Introduction
Cardiovascular disease is a common and frequently
encountered disease, with the highest morbidity and mortality rates
worldwide. Thus, treatment for cardiovascular disease has become an
increasingly important not only for clinicians, but also for the
pharmaceutical industry. Ligustilide (LIG) has been reported to
have a number of biological activities, including anti-spasm,
alleviating pain and relieving asthma functions (1). LIG can inhibit the metabolism of
platelet arachidonic acid, preventing platelet aggregation
(2). LIG can also reduce vascular
resistance, increase blood flow and improve microcirculation
(3,4). Furthermore, LIG has been shown to
exhibit antioxidant effects, thus, can antagonize free
radical-induced tissue damage (5).
In addition, LIG has been demonstrated to exhibit an inhibitory
effect on vascular cell proliferation (6,7). In
a previous study, LIG was found to significantly inhibit vascular
smooth muscle cell proliferation via the mitogen-activated protein
kinase/cycle proteins (p21, CyclinD1 and pRb) and extracellular
signal-regulated kinase signaling pathway. Thus, LIG also exhibits
certain therapeutic effects in cardiovascular diseases (8). The proliferation and migration of
vascular smooth muscle cells is a key process in the formation of
atherosclerosis. In addition, cardiac hypertrophy and fibrosis
results in diseases, including systolic rhythm imbalance. In this
respect, the present study analyzed whether LIG exhibits a similar
therapeutic effect in heart disease, such as the proliferation,
migration and invasion of vascular smooth muscle cells, and the
cardiac hypertrophy. LIG may have a large clinical value if it does
have an inhibitory effect on cardiac hypertrophy. Thus, in the
present study, the effects of LIG on angiotensin II (Ang
II)-induced hypertrophy of myocardial cells was preliminarily
investigated, as well as the possible underlying mechanisms, in
order to provide a scientific basis for the development of a novel
drug for the treatment of cardiovascular disease.
Materials and methods
Animals and reagents
Sprague-Dawley (SD) neonatal rats (age, 1–3 days)
were provided by Guangzhou University of Chinese Medicine
(Guangzhou, China), the study was approved by the ethics committee
of Guangdong Phamaceutical University, (Guangzhou, China). Animals
were treated according to the animal care guidelines of Guangdong
Pharmaceutical University. Ang II was purchased from Alexis
Corporation (Leistal, Switzerland) and ligustilide was purchased
from Tianjin Hualida Biotechnology Co., Ltd. (Tianjin, China).
Fetal bovine serum (FBS) was obtained from Zhejiang Tianhang
Biological Technology Co., Ltd. (Hangzhou, China) and Dulbecco’s
modified Eagle’s medium (DMEM) was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). Primary antibodies against p53,
Bax and Bcl-2, as well as a secondary antibody, were purchased from
Wuhan Boster Bioengineering Co., Ltd. (Wuhan, China). A cytometric
bead array (CBA) assay kit and 3,3′-diaminobenzidine (DAB) kit were
purchased from the Beyotime Institute of Biotechnology (Shanghai,
China).
Preparation of primary myocardial
cells
Cardiac ventricles from the neonatal SD rats were
removed under sterile conditions and placed into pre-cooled
D-Hanks’ medium. Following cutting into sections and digestion with
0.125% trypsin, the cell suspension was filtered and centrifuged at
1,000 × g for 10 min. The cells were then resuspended in DMEM
containing 20% FBS and incubated in an atmosphere of 37°C and 5%
CO2 for 90 min to allow for cell adherence. Next, the
supernatant containing the inadherent cells was harvested and
cultured with 0.1 mmol/l bromodeoxyuridine for 24 h to suppress the
proliferation of non-myocardial cells. The medium was replaced with
serum-free DMEM and incubated in an incubator with saturated
humidity and 5% CO2 at 37°C. Following incubation for 24
h, the myocardial cells were prepared for the following stimulation
experiment.
Treatment
Prepared myocardial cells were divided into three
groups and treated with various stimuli. The control group was
cultured normally. The Ang II group was treated with 1 μg/ml Ang
II, while the Ang II + LIG group was subdivided into three
subgroups and treated with 1 μg/ml Ang II and various doses of LIG
(25, 50 and 100 μg/ml). Each group and subgroup was incubated in an
incubator with saturated humidity and 5% CO2 at 37°C for
one to three days. The cell surface area, total protein content,
apoptosis rate and the expression levels of p53, Bax and Bcl-2 of
the cultured cells were then detected.
Effects of LIG on the hypertrophy of
cardiomyocytes
Following incubation for one to three days with
various stimuli, the cells were observed under an inverted
microscope.
