Introduction
The differential diagnosis of benign and malignant
tumours is challenging in clinical practice. Positron emission
tomography (PET) and computed tomography (CT) are revolutionary in
the field of medicine since these procedures are able to detect the
metabolism of glucose, amino acids and macromolecules, enabling the
diagnosis of diseases at the molecular and genetic levels (1,2).
18F-fluorodeoxyglucose
(18F-FDG) is the most widely used tracer. During
glycolysis, the expression levels of the glucose transporter
protein increase, resulting in the 18F-FDG uptake by
malignant tumours being higher compared with that of normal tissues
(3,4). However, 18F-FDG is not a
tumour-specific tracer and may produce certain false-positive
results (5,6).
In previous years, numerous studies have focused on
the research and development of nucleoside metabolic tracers, of
which 3′-deoxy-3′-18F-fluorothymidine
(18F-FLT) is the most promising. FLT is a pyrimidine
analogue that is catalysed by thymidine kinase 1 (TK1) to induce
the phosphorylation of FLT into a single phosphate and an
intracellular strand. TK1 is a key enzyme in the salvage synthesis
pathway of DNA and TK1 activity in malignant tumour cells is
3–10-fold higher than in benign cells (7–9).
Therefore, TK1 activity indirectly reflects the state of tumour
cell proliferation (10).
The majority of studies consider 18F-FLT
to have 100% specificity for malignant tumours (11). However, cell proliferation is not
tumour-specific and bacterial and viral growth also involve DNA
replication. In addition, a previous study reported a case of
18F-FLT uptake in pulmonary tuberculosis (12,13).
However, the animal models used in previous studies have certain
limitations (12,13). In one study (12), inflammation was induced by
turpentine, which is a physical or chemical stimulus and causes
nonbacterial inflammation. In the other study (13), the experiment did not include a
bacterial breeding period. Therefore, the design of a variety of
models of inflammation is required, with 18F-FLT imaging
being performed at various time points to determine the specificity
of 18F-FLT for tumour cells.
Materials and methods
Experimental animals
Rabbits (age, 6–8 weeks; T739 inbred strain; either
gender; weight, 22–25 g) were provided by the Cancer Institute of
the Chinese Academy of Medical Science (SCXK11-00-0005; Beijing,
China). The study was conducted in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (1995). The animal use
protocol was reviewed and approved by the Institutional Animal Care
and Use Committee of Xuzhou Central Hospital (Xuzhou, China).
Experimental design
The experimental animals were divided into groups A
and B, with eight rabbits in each group. Group A (right tibia) was
implanted with calcium sulphate + gentamicin tablets, whereas group
B (left tibia) was implanted with Staphylococcus aureus (S.
aureus).
Establishment of an inflammation
model
Animals were anaesthetised with a mixture of 2 ml
ketamine and 1.5 ml sumianxin (MeiDa high-tech company, Beijing,
China) at 0.3 ml/kg body weight. For local skin preparation, the
animals were fixed in a supine position, regularly disinfected and
then covered with sterile towels. Next, a tibial patellar medial
inferior longitudinal incision, ~3 cm in length, was made to expose
the medial proximal tibia, and a window was created in the proximal
tibia using a 1-mm diameter drill bit. Intramedullary tissue and a
section of the cancellous bone were scraped with a small curette to
create a 3×10 mm rectangular bone window. Based on the experimental
design, group A received gentamicin sustained-release tablets (2
mg; MeiDa high-tech company) and calcium sulphate (2 mg), whereas
group B was injected with 0.15 ml sodium salts of the fatty acids
of cod liver oil (MeiDa high-tech company), 0.2 ml S. aureus
(5×106 CFU) and 1 ml physiological saline. The bone
window was then closed with bone wax and the wounds were sutured
after flushing with physiological saline. Following surgery, the
animals were fed a conventional diet for four weeks. No animal was
administered intravenous or oral antibiotic therapy.
PET imaging
18F-FLT PET (Phillips, Amsterdam,
Netherlands) imaging was performed four weeks following surgery.
