Introduction
Rapidly growing mycobacteria (RGM) are characterized
by rapid growth on standard media and a lack of pigmentation. They
now include three clinically relevant species: Mycobacterium
fortuitum (M. fortuitum), Myobacterium abscessus (M.
abscessus) and Mycobacterium chelonae (M. chelonae),
which are environmental microorganisms present in media including
soil, bioaerosols and chlorinated water (1). RGM are able to cause chronic
infections in the skin, soft tissues and lungs and these are widely
reported in immunocompromised individuals (2). The present methods used for diagnosis
of nontuberculous mycobacteria (NTM) are biochemical tests,
quantitative polymerase chain reaction (qPCR) assays and
high-performance liquid chromatography (HPLC). Due to the high
homology and similarity in the clinical manifestations of M.
abscessus with other NTM, it is difficult to identify them at
the species level (3). In
addition, the sequencing methods, including the ribonucleic acid
polymerase beta subunit (rpoB) gene, heat-shock protein 65 gene
(hsp65) gene and 16S rDNA sequencing methods, are lacking in
standardized criteria for diagnosis (4). Therefore, accurate molecular
techniques are urgently needed for rapid and precise diagnosis of
NTM infections. In the present study, a case of a skin infection
caused by M. abscessus is reported, which was identified by
16S rDNA sequence analysis and the citrate utilization test.
Informed consent was obtained from the patient.
Case report
A 69-year-old female was admitted to the General
Hospital of Chengdu Military Region of PLA (Chengdu, China) due to
swelling, nodules, ulcers and pain in the right leg. Six months
previously, the patient had been impaled by a bamboo pole on the
tibialis anterior of the right leg. This was followed by the
gradual emergence of skin redness, suppuration and ulceration.
Anti-infective medications at local clinics resulted in no clinical
improvement. Three months prior to admission, the patient was noted
to have a fasting blood glucose level of 18.0 mmol/l. Insulin
treatment was administered and a scab formed on the wound in the
leg. Approximately one month following this, several painless and
erythematous subcutaneous nodules appeared on the patient’s lower
right leg. Several of the nodules ulcerated and a mixture of blood
and pus was exuded. There was no itching reported. The patient was
diagnosed with diabetes and diabetic foot, and was given treatments
for anti-infection, insulin, blood circulation activation and
debridement for half a month. The blood glucose level returned to
normal. When the patient was discharged, the swelling on the right
leg had disappeared, although the nodules persisted and the sores
had formed a crust. One month prior to admission, the number of
nodules on the right leg gradually increased. There was
seropurulent discharge from some of the lesions. At admission,
three irregularly-shaped, dark red papules (with an approximate
diameter of 1.5 cm) emerged near the right knee.
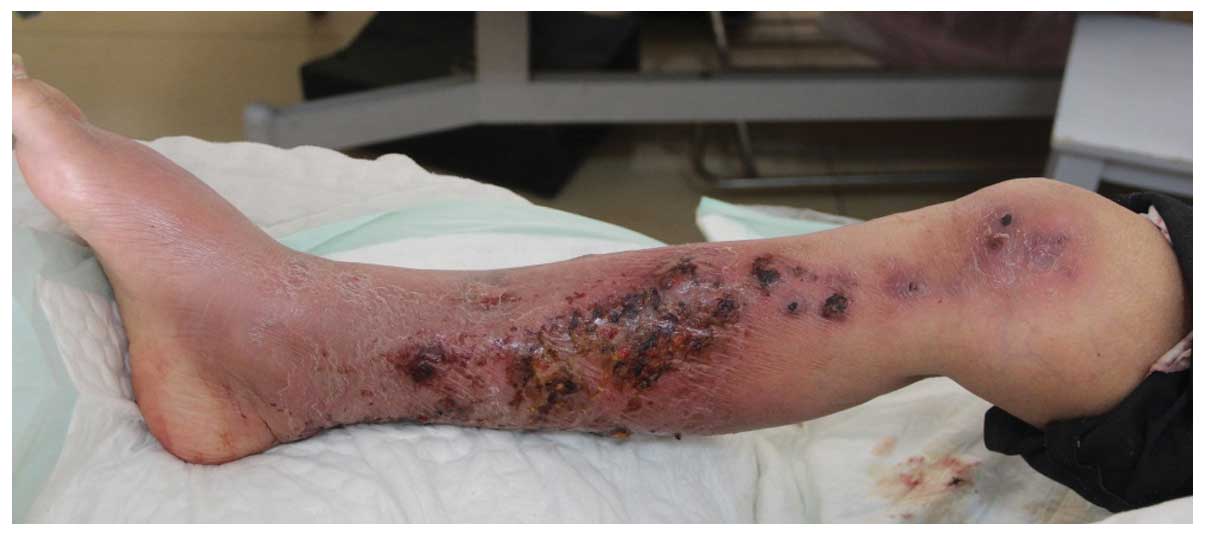
Inspection of the lower extremities revealed
multiple, painless, purple-brown colored, circular and clearly
delineated nodular lesions, 2×2 cm in size, which were localized to
the lower right leg and foot (Fig.