Detection of the myocardial cell surface
area
Following incubation for two days with various
stimuli, cells were stained with hematoxylin and eosin and observed
under an inverted microscope. The myocardial cell surface area was
detected using an Image-Pro Plus image analysis system. Five fields
were selected randomly for detection and between 10 and 15 cells in
each field were randomly selected and measured. Each group
measurements were performed in triplicate.
Determination of the cellular total
protein concentration
Following incubation for two days with various
stimuli, the cells were harvested and washed with
phosphate-buffered saline (PBS) three times. Cells were then lysed
with radioimmunoprecipitation assay buffer for 10 min and
centrifuged at 13,400 × g at 4°C for 3 min. The supernatant was
collected and the total protein content was determined with a CBA
determination assay kit.
Determination of the apoptotic rate
Following incubation for two days with various
stimuli, the cells were harvested and rinsed twice with PBS. The
cell concentration was adjusted to 1×105 cells/ml, and
100 μl cell suspension was mixed with Tris-HCl buffer (containing
1% RNase) and incubated for 10 min. Next, 5 μl annexin and 5 μl
propidium iodide (PI) were added to the cell suspension. Following
incubation at 37°C for 30 min in dark, the apoptotic rate was
detected using flow cytometry (FACS Calibur cytometer; BD
Biosciences, Franklin Lakes, NJ, USA).
Immunocytochemistry analysis
Following incubation for one day with various
stimuli, the cells cultured on the coverslips were fixed with 4%
paraformaldehyde for 30 min, which was followed by incubation with
3% H2O2 (H2O2:methanol,
1:50) at room temperature for 20 min to block endogenous
peroxidase. Following washing with distilled water and soaking with
PBS, the cells were incubated with 5% bovine serum albumin at 37°C
for 30 min. The cells were then incubated with anti-p53, anti-Bax