The animals were injected with 50 μCi/100 μl 18F-FLT in
the tail vein and were subjected to general anaesthesia via the
intramuscular injection of a mixed solution of 2 ml ketamine and
1.5 ml sumianxin. After 10 min, the animals were fixed in an ECAT
Exact HR+ PET imaging system (Siemens AG, Munich, Germany) that was
equipped with a 3D mode image acquisition system. Following
dispersion, random counting and dead time correction, all
measurements were analysed through the reconstruction of coronal,
transverse and sagittal sectional images by the filter-rejected
projection method.
Gross observation
Following anaesthesia, the animals underwent surgery
and samples were collected. No inflammation of the local limb soft
tissue, bone tissue or local pus was observed. Tibia samples of ~2
cm were collected to identify abnormal changes in the marrow
cavity. The samples were divided into two sections to monitor the
development of bone defects and osteomyelitis.
Histological observation
Specimens were fixed with 10% neutral formaldehyde
and decalcified for 20 days using a decalcifying fluid containing
EDTA. The samples were then embedded in paraffin, conventionally
sectioned and subsequently stained with hematoxylin and eosin. A
light microscope was used to observe the repair of bone defects, as
well as bone infection, local abscess, inflammatory cell
infiltration, dead bone and new bone formation.
Bacterial and cancer cell culture
Reproductive-stage Escherichia coli (E. coli)
and A549 lung cancer cells were incubated with 18F-FLT
for 1 h in RMPI 1640 conataining 13% IV-type collagenase and 10%