1). Crimson liquid was exuding from certain lesions and some
crusts had formed. Laboratory investigations revealed that the
blood glucose level was normal. No abnormalities in the biochemical
and urine tests were identified. Examination of autoantibodies also
revealed no abnormalities and the X-rays of the chest were
unremarkable. A plain film of the right leg revealed a small area
of shadow in the soft tissue area, which was considered as a
foreign material. Gross pathological changes in the bones and
joints were not identified. Magnetic resonance angiography (MRA) of
the lower extremity vasculature revealed that stenosis was present
in the peroneal artery of the lower right leg. An ultrasound scan
of the lower extremity vasculature demonstrated extensive
thrombosis involving the right calf muscle veins. A skin biopsy
revealed signs consistent with a suppurative inflammation process
in the skin, with a large number of inflammatory cells (mainly
small lymphocytes) present.
The diagnosis of sporotrichosis and diabetes (with
deep vein thrombosis) was considered. Pus was collected from the
draining lesions. Fungal tests under direct microscopic examination
and fungal cultures were repeatedly negative. Pus cultured on a
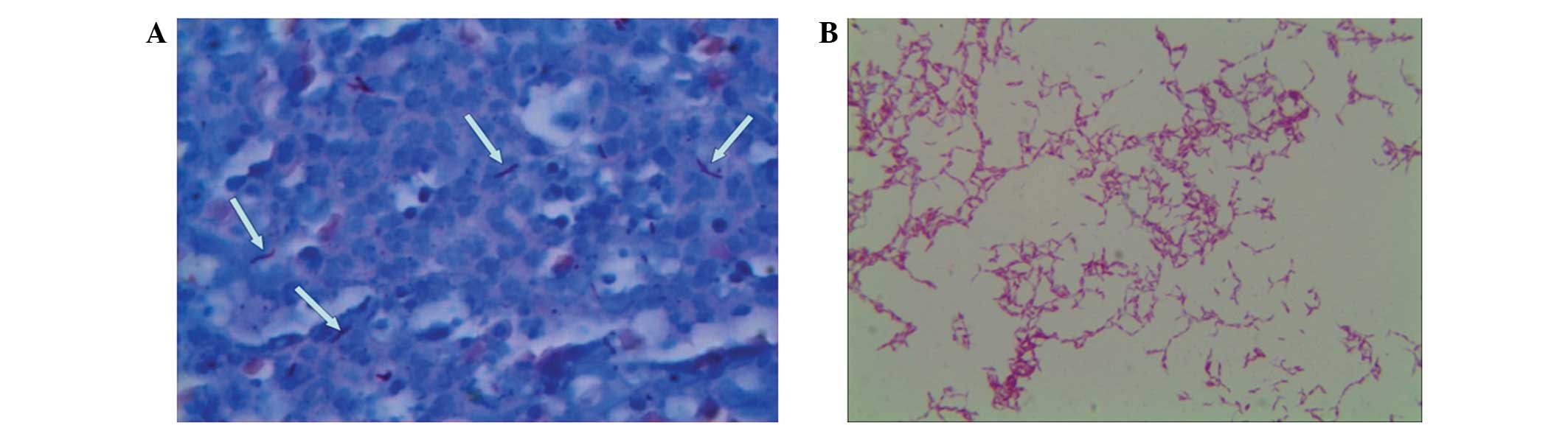
common medium for 48 h revealed no bacterial growth. Ziehl-Neelsen
staining of purulent material obtained from a draining lesion
revealed the presence of multiple acid-fast bacilli (Fig. 2). Cultures of the pus on Sabouraud
medium at 28°C for five days yielded a rapidly growing,
nontuberculous mycobacterium. Direct microscopic examination
following Ziehl-Neelsen staining was positive for acid-fast bacilli
(Fig. 2). This bacterium grew well
on blood, MacConkey, Sabouraud and nutrient agars at 28°C on day
five. A biofilm was formed when the pus was cultured in nutrient
broth for three days at 28°C. Thus, a diagnosis of ‘RGM growth’ was
made.
