Introduction
The neutron capture reaction in boron
[10B(n,α)7Li] is, in principle, very
effective in destroying tumors, provided that a sufficient amount
of 10B is accumulated in the target tumor and a
sufficient number of very-low-energy thermal neutrons are
delivered. The two particles generated in this reaction have a high
linear energy transfer (LET) and have a range of ~1–2 tumor cells
in diameter. It is theoretically possible to destroy tumor cells
without affecting adjacent healthy cells if 10B atoms
are selectively accumulated in the interstitial space of tumor
tissue and/or intracellular space of tumor cells. Thus, successful
boron neutron capture therapy (BNCT) requires the selective
delivery of large amounts of 10B to tumor cells
(1).
The two most common 10B-carriers used in
clinical BNCT trials, designed for the treatment of malignant
gliomas, melanomas, inoperable head and neck tumors, and oral
cancer, are sodium mercaptoundecahydrododecaborate-10B
(BSH, Na210B12H11SH)
and L-para-boronophenylalanine-10B (BPA,
C9H1210BNO4) (1). The delivery of 10B via BSH
relies on passive diffusion from the blood to the brain tumor
through a disrupted blood-brain barrier (2). Thus, the use of BSH results in a high
concentration of boron in the blood and subsequent vascular damage
during BNCT (3). BPA is designed
to be taken up mainly by active transport across the cancer cell
membrane (4). However, the
transport mechanism is operative even in normal cells leading to
the accumulation of BPA in normal brain tissues, although at a
lower rate. Therefore, it has been suggested that tumor response
may be improved by combining BSH and BPA (5).
Antiangiogenic therapy has been hypothesized to
prevent vascular tumor growth and proliferation, thus depriving the
tumor of the oxygen and nutrients necessary for survival (6). However, a subsequent study suggested
that antiangiogenic therapy may also ‘normalize’ the tumor
vasculature for a short period of time, thereby providing a window
of opportunity for improved drug delivery and enhanced sensitivity
to radiation (6,7). Tumor hypoxia results from either
limited oxygen diffusion (chronic hypoxia) or limited perfusion
(acute hypoxia) (8). Previous
studies have reported that acute and cyclic, but not chronic,
hypoxia significantly increased the number of spontaneous lung
metastases and that this effect was due to the influence of acute
hypoxia treatment on the primary tumor (9,10).
In the present study, the efficiency of
administering the vascular endothelial growth factor (VEGF)
inhibitor, bevacizumab, in combination with BNCT, and further
combined with the acute hypoxia-releasing agent, nicotinamide, or
mild temperature hyperthermia (MTH) was evaluated. MTH has already
demonstrated the potential to release tumor cells from
diffusion-limited chronic hypoxia (11,12)
in terms of local tumor response and lung metastasis. In addition,
with regards to the local tumor response, the effect not only on
the total tumor cell population [proliferating (P) + quiescent
(Q)], but also on the Q cell population alone, was evaluated using
an original method for selectively detecting the response of Q
cells in solid tumors (13).
Materials and methods
Mice and tumors
B16-BL6 murine melanoma cells (Institute of
Development, Aging and Cancer, Tohoku University, Sendai, Japan)
derived from C57BL/6 mice were maintained in vitro in
RPMI-1640 medium supplemented with 10% fetal bovine serum. Tumor
cells (1.25×105) were inoculated subcutaneously into the
left hind leg of 8-week-old syngeneic female C57BL/6 mice (Japan
Animal Co., Ltd., Osaka, Japan). Eighteen days later, the tumors,
~7 mm in diameter, were employed for treatment. The body weight of
the tumor-bearing mice was 20.1±2.3 g (mean ± standard error). The
mice were handled according to the Recommendations for Handling of
Laboratory Animals for Biomedical Research, compiled by the
Committee on Safety Handling Regulations for Laboratory Animal
Experiments at Tohoku University. The p53 of the B16-BL6 tumor
cells was the wild-type (14).
Labeling with bromodeoxyuridine
(BrdU)
Twelve days following inoculation, mini-osmotic
pumps (Durect Corporation, Cupertino, CA, USA) containing BrdU
dissolved in physiological saline (250 mg/ml) were implanted
subcutaneously into the backs of the animals for six days in order
to label all P cells. The percentage of labeled cells following the
continuous treatment with BrdU reached a plateau at this stage.
Therefore, tumor cells not incorporating BrdU following continuous
labeling were regarded as Q cells.
Treatment
Fifteen days following the tumor cell inoculation,
bevacizumab (a humanized monoclonal antibody against VEGF;
Hoffmann-La Roche AG, Basel, Switzerland) dissolved in
physiological saline was intravenously administered through a tail
vein, at a dose of 10 mg/kg in a single injection. Bevacizumab has
previously been demonstrated to induce a period of vascular
normalization in B16-F10 murine melanoma tumors originating from
B16-F1 murine melanoma cells (15). Thus, in B16-BL6 tumors also
originating from B16-F1 murine melanoma cells, it was hypothesized
that bevacizumab would exhibit the same effect as in B16-F10
tumors. After three days (18 days following inoculation, on day
18), the percentages of labeled cells following the continuous
administration of BrdU for six days were 60.1±6.8 and 54.3±6.1% for
those treated with bevacizumab and those not, respectively.
BSH and BPA were purchased from KatChem Ltd.
(Prague, Czech Republic), and prepared by dissolving in
physiological saline for BSH (125 mg/kg) and as a complex with 3%
fructose for BPA (250 mg/kg). They were injected intraperitoneally
in a volume of 0.02 ml/g of mouse body weight. In accordance with
previous studies (16), no overt
toxicity was observed at a dose of <500 mg/kg for BSH and
<1,500 mg/kg for BPA. Based on the certificate of analysis and
material safety data sheet, provided by the manufacturer,
borocaptate dimer (BSSB;
10B24H22S24−)
was not present as a contaminant. The intratumor 10B
concentration during neutron irradiation is a crucial determinant
of the cell destruction effect of BNCT. In order to obtain similar
intratumor 10B concentrations during exposure to the
neutron beam, irradiation was initiated at selected time points
following the intraperitoneal injection of the
10B-carriers at a selected dose of 10B. Based
on a preliminary study of the biodistribution of 10B,
irradiation was initiated from 60 min following intraperitoneal
injection of 125 and 250 mg/kg (71.0 and 12.0 mg 10B/kg)
of BSH and BPA, respectively. 10B concentrations were
determined using a thermal neutron guide tube installed at the
Kyoto University Research Reactor (Osaka, Japan) (17).
Certain tumor-bearing mice also received an
intraperitoneal administration of nicotinamide (1,000 mg/kg)
dissolved in physiological saline 1 h prior to neutron irradiation.
Others were subjected to local MTH at 40°C for 1 h by immersing the
implanted tumor in a water bath prior to irradiation (18). Temperatures in the center of the
tumors equilibrated within 3–4 min following immersion in the water
bath and remained at 0.2–0.3°C below the temperature of the bath.
The water temperature in the bath was maintained at 0.3°C above the
desired tumor temperature (18).