or anti-Bcl-2 antibodies at 4°C overnight. After washing with PBS
three times, the coverslips were incubated with a secondary
polymeric, peroxidase-labeled antibody for 1 h. The positive
signals were detected with a DAB kit and observed under a light
microscope.
Statistical analysis
Statistical analysis was conducted with three or
more groups using one-way analysis of variance and Dunnett’s test.
Data are expressed as the mean ± standard deviation and P<0.05
was considered to indicate a statistically significant difference.
SAS System for Elementary Statistical Analysis was provied by SAS
company (Cary, NC, USA).
Results
LIG restores Ang II-induced hypertrophy
of myocardial cells
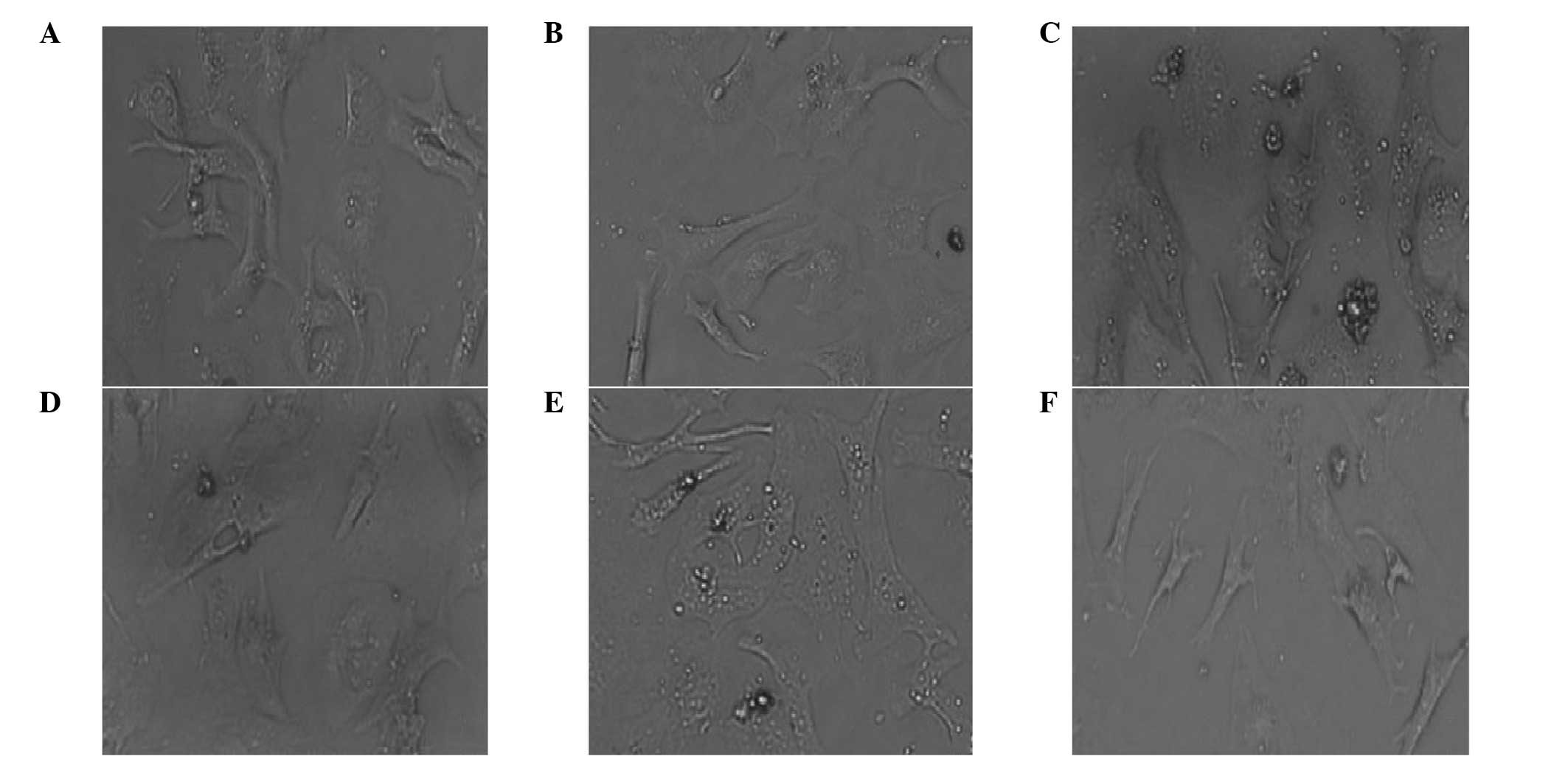
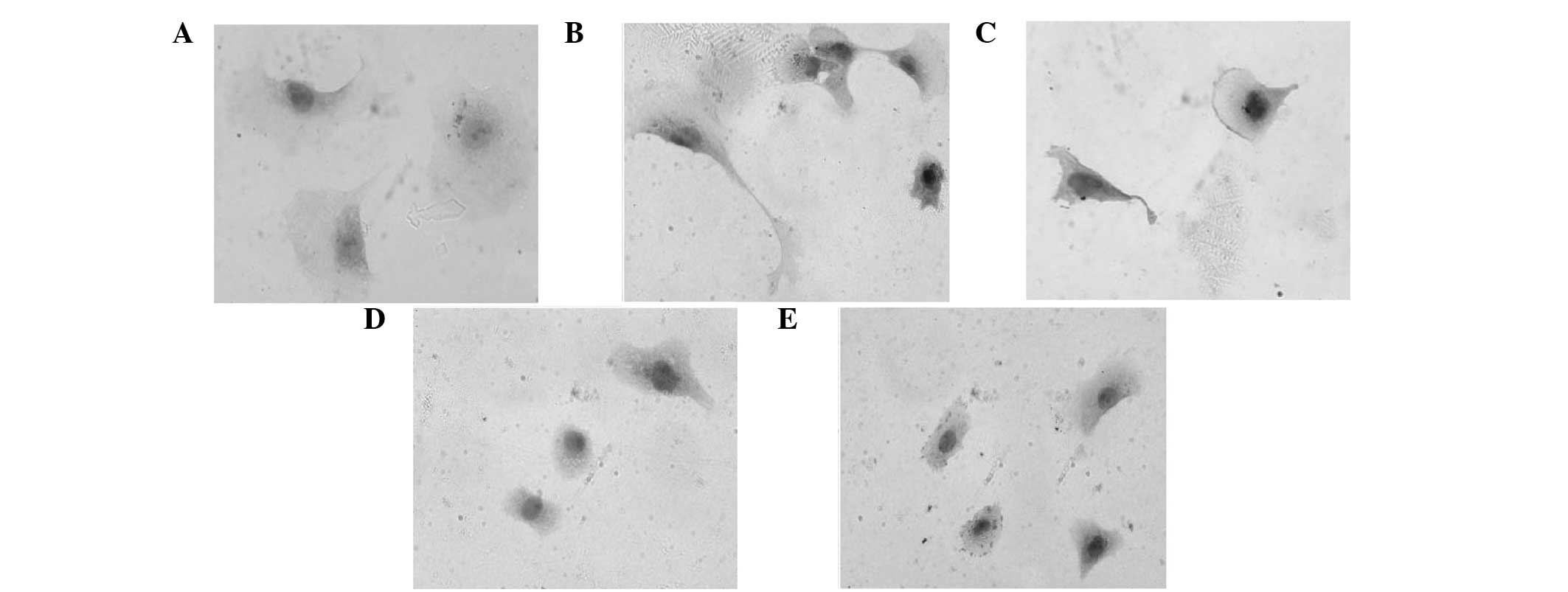
Myocardial cells isolated from neonatal SD rats were
cultured normally or treated with Ang II and/or LIG. Control
myocardial cells grew adherently and extended their pseudopodia
normally. Following two days of culture, the cells became
triangular or polygon-shaped and a few single cells even beat
spontaneously. Pseudopodia were further interwoven into a network
and gradually formed clusters or a monolayer, which appeared
radiate in concentric circles. Cells pulsed in synchronicity with
complete morphology and good vitality (Fig. 1A and B). However, the cells treated
with Ang II exhibited evident distortion and fusion, and the cell
surface area increased significantly, indicating that hypertrophy
occurred in the primary myocardial cells following treatment with
Ang II. Following incubation with Ang II for two or three days, the
cells became over-hypertrophic. No clear interval between the cells
was observed and the fine pseudopodia were integrated into the
intercellular space (Fig. 1C and
D). Cells also lost their spontaneous beating ability. However,
with regard to the cells treated with 1 μg/ml Ang II and 100 μg/ml
LIG for three days, the hypertrophic cells restored their original
state of normal myocardial cells (Fig.