The identification of this RGM isolate was further
confirmed by DNA sequence analysis of 16S rDNA (corresponding to
E. coli positions 27 to 1492 bp) using qPCR primers 27F
(5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGYTACCTTGTTACGACTT-3′).
The results revealed a sequence similarity of 100% with M.
abscessus (GenBank accession no. NR_074427.1) and 99% with
M. chelonae (GenBank accession no. AM884326.1). A citrate
utilization test was then carried out and the result was negative.
The pathogenic bacterium was ultimately identified as M.
abscessus. Treatment was initiated with intravenous
sulfamethoxazole 800 mg twice daily and imipenem 1 g twice daily in
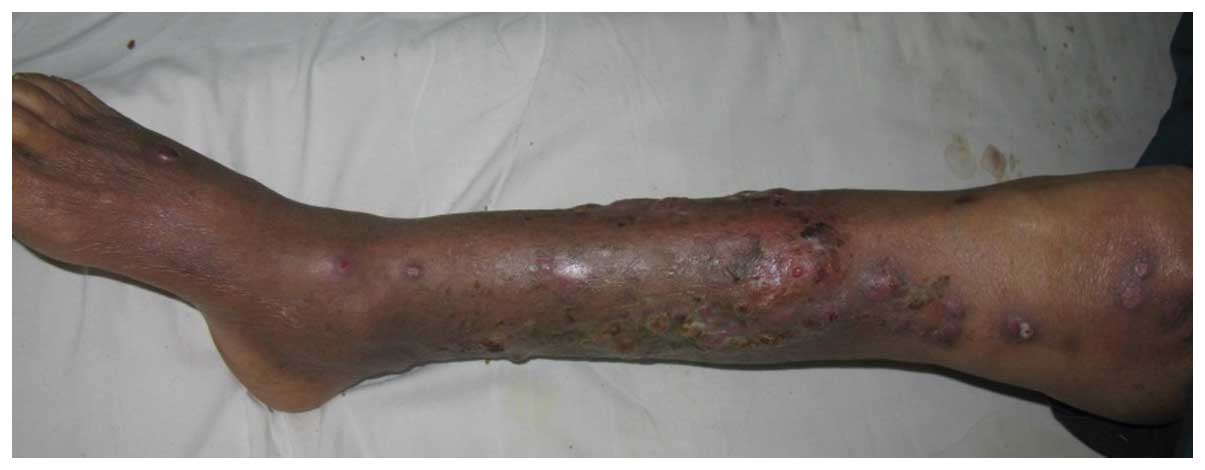
combination with debridement. One week later the condition of the
patient was significantly improved with exudate cessation and
diminishing cutaneous lesions (Fig.
3).
Following the identification of the microorganism as
M. abscessus and susceptibility testing, the patient was
discharged on sulfamethoxazole 800 mg twice daily by oral
administration for an additional three months. Upon several
follow-up visits within one year, the patient maintained a range of
healthy blood sugar levels and the cutaneous lesions were gradually
eliminated.
Discussion
In the present report, the patient had a history of
diabetes that was under control. To the best of our knowledge,
there is no direct association between diabetes and hypoimmunity.