For irradiation of the tumors implanted into the
left hind legs of the mice, a device composed of acrylic resin and
capable of holding 12 mice was used. The tumor-bearing mice were
irradiated with a reactor neutron beam at a power of 1 MW at Kyoto
University Research Reactor after being fixed in position with
adhesive tape. A lithium fluoride (LiF) thermoplastic shield was
employed to avoid irradiating body parts other than the implanted
solid tumors. Neutron irradiation was performed using a reactor
neutron beam with a cadmium ratio of 9.4. The neutron fluence was
measured from the radioactivation of gold foil at both the front
and back of the tumors. Since the tumors were small and located
just beneath the skin surface, the neutron fluence was assumed to
decrease linearly from the front to the back of the tumors. Thus,
the average neutron fluence, determined from the values measured at
the front and back of the tumor, was used. Contaminating γ-ray,
including secondary γ-ray, doses were measured with a
thermoluminescence dosimeter (TLD) powder placed at the back of the
tumors. The TLD used was beryllium oxide (BeO) enclosed in a quartz
glass capsule (Panasonic Corporation, Osaka, Japan). The BeO itself
was not sensitive to thermal neutrons. The thermal neutron fluence,
of 8×1012/cm2, was equal to ~1 cGy γ-ray
dose. TLD was normally used together with the gold activation foil
for the neutron-sensitivity correction in the current study. The
details were described in the study by Sakurai and Kobayashi
(19). For the estimation of
neutron energy spectra, eight types of activation foil and 14 types
of nuclear reaction were used (19). The absorbed dose was calculated
using the flux-to-dose conversion factor (20). The tumors contained H (10.7% in
terms of weight), C (12.1%), N (2%), O (71.4%), and others (3.8%)
(21). The average neutron flux
and kerma rate of the employed beam were 1.0×109
n/cm/sec and 48.0 cGy/h for the thermal neutron range (<0.6 eV),
1.6×108 n/cm/sec and 4.6 cGy/h for the epithermal
neutron range (0.6–10 keV), and 9.4×106 n/cm/sec and
32.0 cGy/h for the fast neutron range (>10 keV), respectively.
The kerma rate for boron dose per φ n/cm/s of thermal neutron flux
for 1 μg/g of 10B was 2.67×10–8φ cGy/h. The
contaminating γ-ray dose rate was 66.0 cGy/h.
Each irradiation group also included mice that were
not pre-treated with BrdU.
Immunofluorescence staining of
BrdU-labeled cells and micronucleus (MN) assays
Immediately following irradiation, selected tumors
were removed from the mice given BrdU. They were homogenized and
trypsinized using 0.05% trypsin and 0.02%
ethylenediaminetetraacetic acid (EDTA) in phosphate-buffered saline
(PBS) at 37°C for 15 min. Tumor cell suspensions were incubated for
72 h in tissue culture dishes containing complete medium and 1.0
μg/ml of cytochalasin B in order to inhibit cytokinesis while
allowing nuclear division. The cultures were trypsinized and cell
suspensions were fixed and resuspended with cold Carnoy’s fixative
(ethanol:acetic acid, 3:1 by volume). Each suspension was placed on
a glass microscope slide, dried at room temperature and treated
with 2 M hydrochloric acid for 60 min at room temperature in order
to dissociate the histones and partially denature the DNA. The
slides were immersed in borax-borate buffer (pH 8.5) to neutralize
the acid. BrdU-labeled tumor cells were detected by indirect
immunofluorescence staining using a monoclonal anti-BrdU antibody
(BD Biosciences, San Jose, CA, USA) and a fluorescein
isothiocyanate (FITC)-conjugated antimouse IgG antibody (Sigma, St.
Louis, MO, USA). To distinguish the tumor cells stained with
green-emitting FITC and observe them separately, cells on the
slides were treated with red-emitting propidium iodide (PI; 2 μg/ml
in PBS) as a background stain and monitored under a fluorescence
microscope (Olympus Bioimaging, Tokyo, Japan).
When cell division is disrupted, or the chromosomes
are broken or damaged by chemicals or radiation, the distribution
of genetic material between the two daughter nuclei during cell
division is affected and pieces or entire chromosomes fail to be
included in either of the two daughter nuclei. The genetic material
that is not incorporated into a new nucleus forms a ‘MN’. Thus, the
frequency of MN formation accurately reflects the genotoxicity of a
chemical compound and radiation. The MN frequency in cells not
labeled with BrdU was examined by counting the micronuclei in the
binuclear cells that showed only red fluorescence. The MN frequency
was defined as the ratio of the number of micronuclei in the
binuclear cells to the total number of binuclear cells observed
(13).
The ratios obtained from the tumors not pretreated
with BrdU indicated the MN frequencies at all phases in the total
tumor cell population. More than 300 binuclear cells were counted
to determine the MN frequency.
Clonogenic cell survival assay
Immediately following irradiation, a clonogenic cell
survival assay was performed on the implanted tumors in mice that
were not given BrdU using an in vivo-in vitro assay method.
The BrdU-unlabeled tumors were removed, weighed, homogenized and
disaggregated by stirring for 20 min at 37°C in PBS containing 0.05
% trypsin and 0.02% EDTA. The cell yield was
1.2±0.4×107/g tumor weight. Appropriate numbers of
viable tumor cells from the single cell suspension were plated on
60- or 100-mm tissue culture dishes and 12 days later, colonies
were fixed with ethanol, stained with Giemsa and counted. For the
tumors that received no irradiation, plating efficiencies for the
total tumor cell populations and the MN frequencies for the total
and Q cell populations are displayed in Table I. The plating efficiency indicates
the percentage of cells seeded that grew into colonies when the
tumors received no irradiation. The fraction of cells surviving a
given dose was determined by counting the number of macroscopic
colonies as a fraction of the total number of cells seeded,
followed by allowance, that is, by dividing by the plating
efficiency.
 | Table IPlating efficiency and micronucleus
frequency at 0 Gy. |
Table I
Plating efficiency and micronucleus
frequency at 0 Gy.
| Variable | Without
nicotinamide or MTH | With
nicotinamide | With MTH |
|---|
| Plating efficiency
(%) |
| Without
bevacizumab |
| Without
10B-carrier | 84.4±8.2 | 81.4±7.3 | 83.5±8.7 |
| With
BPAc | 76.9±7.7 | 69.9±6.5 | 73.9±7.3 |
| With
BSHd | 81.4±8.3 | 74.9±6.3 | 78.9±6.8 |
| With
bevacizumab | | | |
| Without
10B-carrier | 80.1±8.0 | 76.3±7.1 | 78.4±8.3 |
| With BPA | 69.6±7.1 | 64.8±6.2 | 68.8±7.0 |
| With BSH | 74.1±7.7 | 69.8±7.2 | 73.8±7.1 |
| Micronucleus
frequency |
| Without
bevacizumab |
| Total cell
population |
| Without
10B-carrier | 0.050±0.008 | 0.057±0.006 | 0.054±0.005 |
| With BPA | 0.063±0.008 | 0.081±0.008 | 0.077±0.007 |
| With BSH | 0.059±0.008 | 0.078±0.009 | 0.074±0.008 |
| Quiescent cell
population |
| Without
10B-carrier | 0.077±0.008 | 0.084±0.009 | 0.081±0.009 |
| With BPA | 0.091±0.009 | 0.110±0.011 | 0.105±0.010 |
| With BSH | 0.095±0.009 | 0.120±0.011 | 0.115±0.011 |
| With
bevacizumab |
| Total cell
population |
| Without
10B-carrier | 0.058±0.008 | 0.068±0.007 | 0.069±0.007 |
| With BPA | 0.071±0.008 | 0.092±0.009 | 0.092±0.009 |
| With BSH | 0.067±0.008 | 0.089±0.009 | 0.089±0.009 |
| Quiescent cell
population |
| Without
10B-carrier | 0.083±0.008 | 0.086±0.009 | 0.087±0.009 |
| With BPA | 0.097±0.009 | 0.112±0.011 | 0.111±0.011 |
| With BSH | 0.101±0.009 | 0.122±0.011 | 0.121±0.011 |
As stated above, the MN frequencies for Q cells were
obtained from BrdU-unlabeled cells in tumors following continuous
BrdU labeling in vivo. The MN frequencies and surviving
fractions (SFs) for the total tumor cell populations were obtained
from cells in tumors not pretreated with BrdU. Thus, the present
study was not able to detect any interaction between BrdU and
irradiation in the data for the MN frequencies and SFs.