1E and F).
LIG reduces the Ang II-induced protein
content increase in the myocardial cells in a dose-dependent
manner
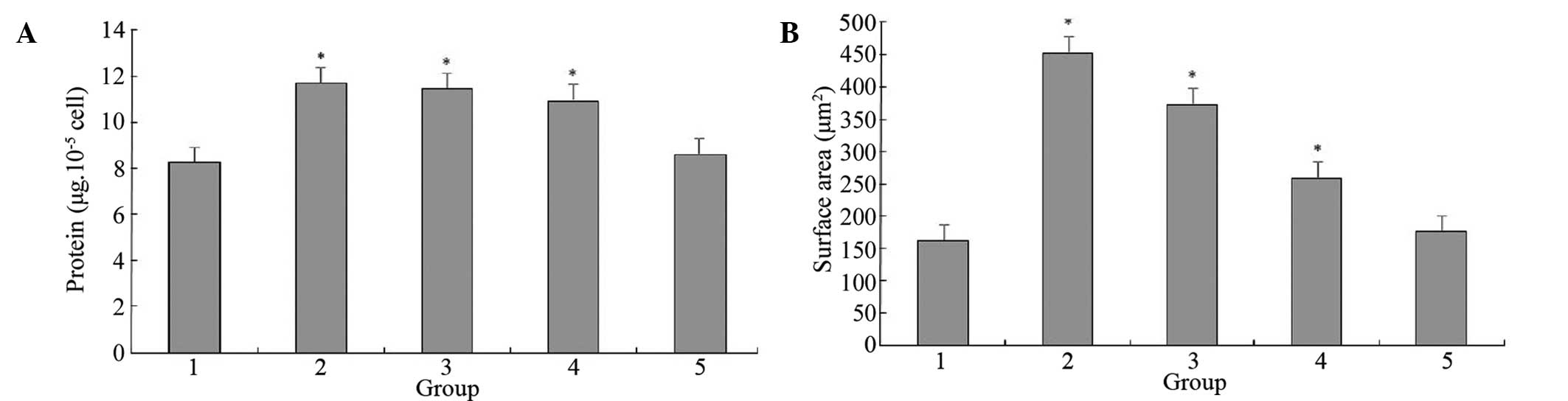
The total protein concentration was determined in
the myocardial cells of the various groups. The total protein
content of the cells treated with Ang II for two days increased
markedly, however, this increase was significantly reduced with the
administration of LIG. Various doses of LIG (25, 50 and 100 μg/ml)
exhibited different inhibitory effects on the hypertrophy and
protein increase induced by Ang II, with inhibitory rates of 18, 43
and 60% for hypertrophy (P<0.05) and 4, 11 and 27% for protein
increment (P<0.05), respectively (Fig. 2). The protein content and cell
surface area of the cells treated with Ang II and 100 μg/ml LIG
were not significantly different from those of the control group
(P>0.05), indicating that LIG can effectively inhibit the Ang
II-induced increase in protein synthesis and cell surface area in
myocardial cells.