FBS (Gibco, Carlsbad, CA, USA). The samples were then centrifuged
and the supernatant was removed. A γ-well counter (162 factory,
Beijing, China) was then used to determine the radioactive
counts.
Statistical analysis
Statistical analyses were performed using the SPSS
statistical software, version 12.0 (SPSS, Inc., Chicago, IL, USA).
Data between the FLT and calcium sulphate groups were compared
using a paired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
General observations
In group A, no bone destruction or marrow cavity pus
was identified in the medullary cavity. The animals in this group
exhibited normal bone tissue, red colouration, loose tissue, clear
boundaries of the intramedullary tissue and cortical bone, wreckage
remains of the implanted calcium sulphate following partial
degradation, largely degraded antibiotic sustained-release tablets
and mostly repaired defects.
In group B, local soft tissue inflammatory oedema
was observed and the control group exhibited intramedullary tissue
swelling, a large amount of pus, necrotic intramedullary tissue and
dead bone formation in the proximal tibial specimen. A large number
of S. aureus bacteria were visible in the bacterial
culture.
Histological examination
Bone tissue sections were demineralised four weeks
following surgery and histological changes in the bone pathology
were observed under a light microscope.
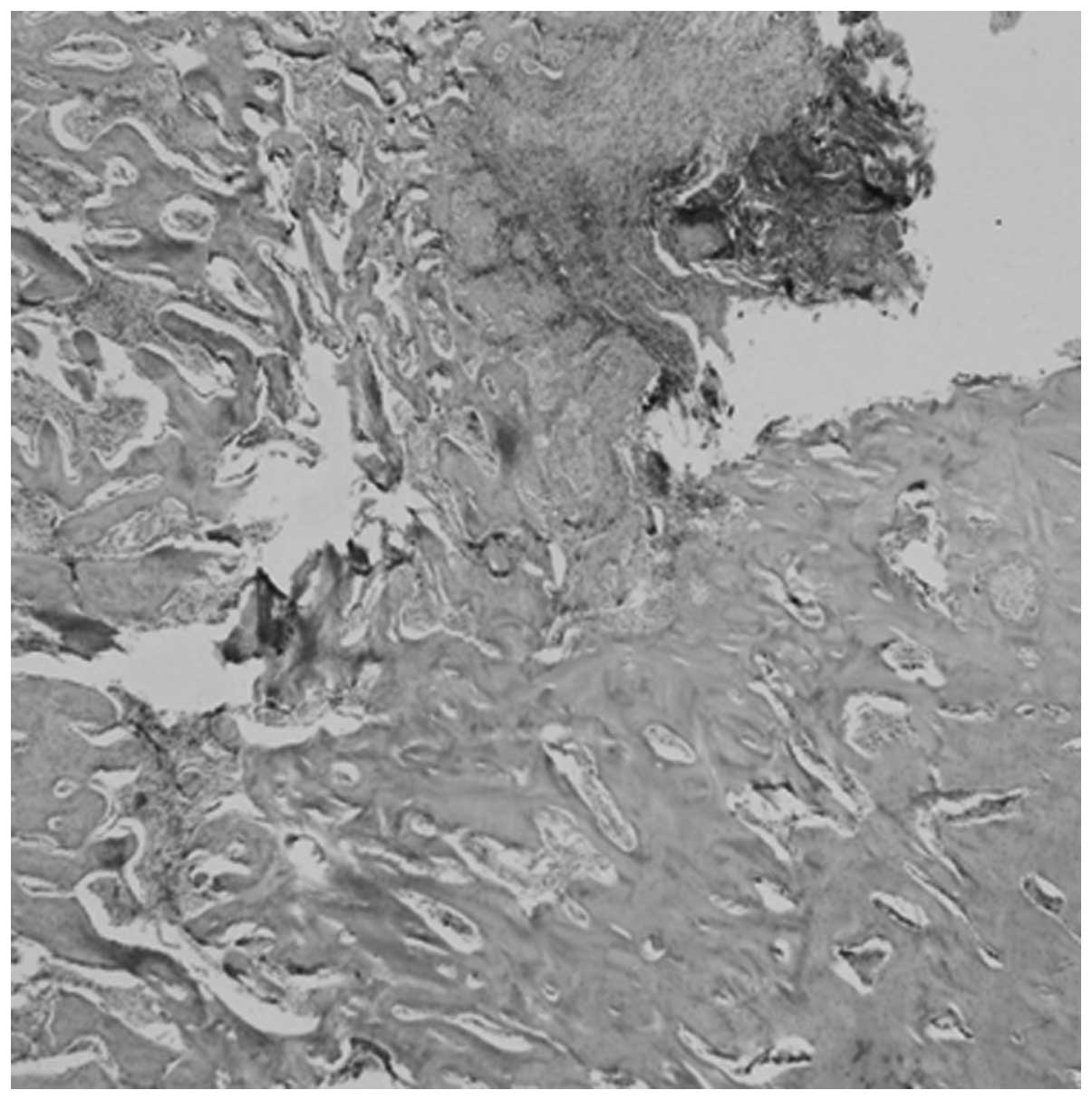
In group A, calcium sulphate and the degradation
products were surrounded by fibrous tissue and there were no
inflammatory cells, necrosis of the bone or local abscesses present
in the medullary cavity (Fig. 1).