Metastasis assessment
Seventeen days following irradiation (35 days
following the inoculation of B16-BL6 melanoma cells), the
tumor-bearing mice were sacrificed by cervical dislocation. Their
lungs were removed, briefly washed with distilled water, cleaned of
extraneous tissue, fixed overnight in Bouin’s solution (Sigma), and
stored in buffered formalin 10% (Sigma) until metastases were
counted. Macroscopically visible metastases were counted using a
dissection microscope (22).
Eighteen days following inoculation and immediately prior to
exposure to the neutron beam, macroscopic lung metastases were also
counted as background data; the number was 7.5±2.2.
Data analysis and statistics
Three mice with a tumor in the left hind leg were
used to assess each set of conditions and each experiment was
repeated three times. Thus, in total, nine mice were used for each
set of conditions. To examine the differences between pairs of
values, the Student’s t-test was used when variances of the two
groups were assumed to be equal; otherwise the Welch’s t-test was
used. P-values were obtained from two-sided tests. P<0.05 was
considered to indicate a statistically significant difference. The
data on cell survival and MN frequencies were fitted to the
linear-quadratic dose relationship (23).
Results
Toxicity of 10B-carriers and
bevacizumab
Table I shows the
plating efficiencies for the total tumor cell population and the MN
frequencies without irradiation for the total and Q cell
populations. The Q cell population revealed significantly higher MN
frequencies than the total cell population under each set of
conditions (P<0.05). The combination with bevacizumab increased
the sensitivity of the total cells more than that of the Q cells,
especially when BPA was employed compared with BSH or no
10B-carrier. The difference, however, was not
significant.
10B concentrations and
10B dose rate during irradiation
Table II shows the
10B concentrations and boron dose rates in irradiated
tumors for each set of conditions. The values are averages obtained
using the 10B concentrations at the start and end points
of the irradiation time. The combination with bevacizumab increased
the concentration, especially when BPA was employed compared with
BSH. When BPA was used as a 10B-carrier, MTH increased
the concentration more than nicotinamide. By contrast, with BSH as
the 10B-carrier, nicotinamide increased the
concentration more than MTH. However, again, these differences were
not significant.
 | Table II10B concentration
(μg/g=ppm) in tumors and boron dose rate (cGy/h). |
Table II
10B concentration
(μg/g=ppm) in tumors and boron dose rate (cGy/h).
| Variable | Without
nicotinamide or MTH | With
nicotinamide | With MTH |
|---|
| 10B
concentration (μg/g) |
| Without
bevacizumab |
| BPA | 6.9±0.8 | 7.3±1.0 | 8.3±1.1 |
| BSH | 7.2±0.9 | 8.4±1.1 | 7.9±1.0 |
| With
bevacizumab |
| BPA | 7.2±0.9 | 7.4±1.1 | 8.6±1.2 |
| BSH | 8.0±1.0 | 8.6±1.2 | 8.7±1.2 |
| Boron dose rate
(cGy/h) |
| Without
bevacizumab |
| BPA | 184.2±21.4 | 194.9±26.7 | 221.6±29.4 |
| BSH | 192.2±24.0 | 224.3±29.4 | 210.9±26.7 |
| With
bevacizumab |
| BPA | 192.2±24.0 | 197.6±29.4 | 229.6±32.0 |
| BSH | 212.7±26.7 | 229.6±32.0 | 231.3±32.0 |
Initial tumor response
Fig. 1 shows cell
survival curves for the total cell population as a function of the
absorbed dose of neutron beam irradiation with or without a
10B-carrier, in combination with nicotinamide or MTH,
and in the presence or absence of bevacizumab. Fig. 2 shows net MN frequencies as a
function of irradiated absorbed dose with or without a
10B-carrier, in combination with nicotinamide or MTH,
and in the presence or absence of bevacizumab in the total and Q
tumor cell populations. The net MN frequency was the MN frequency
in tumors that received irradiation minus the MN frequency in
tumors that did not. Overall, the net MN frequencies were
significantly smaller in Q cells than in the total cell population
(P<0.05).
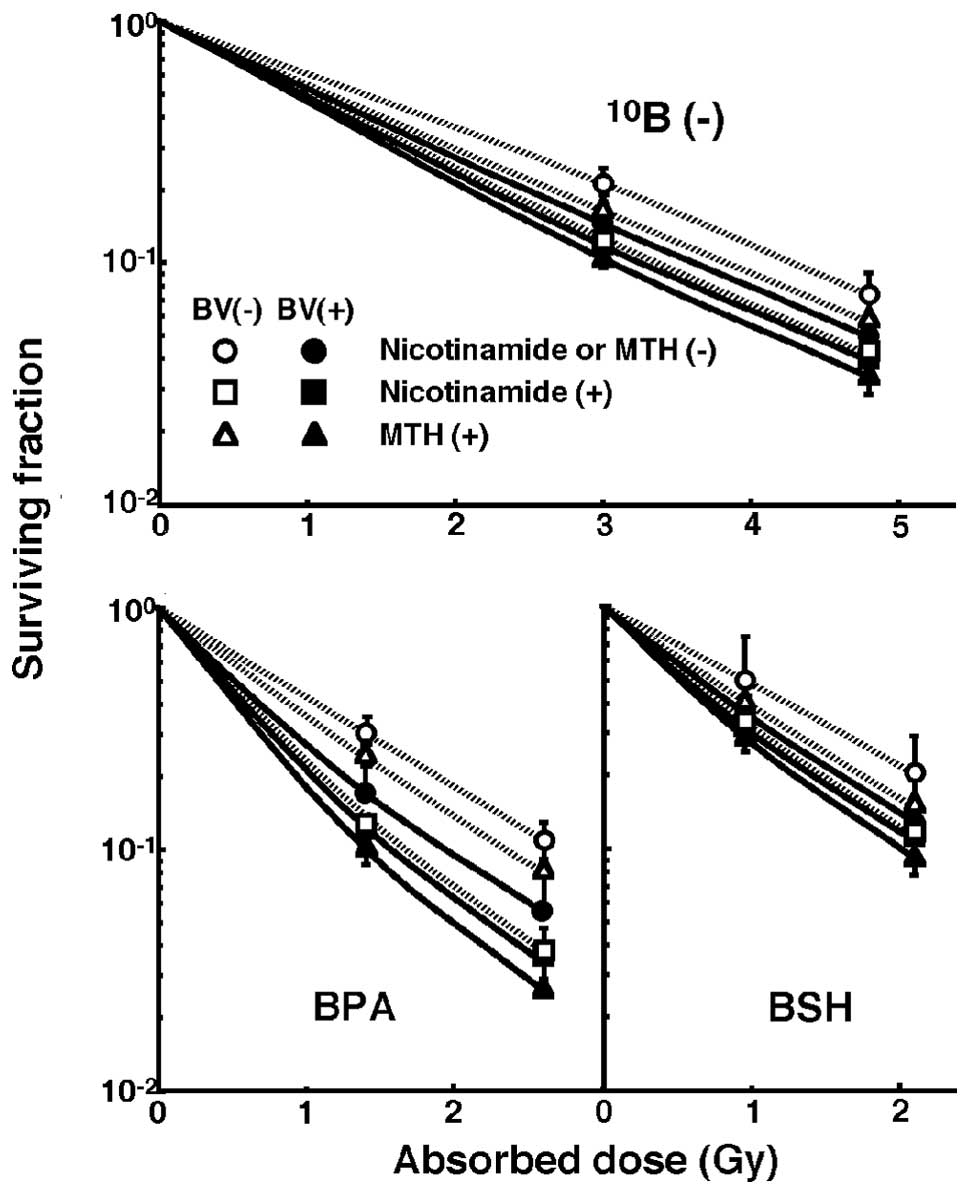 | Figure 1Cell survival curves for the total
cell population from B16-BL6 tumors irradiated with reactor neutron
beams following the administration of a 10B-carrier in
combination with nicotinamide treatment, mild temperature
hyperthermia (MTH), or bevacizumab treatment on day 18 following
tumor cell inoculation. Open and solid symbols represent
irradiation without and with bevacizumab, respectively. Circle,
square, and triangle symbols represent irradiation without
nicotinamide or MTH, with nicotinamide, and with MTH, respectively.