LIG inhibits the Ang II-induced apoptosis
of myocardial cells
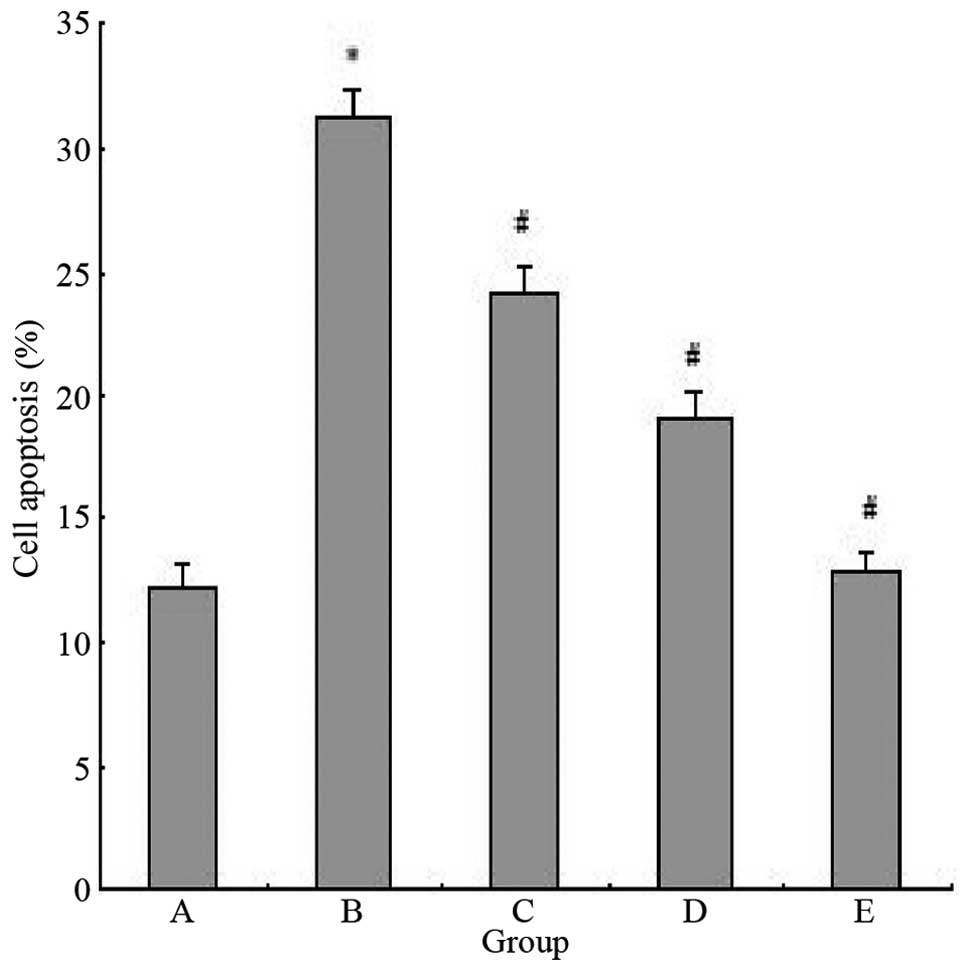
In order to determine whether LIG also affects the
Ang II-induced apoptosis of myocardial cells, the apoptotic rates
of cells treated with Ang II and/or various doses of LIG were
determined. The apoptotic rate of myocardial cells induced by Ang
II was markedly higher when compared with the control group
(P<0.05). However, the apoptotic rate was significantly reduced
with LIG administration (P<0.05; Fig. 3).
Effect of LIG on the expression levels of
the apoptosis-associated proteins, p53, Bcl-2 and Bax
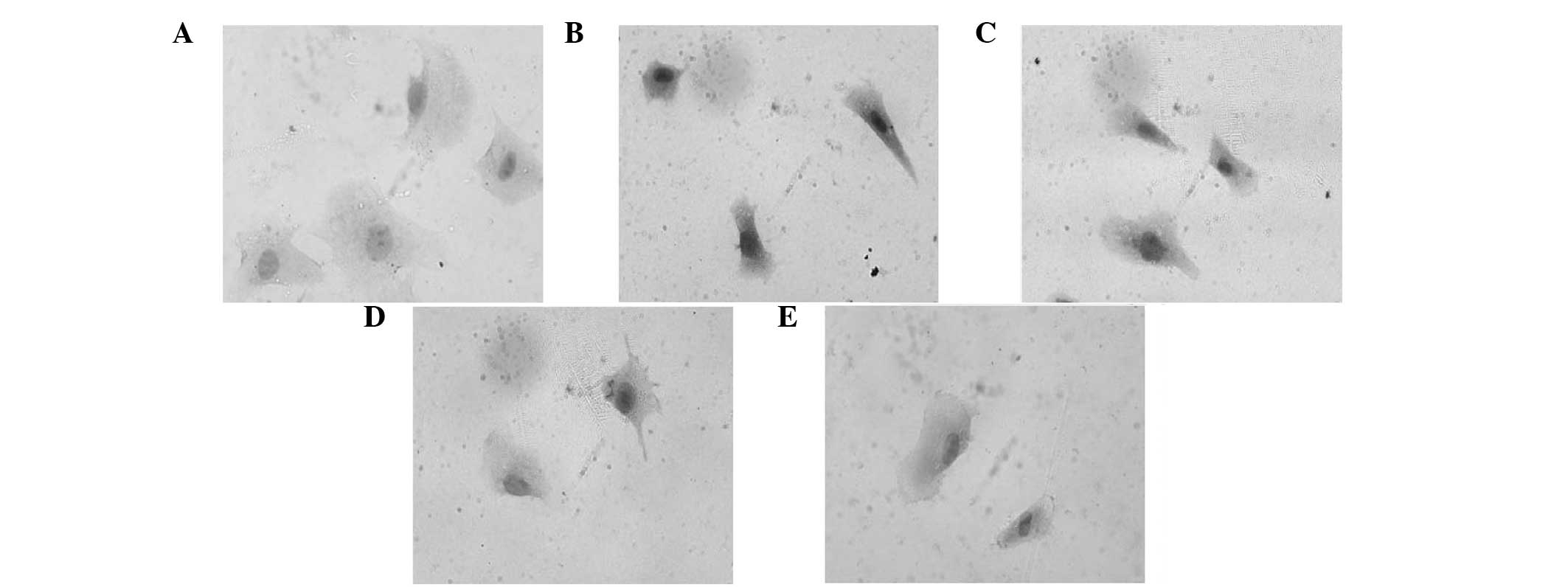
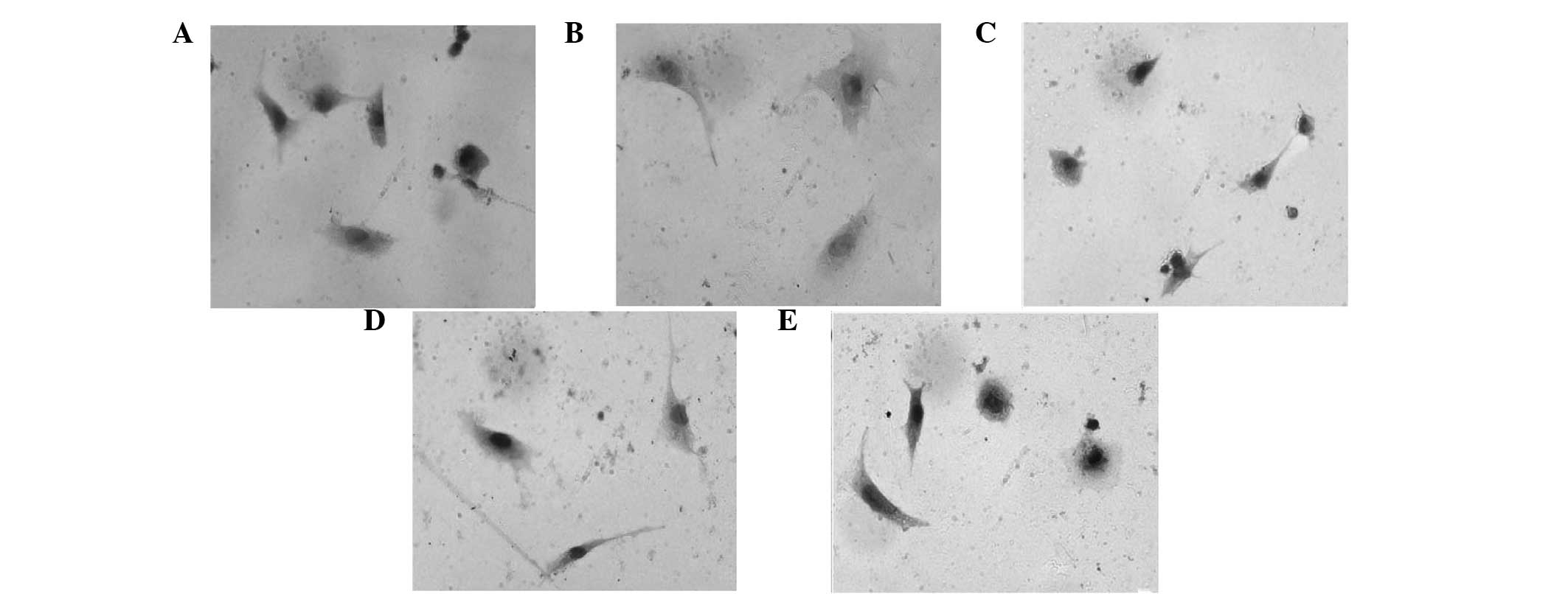
Expression levels of p53, Bcl-2 and Bax in the
primary rat myocardial cells were detected by immunocytochemistry
analysis. The expression of p53 and Bax in the myocardial cells was
markedly induced with Ang II treatment, which was observed as black
staining in the nuclei. However, the expression of these proteins
was inhibited with various doses of LIG, with administration of 100
μg/ml LIG restoring the expression levels of p53 and Bax almost to
the levels in the normal myocardial cells (Figs. 4 and 5). By contrast, the expression of Bcl-2
was significantly suppressed with Ang II treatment, although this
was restored by various doses of LIG. With increasing doses of LIG,
the expression levels of Bcl-2 gradually increased, as shown by the
black staining (Fig. 6).