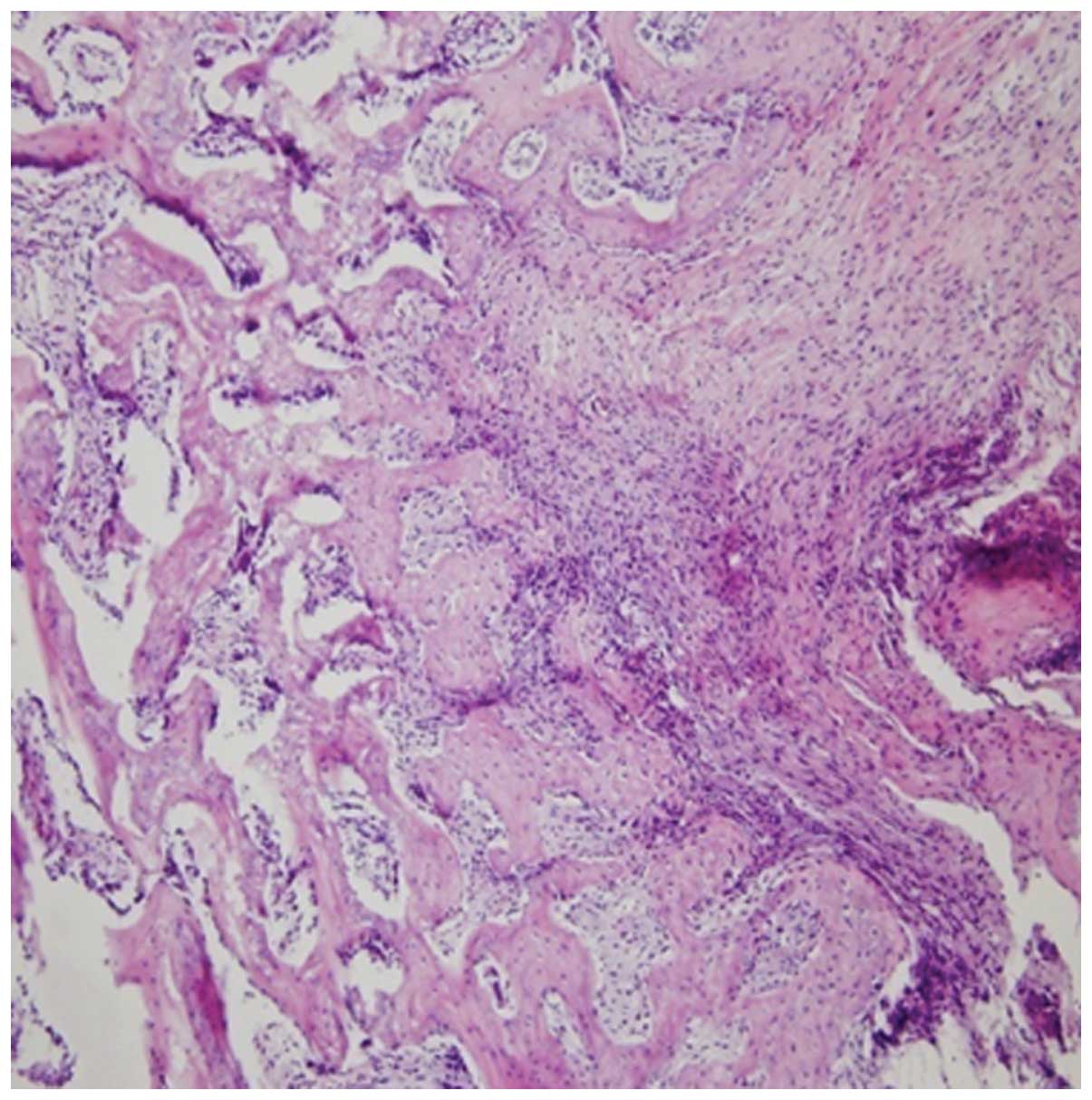
In group B, a certain amount of inflammatory cells and S.
aureus abscess formation were visible. In addition, a small
degree of new bone formation over the scar tissues was observed
(Fig. 2).
PET imaging
Uptake of 18F-FLT by the abscess was
markedly higher compared with that by the granuloma [maximum
standardised uptake value (SUVmax), 5.76±0.25 and
1.15±0.32, respectively; P<0.01; Fig. 3].
Bacterial and cancer cell culture
The 18F-FLT uptake by E. coli was
five times higher compared with that by tumour cells (35,680 and
7,200, respectively; P<0.01).
Discussion
18F-FDG, which has been referred to as
the ‘molecule of the century,’ is a highly sensitive tracer, but is
prone to producing false-positive results (14). 18F-FLT is an analogue of
thymidine, which is involved in DNA synthesis and is an indicator
of cell proliferation (15). The
majority of studies consider the tumour specificity of this tracer
to be 100% (16–18). Carter et al (11) used 18F-FLT and
18F-FDG to study inflammation and tumour models in rats.