Bars represent standard errors (n=9). 10B (−), no
10B-carrier; BPA,
L-para-boronophenylalanine-10B; BSH, sodium
mercaptoundecahydrododecaborate-10B; MTH, mild
temperature hyperthermia; BV, bevacizumab. |
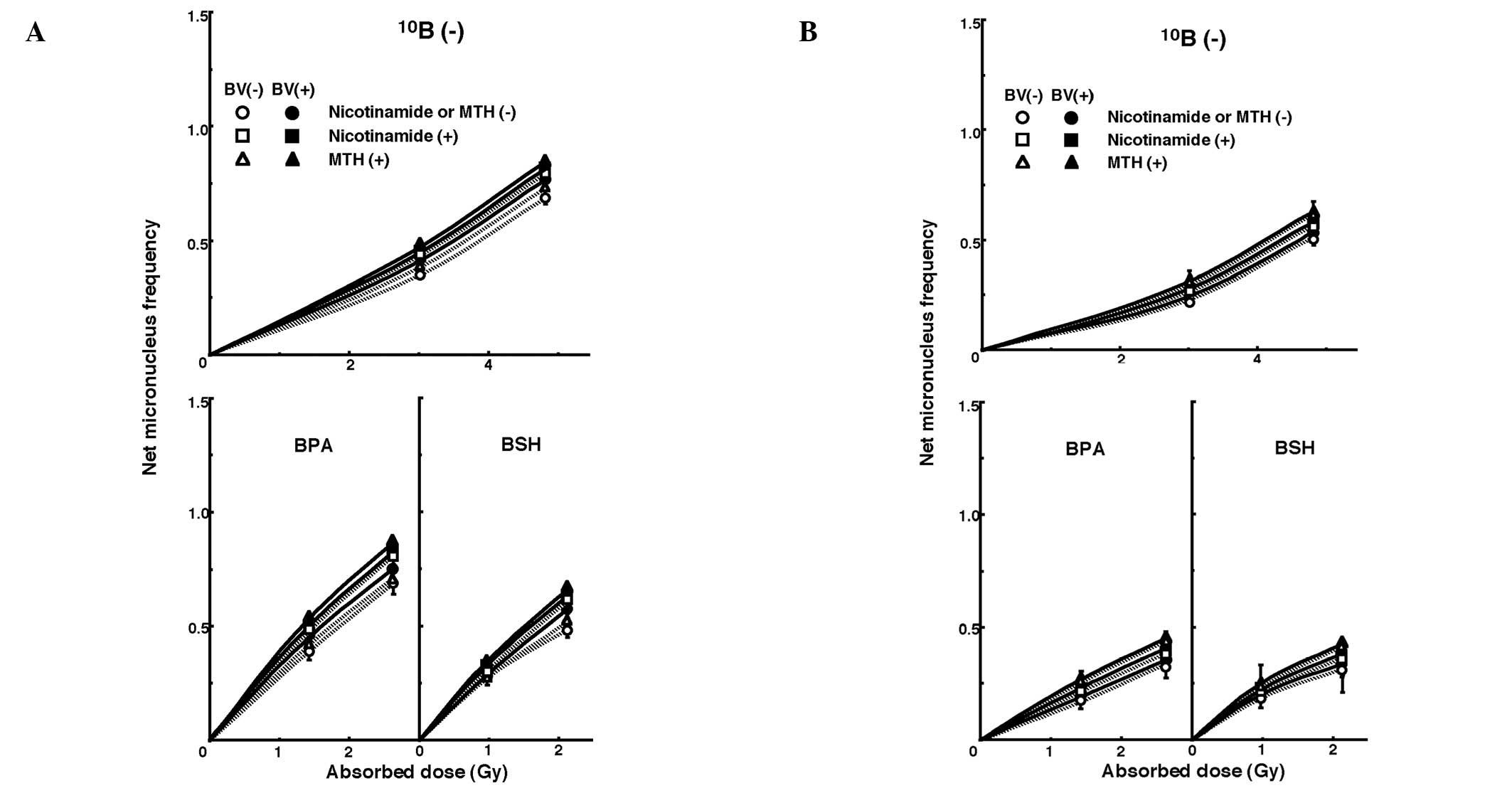 | Figure 2Dose response curves of the net
micronucleus frequency for (A) total and (B) quiescent cell
populations from B16-BL6 tumors irradiated with reactor neutron
beams following the administration of a 10B-carrier in
combination with nicotinamide treatment, mild temperature
hyperthermia (MTH) or bevacizumab treatment on day 18 following
tumor cell inoculation. Open and solid symbols represent
irradiation without and with bevacizumab, respectively. Circle,
square, and triangle symbols represent irradiation without
nicotinamide or MTH, with nicotinamide, and with MTH, respectively.
Bars represent standard errors (n=9). 10B (−), no
10B-carrier; BPA,
L-para-boronophenylalanine-10B; BSH, sodium
mercaptoundecahydrododecaborate-10B; MTH, mild
temperature hyperthermia; BV, bevacizumab. |
Lung metastases from local tumors. To estimate the
radio-enhancing effect of the 10B-carriers, irradiation
with BPA and BSH in both the total and Q cell populations was
compared with neutron beam irradiation only, using the data
obtained without nicotinamide or MTH shown in Figs. 1 and 2 (Table
III). Both BPA and BSH enhanced the sensitivity of the total
cell population significantly more than the Q cell population
(P<0.05). Furthermore, BPA demonstrated a tendency to affect the
sensitivity of the total cell population more than BSH. By
contrast, the sensitivity of the Q cells was enhanced more by BSH
than BPA. When bevacizumab was combined, the sensitivity of the
total cells was enhanced more than that of the Q cells, especially
when BPA was used compared with BSH. However, these differences
were not statistically significant.
 | Table IIIEnhancement ratiosa due to combination with a
10B-carrier. |
Table III
Enhancement ratiosa due to combination with a
10B-carrier.
|
10B-carrier |
|---|
|
|
|---|
| Variable | BPA | BSH |
|---|
| Surviving fraction
= 0.3 |
| Total cell
population |
| Without
bevacizumab | 1.7±0.1b,c | 1.5±0.1c |
| With
bevacizumab | 2.0±0.15b,d | 1.65±0.15d |
| Net micronucleus
frequency = 0.3 |
| Total cell
population |
| Without
bevacizumab | 2.45±0.15 | 2.25±0.15 |
| With
bevacizumab | 2.6±0.2 | 2.35±0.15 |
| Quiescent
cells |
| Without
bevacizumab | 1.5±0.1e | 1.8±0.1e |
| With
bevacizumab | 1.6±0.15 | 1.85±0.15 |
The data in Figs. 1
and 2 were used to estimate the
radio-enhancing effect of combined treatment with bevacizumab in
both the total and Q cell populations (Table IV). With or without a
10B-carrier, the sensitivity of the total cell
population was enhanced when bevacizumab was administered,
especially when BPA and/or MTH were combined. When nicotinamide and
MTH were added, sensitivity was more suppressed and enhanced,
respectively, in the total cells than in the Q cells. However,
again, the differences were not significant.