Discussion
It is generally hypothesized that Ang II is a
classic inducer of cardiac hypertrophy. The results of the present
study demonstrated that the cell surface area and the total protein
content of the neonatal rat myocardial cells increased
significantly when the cells were incubated with Ang II for 48 h,
which was consistent with the results reported by previous studies
(9–12). In the current study, an
experimental model of cardiac hypertrophy induced by Ang II was
successfully established. The observations demonstrated that LIG
significantly inhibited the process of cardiac hypertrophy. In
addition, LIG inhibited the Ang II-induced increase in cell surface
area and protein concentration in myocardial cells in a
dose-dependent manner.
Previous studies have demonstrated that the
apoptosis and proliferation of myocardial cells occurs in
overload-induced ventricular hypertrophy (13,14).
Apoptosis, proliferation and hypertrophy are processes that are
closely associated with each other, since the developmental stages
of the ventricular and are changes in ventricular remodeling.
Apoptosis is an initiative cellular suicide process that is
regulated by a number of genes, the majority of which are
tumor-associated genes, including proto-oncogene and anti-oncogene.
Bcl-2, p53 and Bax genes are apoptosis-associated genes.
Overexpression of p53 can not only trigger the apoptotic process,
but can also inhibit cell cycle progression from the G1 phase to
the S phase (14,15). A low expression level of Bcl-2 and
a high expression level of Bax promotes the occurrence of apoptosis
(16–18).
In China, Suxiao Jiuxin pills and compound Danshen
(Salvia miltiorrhiza) tablets are widely administered for
the treatment of cardiovascular disease. These treatments
significantly improve the patient’s clinical symptoms, signs and
electrocardiograms. It has been reported that Danshen (19,20)
and Chuanxiong (Szechwan Lovage Rhizome) (21,22)
exhibit the characteristics of anti-ischemia, anti-hypoxia and
calcium channel blockers. These treatments have been shown to block
the L-type calcium current in the ventricular myocytes and
antagonize Ca2+ influx in myocardial cells (23). Previous studies have also
demonstrated that Danshen and Chuanxiong have an inhibitory effect
on cardiac hypertrophy, as they have been shown to inhibit the
formation of left ventricular hypertrophy in spontaneously
hypertensive rats (19–22). LIG is one of the active ingredients
in Danshen and Chuanxiong. In the present study, LIG was shown to
markedly reduce the cell surface area and protein concentration of
hypertrophic myocardial cells. The topic investigated the effects
of LIG on myocardial cells, the cell surface area, the
intracellular protein concentration, the rate of apoptosis and the
expression levels of p53, Bcl-2 and Bax were determined. The
results demonstrated that LIG reversed the Ang II-induced
hypertrophy in cardiomyocytes and restored the expression levels of
the apoptosis-associated proteins, p53, Bcl-2 and Bax, indicating
that LIG exhibits a significant preventive effect on cardiac
hypertrophy. These observations may be associated with the
inhibitory effects that LIG exhibits on the apoptosis of myocardial
cells. However, the specific underlying mechanism of LIG requires
further investigation.
Acknowledgements
The study was supported by a grant from the
Technological Bureau of Guangdong (no. 2010B031600292).
References
|
1
|
Yin J, Wang C, Mody A, et al: The effect
of Z-ligustilide on the mobility of human glioblastoma T98G gells.
PLoS One. 8:e665982013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang L, Du JR, Wang J, et al: Errata:
Z-ligustilide extracted from Radix Angelica Sinensis decreased
platelet aggregation induced by ADP ex vivo and arterio-venous
shunt thrombosis in vivo in rats. Yakugaku Zasshi. 134:4552014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng B, Zhao P, Lu YP, et al:
Z-ligustilide activates the Nrf2/HO-1 pathway and protects against
cerebral ischemia-reperfusion injury in vivo and in vitro. Brain
Res. 1520:168–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng Z, Lu Y, Wu X, et al: Ligustilide
alleviates brain damage and improves cognitive function in rats of
chronic cerebral hypoperfusion. J Ethnopharmacol. 144:313–321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang YH, Wei YQ, Rong R and Yuan JR:
Determination of ligustilide in SFE extrate of SWT with HPLC.