The rat inflammation model was prepared through the injection of
Escherichia coli (E. coli) in the rat thigh, while the
tumour model was thigh sarcoma. The results indicated that the
18F-FDG tumour/non-tumour ratio was 7.39, which was
higher than the 2.76 of 18F-FLT. 18F-FDG
uptake was slightly lower in inflammatory tissues compared with
that in tumour tissues; the inflammation-to-normal tissue ratio was
3.29, whereas the 18F-FLT inflammatory tissue/normal
tissue uptake ratio was only 1.14. van Waarde et al
(12) also compared
18F-FLT and 18F-FDG uptake in inflammatory
and tumour tissues in a rat model. The tumour model was prepared by
injecting C6 nerve glioma into the right shoulder of the rats,
while the inflammation model was established by injecting
turpentine oil into the left calf. In this study (12), the tumour/muscle ratio of
18F-FDG was 13, which was higher than the 8 of
18F-FLT. The radioactive uptake of 18F-FDG
significantly increased at the site of inflammation, whereas no
significant uptake of 18F-FLT was observed in the
inflammatory sites.
It may be hypothesised that the E. coli
implanted in the study by Carter et al (11) was undetectable at the reproductive
stage. Turpentine is an aseptic chemical irritant that causes local
plasma extravasation and neutrophil migration. These inflammatory
cells originate in the blood. The site of inflammation contains no
bacteria and, thus, does not accurately reflect bacterial
inflammation.
In the present study, bacteria was shown to take up
a large amount of 18F-FLT in the reproductive stage, as
shown by the E. coli cell culture assay and the rabbit S.
aureus abscess model. These results indicate that
18F-FLT is not a tumour-specific tracer.
In the E. coli and A549 cell culture assay, the
results indicated that 18F-FLT uptake by E. coli
was five times higher compared with that by tumour cells (35,680
and 7,200, respectively; P<0.01). This result indicates that
bacteria in a reproductive stage take up a significantly higher
amount of 18F-FLT compared with that taken up by tumour
cells. In the rabbit S. aureus abscess model in the present
study, the animals underwent 18F-FLT PET imaging after
four weeks. The lesions were then removed for gross observation and
histological examination. Samples were compared with lesions due to
calcium sulphate + gentamicin to investigate the difference in the
18F-FLT uptake between the abscess and granuloma. After
40 days, the pathological changes in the right and left tibiae
varied. In the side injected with S. aureus, a large number
of inflammatory cells aggregated in the medullary cavity and
abscess formation, necrotic bone formation and partial chronic
osteomyelitis were also observed. These observations indicate that
inflammatory changes and tissue repair were occurring
simultaneously. In addition, a small degree of new bone formation
was observed, replacing the granulation of fibrous tissue. In the
side that received calcium sulphate and antibiotic, calcium
sulphate was visible and the degradation products were surrounded
by fibrous tissue. No inflammatory cells, local abscesses or bone
necrosis were observed in the medullary cavity. Bilateral PET
imaging results demonstrated that 18F-FLT uptake in the
S. aureus abscess side was significantly higher compared
with the calcium sulphate side (SUVmax, 5.76±0.25 and
1.15±0.32, respectively; P<0.01).
18F-FLT is an indicator of cell
proliferation, which is not unique to tumours. 18F-FLT
uptake by inflammatory lesions depends on the growth period of the
bacteria implanted in the animal model (19). During the bacterial reproductive
period, the inflammatory bacteria exhibit exponential growth,
accelerated DNA synthesis and increased 18F-FLT uptake.
However, during the bacterial nonreproductive period or chronic
period of bacterial inflammation, no bacterial reproduction occurs.