 | Table IVEnhancement ratiosa due to combined treatment with
bevacizumab. |
Table IV
Enhancement ratiosa due to combined treatment with
bevacizumab.
| Variable | Without
nicotinamide or MTH | With
nicotinamide | With MTH |
|---|
| Surviving fraction
= 0.3 |
| Total cell
population |
| Without
10B-carrier | 1.25±0.1 | 1.05±0.1 | 1.3±0.1 |
| With BPA | 1.55±0.2 | 1.05±0.1 | 1.7±0.15 |
| With BSH | 1.35±0.1 | 1.05±0.15 | 1.35±0.15 |
| Net micronucleus
frequency = 0.3 |
| Total cell
population |
| Without
10B-carrier | 1.1±0.1 | 1.05±0.1 | 1.2±0.1 |
| With BPA | 1.2±0.2 | 1.05±0.1 | 1.3±0.15 |
| With BSH | 1.15±0.1 | 1.05±0.1 | 1.25±0.15 |
| Quiesent cell
population |
| Without
10B-carrier | 1.05±0.1 | 1.05±0.1 | 1.05±0.1 |
| With BPA | 1.05±0.2 | 1.05±0.1 | 1.1±0.1 |
| With BSH | 1.1±0.1 | 1.05±0.1 | 1.2±0.1 |
The data in Figs. 1
and 2 were also used to estimate
the radio-enhancing effect of combined treatment with nicotinamide
or MTH in the total and Q cell populations (Table V). With BSH and without a
10B-carrier, the sensitivity of the total cell
population was more enhanced with nicotinamide than with MTH. With
BPA, the sensitivity of the total cell population was more enhanced
with MTH than with nicotinamide. By contrast, the sensitivity of
the Q cell population was more enhanced with MTH than with
nicotinamide. Notably, with BPA or BSH as the
10B-carrier, MTH enhanced the sensitivity of the Q cell
populations significantly more than the total cell populations
(P<0.05). When bevacizumab was used, the enhancing effect of
nicotinamide was suppressed, especially in the total cell
population, although not significantly. However, the combination
with bevacizumab revealed no significant effect on the enhancing
effect of MTH.
 | Table VEnhancement ratiosa due to combined treatment with
nicotinamide or mild temperature hyperthermia. |
Table V
Enhancement ratiosa due to combined treatment with
nicotinamide or mild temperature hyperthermia.
| Variable | Nicotinamide | MTH |
|---|
| Surviving fraction
= 0.3 |
| Total cell
population |
| Without
bevacizumab |
| Without
10B-carrier | 1.3±0.15 | 1.2±0.1 |
| With BPAc | 1.25±0.1 | 1.4±0.15 |
| With BSHd | 1.5±0.15 | 1.2±0.1 |
| With
bevacizumab |
| Without
10B-carrier | 1.2±0.1 | 1.1±0.1 |
| With BPA | 1.15±0.1 | 1.35±0.15 |
| With BSH | 1.3±0.15 | 1.15±0.1 |
| Net micronucleus
frequency = 0.3 |
| Total cell
population |
| Without
bevacizumab |
| Without
10B-carrier | 1.25±0.1 | 1.15±0.1 |
| With BPA | 1.15±0.1 | 1.3±0.1 |
| With BSH | 1.4±0.1 | 1.2±0.1b |
| With
bevacizumab |
| Without
10B-carrier | 1.1±0.1 | 1.15±0.1 |
| With BPA | 1.1±0.1 | 1.2±0.1 |
| With BSH | 1.2±0.1 | 1.15±0.1c |
| Quiescent cell
population |
| Without
bevacizumab |
| Without
10B-carrier | 1.1±0.1 | 1.2±0.1 |
| With BPA | 1.15±0.1d | 1.4±0.1d |
| With BSH | 1.2±0.1e | 1.45±0.15b,e |
| With
bevacizumab |
| Without
10B-carrier | 1.05±0.1 | 1.15±0.1 |
| With BPA | 1.1±0.1f | 1.35±0.15f |
| With BSH | 1.15±0.1g | 1.4±0.15c,g |
To examine the difference in radio-sensitivity
between the total and Q cell populations, dose-modifying factors
were calculated using the data in Figs. 1 and 2 (Table
VI). Overall, the values obtained were significantly >1.0
(P<0.05). Regardless of the 10B-carrier used, the
difference in radio-sensitivity significantly increased
(P<0.05), although the difference was smaller with BSH than with
BPA. The difference in radio-sensitivity, especially without
bevacizumab, was increased with nicotinamide and reduced with MTH.
However, the difference following irradiation without nicotinamide
or MTH or, in particular, with MTH, was increased with bevacizumab.
By contrast, the difference following irradiation and nicotinamide
administration was not significantly altered with bevacizumab.
 | Table VIDose-modifying factors for quiescent
cells relative to the total cell populationa (net micronucleus frequency =
0.3). |
Table VI
Dose-modifying factors for quiescent
cells relative to the total cell populationa (net micronucleus frequency =
0.3).
| Variable | Without
nicotinamide or MTH | With
nicotinamide | With MTH |
|---|
| Without
bevacizumab |
| Without
10B-carrier | 1.3±0.1 | 1.45±0.1 | 1.25±0.1 |
| With BPA | 2.1±0.2 | 2.35±0.2 | 1.7±0.15 |
| With BSH | 1.6±0.15 | 1.75±0.15 | 1.25±0.1 |
| With
bevacizumab |
| Without
10B-carrier | 1.4±0.1 | 1.5±0.15 | 1.4±0.1 |
| With BPA | 2.3±0.2 | 2.45±0.2 | 2.1±0.2 |
| With BSH | 1.65±0.15 | 1.75±0.15 | 1.45±0.1 |
Lung metastases from local tumors
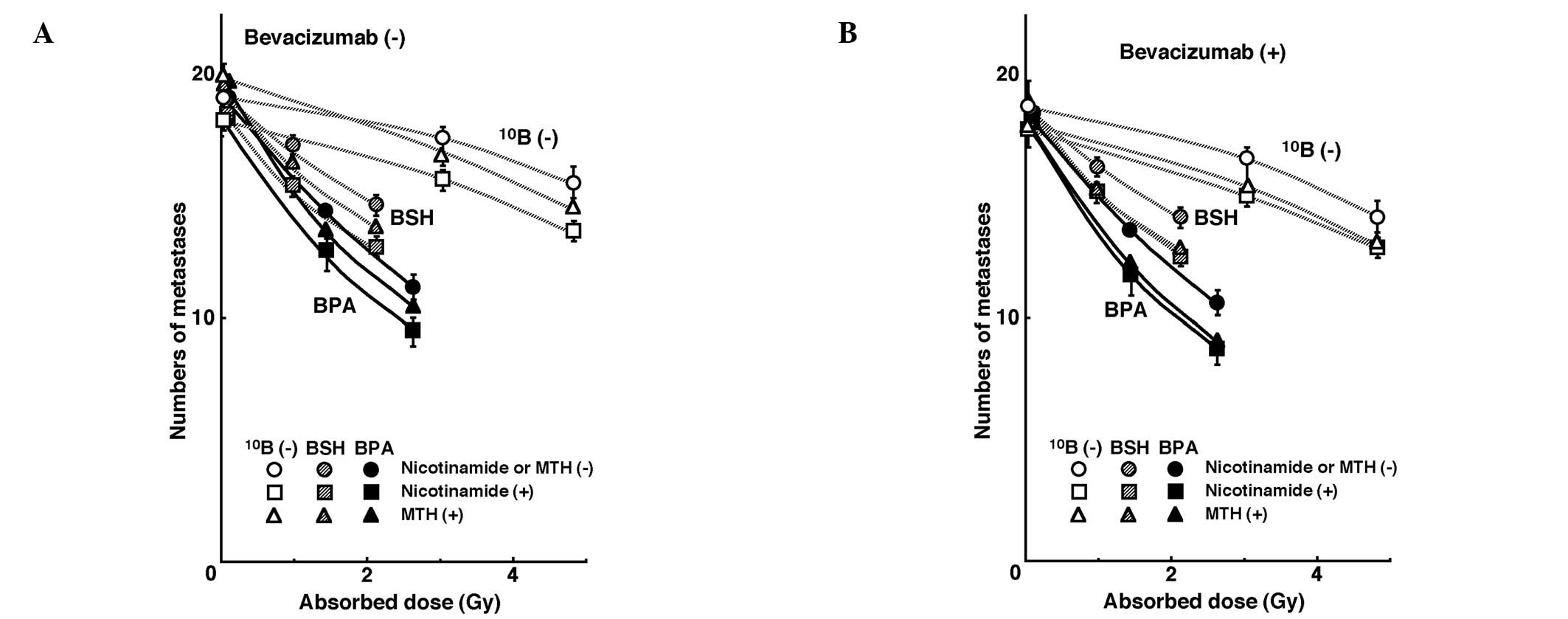
Fig. 3 shows the
numbers of lung metastases on day 35 following inoculation as a
function of the absorbed dose of neutron beam irradiation with or
without a 10B-carrier, in combination with nicotinamide
or MTH, and in the presence or absence of bevacizumab treatment.