Shanghai J Tradit Chin Med. 38:47–49. 2004.
|
|
6
|
Hou YZ, Zhao GR, Yuan YJ, Zhu GG and
Hiltunen R: Inhibition of rat vascular smooth muscle cell
proliferation by extract of Ligusticum chuanxiong and Angelica
sinensis. J Ethnopharmacol. 100:140–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Q and Lou SH: Ligustilide regulates
vascular smooth muscle cells proliferation by MAPK signaling
pathway. Journal China Phamaceutical University. 26:632–634.
2010.
|
|
8
|
Lu Q, Qiu TQ and Yang H: Ligustilide
inhibits vascular smooth muscle cells proliferation. Eur J
Pharmacol. 542:136–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang L, Yang R, Wang M, et al:
Angiotensin II type-1 receptor-JAK/STAT pathway mediates the
induction of visfatin in angiotensin II-induced cardiomyocyte
hypertrophy. Am J Med Sci. 343:220–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou D, Liang Q, He X and Zhan C: Changes
of c-fos and c-jun mRNA expression in angiotensin II-induced
cardiomyocyte hypertrophy and effects of sodium tanshinone IIA
sulfonate. J Huazhong Univ Sci Technolog Med Sci. 28:531–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang T, Zhang W and Zhang L: L-Arginine-NO
pathway inhibits the hypertrophic response of cultured
cardiomyocytes induced by angiotensin II. Zhongguo Yao Li Xue Tong
Bao. 24:1361–1365. 2008.(In Chinese).
|
|
12
|
Liu J, Shen Q and Wu Y: Simvastatin
prevents cardiac hypertrophy in vitro and in vivo via JAK/STAT
pathway. Life Sci. 82:991–996. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim M, Hunter RW, Garcia-Menendez L, et
al: Mutation in the γ2-subunit of AMP-activated protein kinase
stimulates cardiomyocyte proliferation and hypertrophy independent
of glycogen storage. Circ Res. 114:966–975. 2014.
|
|
14
|
Tu S, Liu ZQ, Fu JJ, Zhu WF, Luo DY and
Wan FS: Inhibitory effect of p53 upregulated modulator of apoptosis
targeting siRNA on hypoxia/reoxygenation-induced cardiomyocyte
apoptosis in rats. Cardiology. 122:93–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vuong L, Brobst DE, Ivanovic I, Sherry DM
and Al-Ubaidi MR: p53 selectively regulates developmental apoptosis
of rod photoreceptors. PLoS One. 8:e673812013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma YX, Guo Z and Sun T: CGRP inhibits
norepinephrine induced apoptosis with restoration of Bcl-2/Bax in
cultured cardiomyocytes of rat. Neurosci Lett. 549:130–134. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu YH, Xiong J, Wang SS, Tang D, Wang RS
and Zhu Q: Calycosin entered HUVECs and ameliorated AGEs-promoted
cell apoptosis via the Bcl-2 pathway. J Nat Med. 68:163–172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng C, Ke Z, Song Y, et al: Annexin A3 is
associated with a poor prognosis in breast cancer and participates
in the modulation of apoptosis in vitro by affecting the Bcl-2/Bax
balance. Exp Mol Pathol. 95:23–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi K, Ouyang X, Komatsu K, et al:
Sodium tanshinone IIA sulfonate derived from Danshen (Salvia
miltiorrhiza) attenuates hypertrophy induced by angiotensin II
in cultured neonatal rat cardiac cells. Biochem Pharmacol.
64:745–749. 2002.PubMed/NCBI
|
|
20
|
Ouyang X, Takahashi K, Komatsu K, et al:
Protective effect of Salvia miltiorrhiza on angiotensin
II-induced hypertrophic responses in neonatal rat cardiac cells.
Jpn J Pharmacol. 87:289–296. 2001.
|
|
21
|
Zengyong Q, Jiangwei M and Huajin L:
Effect of Ligusticum wallichii aqueous extract on oxidative
injury and immunity activity in myocardial ischemic reperfusion
rats. Int J Mol Sci. 12:1991–2006. 2011.
|
|
22
|
Yu L, She T, Li M, Shi C, Han L and Cheng
M: Tetramethylpyrazine inhibits angiotensin II-induced
cardiomyocyte hypertrophy and tumor necrosis factor-α secretion
through an NF-κB-dependent mechanism. Int J Mol Med. 32:717–722.
2013.PubMed/NCBI
|
|
23
|
Roe ND and Ren J: Oxidative activation of
Ca(2+)/calmodulin-activated kinase II mediates ER stress-induced
cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol.
304:H828–H839. 2013.
|