Thus, 18F-FLT uptake is low. In addition, due to the
immune reaction or formation of granulomas in the lesion, the
tracer uptake in certain bacterial and nonbacterial infections
during the development of the disease varies with the disease
progression stage. Pathological changes differ at various stages
(12). In the early stages,
tuberculosis or other types of bacteria may multiply and DNA
synthesis may accelerate. By contrast, endogenous lymphocyte and
macrophage proliferation may form granulomas. In addition, nodular
regions in the lesions at the edge of the calcium sulphate side
exhibit higher 18F-FLT uptake. Thus, the calcium
sulphate side may be the site that forms granulomas. In the chronic
phase, the number of granulomas does not increase and the uptake of
macrophages from the peripheral blood results in the formation of
hard granulomas. During this period, granulomas are mainly formed
when macrophages stop proliferating and bacteria stop reproducing.
As a result, 18F-FLT uptake by the bacteria may stop.
Therefore, granulomatous lesions with different causes may exhibit
various 18F-FLT uptake rates, which should be distinct
according to the different disease stages. All these results
indicate that 18F-FLT is not a specific tumour tracer,
since it is also absorbed by inflammatory lesions associated with
reproductive-stage bacteria or viruses. However, the uptake may
significantly decrease during the chronic phase.
18F-FLT as an imaging agent has an
advantage over 18F-FDG in the diagnosis of tumour cell
proliferation and inflammatory lesions. This is due to the
different levels of 18F-FDG uptake by inflammatory
lesions at various disease stages. At the acute stage, the number
of granulocytes, monocytes and macrophages may increase. These
cells are metabolically active and consume large amounts of glucose
via the hexose monophosphate pathway during chemotaxis and
phagocytosis (20). As a result,
18F-FDG uptake may increase. In the chronic phase,
Sugawara et al (20)
hypothesised that macrophages and neutrophils also take up
18F-FDG. In the chronic granuloma phase, capillary
distribution in the tissue is sparse. In hypoxic or anaerobic
conditions, macrophages can adapt to the hypoxic environment
through anaerobic glycolysis, which results in increased
18F-FDG uptake.
Notably, the Baicalin content in rabbit serum
specimens is 9–16 times higher compared with human sera (21). Large amounts of endogenous
thymidine compete with 18F FLT for the adenosine
transporter and the active site of TK. This competition results in
low 18F-FLT uptake in the rabbit model (21). However, whether the experimental
results of the present study may be applied to clinical practice
requires further investigation.
Acknowledgements
The study was supported by grants from the National
Natural Science Foundation of China (no. 81272557), the Technology
Bureau of Xuzhou City (no. XZZD1125) and the Natural Science Fund
Foundation of Jiangsu Province (no. BK2012647).
References
|
1
|
Strauss LG and Conti PS: The applications
of PET in clinical oncology. J Nucl Med. 32:623–650.
1991.PubMed/NCBI
|
|
2
|
Halter G, Buck AK, Schirrmeister H, et al:
[18F] 3-deoxy-3′-fluorothymidine positron emission
tomography: alternative or diagnostic adjunct to
2-[18F]-fluoro-2-deoxy-D-glucose positron emission
tomography in the workup of suspicious central focal lesions? J
Thorac Cardiovasc Surg. 127:1093–1099. 2004.
|
|
3
|
Haberkorn U, Bellemann ME, Brix G, et al:
Apoptosis and changes in glucose transport early after treatment of
Morris hepatoma with gemcitabine. Eur J Nucl Med. 28:418–425. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burgman P, Odonoghue JA, Humm JL and Ling
CC: Hypoxia induced increase in FDG uptake in MCF7 cells. J Nucl
Med. 42:170–175. 2001.PubMed/NCBI
|
|
5
|
Bakheet SM, Saleem M, Powe J, Al-Amro A,
Larsson SG and Mahassin Z: F-18 fluorodeoxyglucose chest uptake in
lung inflammation and infection. Clin Nucl Med. 25:273–278. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bakheet SM and Powe J: Benign causes of
18-FDG uptake on whole body imaging. Semin Nucl Med. 28:352–358.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minn H, Clavo AC, Grénman R and Wahl RL:
In vitro comparison of cell proliferation kinetics and uptake of
tritiated fluorodeoxyglucose and L-methionine in squamous-cell
carcinoma of the head and neck. J Nucl Med. 36:252–258.