Without bevacizumab or irradiation, irrespective of a
10B-carrier, nicotinamide and MTH decreased and
increased the numbers of macroscopic metastases, respectively. With
bevacizumab, but under no irradiation, both nicotinamide and MTH
decreased the number of metastases. With neutron beam irradiation,
as the absorbed dose increased, the number of metastases decreased.
Furthermore, the number of metastases decreased markedly with a
10B-carrier, especially BPA, than without. There was a
near-parallel shift in the curves and no significant changes in the
slopes of the curves for the tumors treated without a
10B-carrier or with BPA or BSH. This indicates that no
apparent radio-sensitizing or -protecting effect was observed with
or without bevacizumab, nicotinamide or MTH in terms of the numbers
of lung metastases. However, with irradiation, nicotinamide reduced
the numbers of metastatic nodules from the local tumors treated
with the neutron beam only, BPA-BNCT, or BSH-BNCT. The combination
with bevacizumab also reduced the number of metastases from the
local tumors treated with MTH more than those with nicotinamide and
without nicotinamide or MTH.
The numbers of lung metastases from local tumors
that received irradiation under each set of conditions, which
produced an identical SF of 0.3 as an initial effect (Fig. 1), were estimated using the data
shown in Fig. 3 (Table VII). Overall, BNCT with a
10B-carrier, especially BPA, decreased the number of
metastases more than neutron beam irradiation only. Irrespective of
a 10B-carrier, irradiation in combination with
nicotinamide resulted in a smaller number of metastases than any
other combination. Further combination with bevacizumab produced
further decreased numbers.
 | Table VIINumbers of metastases from the
irradiated tumors that received cytotoxic treatment producing a
similar initial local effecta
(surviving fraction = 0.3). |
Table VII
Numbers of metastases from the
irradiated tumors that received cytotoxic treatment producing a
similar initial local effecta
(surviving fraction = 0.3).
| Variable | Without
nicotinamide or MTH | With
nicotinamide | With MTH |
|---|
| Without
bevacizumab |
| Without
10B-carrier | 17.9 | 16.6 | 17.9 |
| With BPA | 15.3 | 14.5 | 15.1 |
| With BSH | 15.9 | 15.2 | 15.5 |
| With
bevacizumab |
| Without
10B-carrier | 17.4 | 16.3 | 16.8 |
| With BPA | 14.5 | 14.2 | 14.3 |
| With BSH | 15.6 | 14.8 | 15.4 |
Discussion
The cellular distribution of 10B from BSH
is considered to be mostly dependent on the diffusion of the drug,
whereas that from BPA is more dependent on the ability of the cells
to take up 10B (2). Q
cell populations have been demonstrated to have a much larger
hypoxic fraction (HF) than total cell populations (11). As hypoxic cells are thought to
exhibit less uptake than aerobic cells (24), it follows that Q cells have a lower
uptake capacity than the total cell population, and that the
distribution of 10B from 10B-carriers into Q
cells is more dependent on the diffusion of the drugs than on the
uptake ability of the cells.
Perfusion-related acute hypoxia is caused by
inadequate blood flow in tissues. Tumor microvasculature frequently
has severe structural and functional abnormalities, such as a
disorganized vascular network, dilations, an elongated and tortuous
shape, an incomplete endothelial lining, a lack of
physiological/pharmacological receptors, an absence of flow
regulation, and intermittent stasis (25). Perfusion-related O2
delivery leads to ischemic hypoxia, which is often transient. Thus,
acute hypoxic areas are distributed throughout the tumor depending
on these causative factors (8,10,14).
Nicotinamide, a vitamin B3 analog, prevents these transient
fluctuations in tumor blood flow that lead to the development of
acute hypoxia (26).
Diffusion-related chronic hypoxia is caused by an increase in
diffusion distances with tumor expansion. This results in an
inadequate O2 supply for cells distant (>70 μm) from
the nutritive blood vessels. Diffusion-related hypoxia may also be
caused by the deterioration of diffusion ‘geometry’ for example,
concurrent versus countercurrent blood flow within the tumor
microvessel network (10,24). MTH prior to irradiation decreased
the HF, even when combined with nicotinamide administration. By
contrast, in a previous study, MTH did not decrease the HF when
tumor-bearing mice were placed in a circulating carbogen (95%
O2/5% CO2) chamber during irradiation
(11). Thus, MTH has been
demonstrated to increase the tumor response to radiation by
improving tumor oxygenation through an increase in tumor blood flow
(27), thereby preferentially
overcoming chronic hypoxia rather than acute hypoxia. Furthermore,
the previous finding that the HFs in the total and Q cell
populations of B16-BL6 tumors are predominantly composed of acute
and chronic HFs, respectively needs to be taken into account
(12).
The recombinant humanized monoclonal antibody,
bevacizumab is composed of the human immunoglobulin G 1 (IgG1)
framework regions and the antigen-binding regions from the murine
IgG1 anti-human VEGF monoclonal antibody (28). In previous animal experiments,
tumor hypoxia decreased two days following antiangiogenic
treatment, such as VEGF-blocking therapy, was almost absent by day
five, and increased again by day eight (7,15).
In addition to reducing hypoxia, antiangiogenic treatment is
considered to be associated with the recruitment of pericytes that
help to support vessel walls to the tumor blood vessel. This
stabilizes the broken and dilated vasculature that is a common
characteristic of tumor vessels (29). Pericyte-covered vessels have also
been reported to decrease in number by day eight following
antiangiogenic treatment (28).
Vascular normalization including the recruitment of pericytes is
thought to occur 2–5 days following the blocking of VEGF (1,3).
During this window, pericyte coverage of tumor vessels (11,12)
and a reduction in tumor vessel permeability and interstitial fluid
pressure (30) occur, resulting in
the normalization of the tumor vessels leading to a release from
acute hypoxia. The decrease in the HFs induced by combining
bevacizumab with MTH was more marked than that achieved by
combining bevacizumab with nicotinamide treatment in both the total
and Q cell populations.