However, certain individuals with diabetes who have a loss in
glucose homeostasis may have a hypoimmunity (5). The patient was impaled by a bamboo
pole and the healing of the puncture wound was delayed.
Endocrinologists often do not pay much attention to the puncture
wound and mistake these lesions for complications caused by
diabetes itself, resulting in a misdiagnosis.
M. abscessus infections have similar clinical
manifestations to those of other RGM infections, including M.
fortuitum and M. chelonae (6). Identifying RGM to the species level
is very important as their therapeutic responses are species
specific. Molecular biological techniques, particularly sequencing
technology, have become very powerful tools in the identification
of pathogens. However, there is a difference of only 4 bp (0.37%)
in the 16s rDNA between M. abscessus and M. chelonae
(7). Therefore, it is insufficient
to identify the pathogenic bacteria solely using 16s rDNA
sequencing. Citrate utilization tests for the identification of RGM
have been performed previously (M. chelonae is citrate
positive; M. abscessus is citrate negative) (8). It is worth noting that 16S rDNA
sequence analysis combined with a citrate utilization test is an
efficient, quick and precise method of identifying the exact
species of RGM. In addition, sequencing the genes of 16S–23S rDNA,
IS6110, rpoB and/or hsp65 has also been suggested in order to
determine the RGM species or subspecies (9,10).
In conclusion, the present case study describes a
quick method for RGM identification. However, this method requires
verification through the analysis of more cases and a future study
to confirm the validity of the method is planned.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (No. 81301445), the Public Health
Department of Sichuan (No. 130318) and the Research Fund of the
General Hospital of Chengdu Military Region of PLA (No.
2013YG-B055).
References
|
1
|
Daley CL and Griffith DE: Pulmonary
disease caused by rapidly growing mycobacteria. Clin Chest Med.
23:623–632. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colombo RE and Olivier KN: Diagnosis and
treatment of infections caused by rapidly growing mycobacteria.
Semin Respir Crit Care Med. 29:577–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park JS, Choi JI, Lim JH, et al: The
combination of real-time PCR and HPLC for the identification of
non-tuberculous mycobacteria. Ann Lab Med. 33:349–352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SY, Kang YA, Bae IK, et al:
Standardization of multilocus sequence typing scheme for
Mycobacterium abscessus and Mycobacterium
massiliense. Diagn Microbiol Infect Dis. 77:143–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Al-Suhaimi EA and Shehzad A: Leptin,
resistin and visfatin: the missing link between endocrine metabolic
disorders and immunity. Eur J Med Res. 18:122013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harris KA, Kenna DT, Blauwendraat C, et
al: Molecular fingerprinting of Mycobacterium abscessus
strains in a cohort of pediatric cystic fibrosis patients. J Clin
Microbiol. 50:1758–1761. 2012.
|
|
7
|
Springer B, Böttger EC, Kirschner P and
Wallace RJ Jr: Phylogeny of the Mycobacterium chelonae-like
organism based on partial sequencing of the 16S rRNA gene and
proposal of Mycobacterium mucogenicum sp nov. Int J Syst
Bacteriol. 4:262–267. 1995.
|
|
8
|
Wallace RJ Jr, Silcox VA, Tsukamura M, et
al: Clinical significance, biochemical features, and susceptibility
patterns of sporadic isolates of the Mycobacterium
chelonae-like organism. J Clin Microbiol. 31:3231–3239.
1993.PubMed/NCBI
|
|
9
|
Arnold C, Barrett A, Cross L and Magee JG:
The use of rpoB sequence analysis in the differentiation of
Mycobacterium abscessus and Mycobacterium chelonae: a
critical judgement in cystic fibrosis? Clin Microbiol Infect.
18:E131–133. 2012.PubMed/NCBI
|
|
10
|
Blauwendraat C, Dixon GL, Hartley JC, et
al: The use of a two-gene sequencing approach to accurately
distinguish between the species within the Mycobacterium
abscessus complex and Mycobacterium chelonae. Eur J Clin
Microbiol Infect Dis. 31:1847–1853. 2012. View Article : Google Scholar : PubMed/NCBI
|