
|
Chinese Pharmacopoeia Committee. Chinese
Pharmacopoeia Commission: Part I. Beijing, China: China Medical
Science and Technology Press; 2010
|
|
21
|
Ghafoori P, Marks LB, Vujaskovic Z and
Kelsey CR: Radiation-induced lung injury. Assessment, management,
and prevention. Oncology (Williston Park). 22:37–47; discussion
52–53. 2008.PubMed/NCBI
|
|
22
|
Vujaskovic Z, Down JD, van t’ Veld AA, et
al: Radiological and functional assessment of radiation-induced
lung injury in the rat. Exp Lung Res. 24:137–148. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahmood J, Jelveh S, Calveley V, Zaidi A,
Doctrow SR and Hill RP: Mitigation of lung injury after accidental
exposure to radiation. Radiat Res. 176:770–780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amălinei C, Căruntu ID, Giuşcă SE and
Bălan RA: Matrix metalloproteinases involvement in pathologic
conditions. Rom J Morphol Embryol. 51:215–228. 2010.
|
|
25
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masson V: Roles of serine proteases and
matrix metalloproteinases in tumor invasion and angiogenesis. Bull
Mem Acad R Med Belg. 161:320–326. 2006.(In French).
|
|
27
|
Watanabe N and Ikeda U: Matrix
metalloproteinases and atherosclerosis. Curr Atheroscler Rep.
6:112–120. 2004. View Article : Google Scholar
|
|
28
|
Foda HD and Zucker S: Matrix
metalloproteinases in cancer invasion, metastasis and angiogenesis.
Drug Discov Today. 6:478–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wright JL, Tai H, Wang R, Wang X and Churg
A: Cigarette smoke upregulates pulmonary vascular matrix
metalloproteinases via TNF-alpha signaling. Am J Physiol Lung Cell
Mol Physiol. 292:L125–L133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nénan S, Boichot E, Lagente V and Bertrand
CP: Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem
Inst Oswaldo Cruz. 100(Suppl 1): 167–172. 2005.
|
|
31
|
Yue J, Zhang K and Chen J: Role of
integrins in regulating proteases to mediate extracellular matrix
remodeling. Cancer Microenviron. 5:275–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang BL, Tu YY, Fu JF, et al: Unbalanced
MMP/TIMP-1 expression during the development of experimental
pulmonary fibrosis with acute paraquat poisoning. Mol Med Rep.
4:243–248. 2011.PubMed/NCBI
|
|
33
|
Selman M, Ruiz V, Cabrera S, et al:
TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A
prevailing nondegradative lung microenvironment? Am J Physiol Lung
Cell Mol Physiol. 279:L562–L574. 2000.PubMed/NCBI
|
|
34
|
Gibbs DF, Shanley TP, Warner RL, Murphy
HS, Varani J and Johnson KJ: Role of matrix metalloproteinases in
models of macrophage-dependent acute lung injury. Evidence for
alveolar macrophage as source of proteinases. Am J Respir Cell Mol
Biol. 20:1145–1154. 1999.
|
|
35
|
Pardo A and Selman M: MMP-1: the elder of
the family. Int J Biochem Cell Biol. 37:283–288. 2005. View Article : Google Scholar : PubMed/NCBI
|