As shown in Table
II, concerning the distribution of 10B in the total
cell population within tumors, a minor improvement was achieved
through the chronic hypoxia-releasing treatment MTH when BPA, which
delivers more 10B to normoxic total tumor cells, rather
than BSH, was used. By contrast, a small improvement was achieved
through the acute hypoxia-releasing agent nicotinamide or
bevacizumab when BSH, which delivers more 10B to hypoxic
Q cells than BPA, was used. Regardless of which
10B-carrier was used, the 10B concentration
in tumors may be raised by combining the 10B-carrier
treatment with a treatment that is able to efficiently release the
hypoxic areas to which the distribution of 10B from each
10B-carrier does not readily occur.
The data in table
III supports this as when bevacizumab was not used, the
distribution of 10B in the tumor from BSH relied mostly
on passive diffusion, whereas that from BPA relied on uptake
capacity in the tumor by active transport. The former resulted in a
greater effect on Q cells and the latter, on the total tumor cell
population. When bevacizumab was used, the increase in the
enhancement ratio was greater in the total cells and with the use
of BPA than that in the Q cells and with the use of BSH,
respectively. This suggests that bevacizumab was able to
efficiently release the acute hypoxia, resulting in a higher uptake
of 10B from BPA than from BSH into oxygenated tumor
cells originating from a state of acute hypoxia. Furthermore, in
Table IV, when bevacizumab was
used, the increase in the enhancement ratio was more marked in the
total cells, with the use of BPA and with MTH, than in the Q cells,
with the use of BSH and with nicotinamide, respectively.
Regardless of the presence of bevacizumab, the
sensitivity-enhancing effect of nicotinamide and MTH on the total
cell population (Table V) almost
paralleled the changes in the 10B concentration in
tumors shown in Table II. Thus,
MTH combined with BPA, and nicotinamide combined with BSH induced a
greater sensitivity-enhancing effect on the total cell population.
In the Q cell population, MTH induced a significantly greater
enhancing effect (P<0.05), regardless of which
10B-carrier was used. When bevacizumab was combined, the
effect of the nicotinamide combination was reduced as both
nicotinamide and bevacizumab demonstrated a similar acute
hypoxia-releasing effect. However, the combination with bevacizumab
had almost no influence on the effect of MTH since the former and
the latter exhibited respective acute and chronic hypoxia-releasing
effects independently. Based on these findings, the difference in
sensitivity between total and Q cell populations increased with
nicotinamide or bevacizumab and decreased with MTH (Table VI). Furthermore, the fact that the
employed reactor neutron beams included not only high
linear-energy-transfer (LET) neutrons but also low LET γ-rays, may
have meant that even when a 10B-carrier was not used,
nicotinamide or bevacizumab and MTH had a marginal
sensitivity-enhancing effect on the total and Q cell populations,
respectively. Thus, even without a 10B-carrier, the
difference in sensitivity between the total and Q cell populations
increased with nicotinamide or bevacizumab and decreased with MTH
(Table VI). Since both
nicotinamide and bevacizumab demonstrated a similar acute
hypoxia-releasing effect, the difference in sensitivity was not
changed significantly in combination with bevacizumab. By contrast,
since bevacizumab and MTH induced respective acute and chronic
hypoxia-releasing effects independently, the difference in
sensitivity became clearer in combination with bevacizumab than
without.
In a previous study BNCT was performed, in
combination with thalidomide as an antiangiogenic drug, on a
short-term basis in order to induce a window of vascular
normalization in the treatment of tumors in a hamster cheek pouch
model of oral cancer. The blood vessel normalization was not
performed to increase the total 10B-carrier uptake, but
to distribute the 10B-carriers effectively to a larger
proportion of the tumor cells by fixing the flawed delivery system
(31). In particular, pretreatment
with thalidomide did not increase the absolute boron content in
oral tumors but improved boron targeting homogeneity. Thus, the
effect of tumor blood vessel normalization in BNCT remains to be
clarified in future studies.
The presence of Q cells is most likely due, at least
in part, to hypoxia and the depletion of nutrition as a consequence
of poor vascular supply (11,30).
As a result, Q cells are viable and clonogenic, but have ceased
dividing. This may promote the formation of micronuclei at 0 Gy in
Q tumor cells (Table I). Q cells
have been revealed to have significantly less radiosensitivity than
the total cell population (11,30,32).
This may also be applicable to BNCT as more Q cells survive BNCT
than do P cells (P<0.05; Fig.
2, Table I). Thus, the control
of chronic hypoxic Q cells has a significant impact on the outcome
of BNCT for controlling local tumors, resulting in the superiority
of BSH as a 10B-carrier in BNCT due to the delivery of
more 10B from BSH in the Q cell population than from
BPA. With or without a 10B-carrier in the boron neutron
capture reaction, nicotinamide and bevacizumab enhanced the
radiosensitivity of the total cell population and MTH enhanced the
sensitivity of Q cell populations. As a result, the use of
nicotinamide or bevacizumab led to an increase, while the use of
MTH led to a reduction, in the difference in radiosensitivity
(Table VI). Although the use of a
10B-carrier in BNCT, especially BPA, significantly
increased the difference in radiosensitivity between total and Q
cell populations (P<0.05), MTH is considered to be more useful
than nicotinamide or bevacizumab in terms of local tumor response
since it reduces the difference in radiosensitivity between
radiosensitive total and radioresistant Q cell populations.
Overall, the use of BSH as a 10B-carrier in combination
with MTH is considered to be advantageous and promising in terms of
local tumor response in BNCT.
Hypoxia is believed to enhance metastasis by
increasing genetic instability (10). Acute, but not chronic, hypoxia has
been reported to increase the number of macroscopic metastases in
mouse lungs (9,10). A previous study reported the
significance of injecting an acute hypoxia-releasing agent,
nicotinamide, into tumor-bearing mice as a combined treatment with
γ-ray irradiation to repress lung metastasis (12). With or without irradiation,
nicotinamide and bevacizumab appeared to reduce the number of
macroscopic metastases in the current study (Fig. 4, Table VII). Without irradiation or
bevacizumab, MTH increased the number of metastases, implying that
the release from chronic hypoxia is not as important in repressing
metastasis as the release from acute hypoxia. However, hyperthermia
is not thought to induce metastasis in the clinical setting
(33). As the delivered total dose
increased with irradiation, the number of macroscopic lung
metastases decreased reflecting the decrease in the number of
clonogenically viable tumor cells in the primary tumor (Fig. 4).
The metastasis-repressing effect achieved through a reduction in
the number of clonogenic tumor cells by irradiation is much greater
than that achieved by releasing tumor cells from acute hypoxia.
However, more 10B from BPA than from BSH could be
distributed into the acute hypoxic total tumor cell population,
resulting in a greater reduction in the number of highly clonogenic
P tumor cells with BPA-BNCT than with BSH-BNCT and with neutron
beam irradiation only. BPA-BNCT rather than BSH-BNCT has certain
potential to decrease the number of lung metastases and an acute
hypoxia-releasing treatment, such as the administration of
nicotinamide or bevacizumab, may be promising for reducing the
number of lung metastases. Consequently, BPA-BNCT in combination
with nicotinamide and/or bevacizumab treatment may demonstrate a
higher potential in reducing the number of metastases. Finally, it
was revealed that control of the chronic hypoxic Q cell population
in the primary solid tumor has the potential to impact the control
of local tumors as a whole and that control of the acute hypoxic
total tumor cell population in the primary solid tumor has the
potential to impact the control of lung metastases.