1995.PubMed/NCBI
|
|
8
|
Eriksson S, Munch-Petersen B, Johansson K
and Eklund H: Structure and function of cellular
deoxyribonucleoside kinases. Cell Mol Life Sci. 59:1327–1346. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rasey JS, Grierson JR, Wiens LW, Kolb PD
and Schwartz JL: Validation of FLT uptake as a measure of thymidine
kinase-1 activity in A549 carcinoma cells. J Nucl Med.
43:1210–1217. 2002.PubMed/NCBI
|
|
10
|
Carter EA, McCusker C, Syed S, Tompkins RG
and Fischman AJ: Comparison of 18FLT with
18FDG for differentiation between tumor and focal sites
of infection in rats. J Nucl Med. 43:2662002.
|
|
11
|
van Waarde A, Cobben DCP, Suurmeijer AJH,
et al: Selectivity of 18F-FLT and 18F-FDG for
differentiating tumor from inflammation in a rodent model. J Nucl
Med. 45:695–700. 2004.
|
|
12
|
Boerman OC, Storm G, Oyen WJ, et al:
Sterically stabilized liposomes labeled with indium-111 to image
focal infection. J Nucl Med. 36:1639–1644. 1995.PubMed/NCBI
|
|
13
|
Francis DL, Visvikis D, Costa DC, et al:
Potential impact of [18F]3′-deoxy-3′-fluorothymidine
versus [18F]fluoro-2-deoxy-D-glucose in positron
emission tomography for colorectal cancer. Eur J Nucl Med Mol
Imaging. 30:988–994. 2003.
|
|
14
|
Munch-Petersen B, Cloos L, Jensen HK and
Tyrsted G: Human thymidine kinase 1. Regulation in normal and
malignant cells. Adv Enzyme Regul. 35:69–89. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Been LB, Suurmeijer AJ, Cobben DC, Jager
PL, Hoekstra HJ and Elsinga PH: [18F]FLT-PET in
oncology: current status and opportunities. Eur J Nucl Med Mol
Imaging. 31:1659–1672. 2004.
|
|
16
|
Shields AF: PET imaging with
18F-FLT and thymidine analogs: promise and pitfalls. J
Nucl Med. 44:1432–1434. 2003.PubMed/NCBI
|
|
17
|
Wagner M, Seitz U, Buck A, et al:
3′-[18F]fluoro-3′-deoxythymidine ([18F]-FLT)
as positron emission tomography tracer for imaging proliferation in
a murine B-cell lymphoma model and in the human disease. Cancer
Res. 63:2681–2687. 2003.
|
|
18
|
Dittmann H, Dohmen BM, Kehlbach R, et al:
Early changes in [18F]FLT uptake after chemotherapy: an
experimental study. Eur J Nucl Med Mol Imaging. 29:1462–1469.
2002.
|
|
19
|
Chen W, Cloughesy T, Kamdar N, et al:
Imaging proliferation in brain tumors with 18F-FLT PET:
comparison with 18F-FDG. J Nucl Med. 46:945–952.
2005.PubMed/NCBI
|
|
20
|
Sugawara Y, Gutowski TD, Fisher SJ, Brown
RS and Wahl RL: Uptake of positron emission tomography tracers in
experimental bacterial infections: a comparative biodistribution
study of radiolabeled FDG, thymidine, L-methionine,
67Ga-citrate, and 125I-HSA. Eur J Nucl Med.
26:333–341. 1999. View Article : Google Scholar
|
|
21
|
Vesselle H, Grierson J, Muzi M, et al: In
vivo validation of 3′deoxy-3′-[(18)F]fluorothymidine ([(18)F]FLT)
as a proliferation imaging tracer in humans: correlation of
[(18)F]FLT uptake by positron emission tomography with Ki-67
immunohistochemistry and flow cytometry in human lung tumors. Clin
Cancer Res. 8:3315–3323. 2002.
|