Acknowledgements
The present study was supported, in part, by a
Grant-in-aid for Scientific Research (C) (23300348) from the Japan
Society for the Promotion of Science.
References
|
1
|
Barth RF, Coderre JA, Vicente MG and Blue
TE: Boron neutron capture therapy of cancer: current status and
future prospects. Clin Cancer Res. 11:3987–4002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soloway AH, Hatanaka H and Davis MA:
Penetration of brain and brain tumor. VII Tumor-binding sulfhydryl
boron compounds. J Med Chem. 10:714–717. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coderre JA, Turcotte JC, Riley KJ, Binns
PJ, Harling OK and Kiger WS III: Boron neutron capture therapy:
cellular targeting of high linear energy transfer radiation.
Technol Cancer Res Treat. 2:355–375. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wittig A, Sauerwein WA and Coderre JA:
Mechanisms of transport of p-borono-phenylalanine through the cell
membrane in vitro. Radiat Res. 153:173–180. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyatake S, Kawabata S, Kajimoto Y, et al:
Modified boron neutron capture therapy for malignant gliomas
performed using epithermal neutron and two boron compounds with
different accumulation mechanisms: an efficacy study based on
findings on neuroimages. J Neurosurg. 103:1000–1009. 2005.
View Article : Google Scholar
|
|
6
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winkler F, Kozin SV, Tong RT, et al:
Kinetics of vascular normalization by VEGFR2 blockade governs brain
tumor response to radiation: role of oxygenation, angiopoietin-1,
and matix metalloproteinases. Cancer Cell. 6:553–563. 2004.
|
|
8
|
Brown JM: Evidence for acutely hypoxic
cells in mouse tumours, and a possible mechanism of reoxygenation.
Br J Radiol. 52:650–656. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cairns BA, Kalliomaki T and Hill RP: Acute
(cyclic) hypoxia enhances spontaneous metastasis of KHT murine
tumors. Cancer Res. 61:8903–8908. 2001.PubMed/NCBI
|
|
10
|
Rofstad EK, Galappathi K, Mathiesen B and
Ruud EB: Fluctuating and diffusion-limited hypoxia in
hypoxia-induced metastasis. Clin Cancer Res. 13:1971–1978. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masunaga S, Ono K, Suzuki M, et al:
Alteration in the hypoxic fraction of quiescent cell populations by
hyperthermia at mild temperatures. Int J Hyperthermia. 13:401–411.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Masunaga S, Matsumoto Y, Hirayama R, et
al: Significance of manipulating intratumor hypoxia in the effect
on lung metastases in radiotherapy, with reference to its effect on
the sensitivity of intratumor quiescent cells. Clin Exp Metastasis.
26:693–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masunaga S and Ono K: Significance of the
response of quiescent cell populations within solid tumors in
cancer therapy. J Radiat Res. 43:11–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan X, Zhang H, Liu B, Li XD, Gao QX and
Wu ZH: Apoptosis of murine melanoma cells induced by heavy-ion
radiation combined with Tp53 gene transfer. Int J Radiat Biol.
84:211–217. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dings RP, Loren M, Heun H, et al:
Scheduling of radiation with angiogenesis inhibitors anginex and
Avastin improves therapeutic outcome via vessel normalization. Clin
Cancer Res. 13:3395–3402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Masunaga S, Ono K, Sakurai Y, et al:
Evaluation of apoptosis and micronucleation induced by reactor
neutron beams with two different cadmium ratios in total and
quiescent cell populations within solid tumors. Int J Radiat Oncol
Biol Phys. 51:828–839. 2001. View Article : Google Scholar
|
|
17
|
Kobayashi T and Kanda K: Microanalysis
system of ppm-order B-10 concentrations in tissue for neutron
capture therapy by prompt γ-ray spectrometry. Nucl Instrum Methods
Phys Res. 204:525–531. 1983.
|
|
18
|
Nishimura Y, Ono K, Hiraoka M, et al:
Treatment of murine SCC VII tumors with localized hyperthermia and
temperature-sensitive liposomes containing cisplatin. Radiat Res.
122:161–167. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakurai Y and Kobayashi T: Characteristics
of the KUR heavy water neutron irradiation facility as a neutron
irradiation field with variable energy spectra. Nucl Instr Meth A.
453:569–596. 2000. View Article : Google Scholar
|
|
20
|
Kobayashi T, Sakurai Y, Kanda K, Fujita Y
and Ono K: The remodeling and basic characteristics of the heavy
water neutron irradiation facility of the Kyoto University Research
Reactor, mainly for neutron capture therapy. Nucl Tech.
131:354–378. 2000.
|
|
21
|
Snyder WS, Cook MJ, Nasset ES, Karhausen
LR, Parry Howells G and Tipton I: Gross and elemental content of
reference man. Report of the task group on reference man. Snyder
WS: Pergamon Press; Oxford, UK: pp. 273–324. 1975
|
|
22
|
De Jaeger K, Kavanagh MC and Hill RP:
Relationship of hypoxia to metastatic ability in rodent tumours. Br
J Cancer. 84:1280–1285. 2001.PubMed/NCBI
|
|
23
|
Hall EJ: Time, Dose, and Fractionation in
Radiotherapy. Radiobiology for the Radiologist. Hall EJ and Giaccia
AJ: 7th edition. Lippincott Williams & Wilkins; Philadelphia,
PA, USA: pp. 391–411. 2012
|
|
24
|
Vaupel P: Tumor microenvironmental
physiology and its implications for radiation oncology. Semin
Radiat Oncol. 14:198–206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaupel P, Kallinowski F and Okunieff P:
Blood flow, oxygen and nutrient supply, and metabolic
microenvironment of human tumors: a review. Cancer Res.
49:6449–6465. 1989.PubMed/NCBI
|
|
26
|
Chaplin DJ, Horsman MR and Trotter MJ:
Effect of nicotinamide on the microregional heterogeneity of oxygen
delivery within a murine tumor. J Natl Cancer Inst. 82:672–676.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song CW, Park H and Griffin RJ:
Improvement of tumor oxygenation by mild hyperthermia. Radiat Res.
155:512–528. 2001.PubMed/NCBI
|
|
28
|
Presta LG, Chen H, O’Conner SJ, et al:
Humanization of an anti-vascular endothelial growth factor
monoclonal antibody for the therapy of solid tumors and other
disorders. Cancer Res. 57:4593–4599. 1997.PubMed/NCBI
|
|
29
|
Ma J and Waxman DJ: Combination of
antiangiogenesis with chemotherapy for more effective cancer
treatment. Mol Cancer Ther. 7:3670–3684. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jain RK, Tong RT and Munn LL: Effect of
vascular normalization by antiangiogenic therapy on interstitial
hypertension, peritumor edema, and lymphatic metastasis: insights
from a mathematical model. Cancer Res. 67:2729–2735. 2007.
View Article : Google Scholar
|
|
31
|
Molinari AJ, Pozzi EC, Monti Hughes A, et
al: Tumor blood vessel “normalization” improves the therapeutic
efficacy of boron neutron capture therapy (BNCT) in experimental
oral cancer. Radiat Res. 177:59–68. 2012.
|
|
32
|
Ando K, Koike S, Ohira C, et al:
Accelerated reoxygenation of a murine fibrosarcoma after carbon-ion
radiation. Int J Radiat Biol. 75:505–512. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Möller MG, Lewis JM, Dessureault S and
Zager JS: Toxicities associated with hyperthermic isolated limb
perfusion and isolated limb infusion in the treatment of melanoma
and sarcoma. Int J Hyperthermia. 24:275–89. 2008.PubMed/NCBI
|