Introduction
Acute leukemia occurs in all age groups and has the
highest mortality rate of all malignant tumors in children and
adults aged <35 years-old (1).
Ecotropic viral integration site-1 (EVI-1) has been recognized as
one of the dominant oncogenes associated with murine and human
myeloid leukemia (2–5). EVI-1 protein is a transcription
factor and contains DNA-binding zinc-finger motifs. It has been
reported that ~10% of acute myeloid leukemia cases exhibit EVI-1
overexpression, which is an independent negative prognostic
indicator of survival in leukemia patients (4).
Arsenic trioxide (ATO) is used in a number of
traditional Chinese remedies and has been found to be an effective
treatment for acute promyelocytic leukemia (APL). ATO is also being
tested for the treatment of other malignancies. The drug has been
reported to induce complete remission in APL patients, particularly
in relapsed and refractory APL (3). A previous study demonstrated that ATO
targets the EVI-1 protein, without affecting EVI-1 mRNA (5).
Apoptosis, also known as programmed cell death, is
an active process of cellular self-destruction that is mediated by
a variety of signaling pathways and genes. Apoptosis plays a key
role in multicellular organisms by maintaining normal development
and homeostasis, and allowing organisms to respond appropriately to
environmental stimuli (6). The
pathogenesis of a number of diseases, including cancer, is
associated with dysregulated apoptosis processes. Numerous
anticancer drugs exert their effects by inducing cancer cell death
via apoptosis.
The aim of the present study was to investigate the
effects of ATO on EVI-1 mRNA and protein expression in THP1 cells.
The apoptotic mechanisms and pathways induced by ATO were also
investigated, with particular focus on the proteins that are
located downstream of EVI-1, including c-Jun N-terminal kinase
(JNK), B-cell lymphoma 2 (Bcl-2) family members and caspases.
Materials and methods
Experimental materials
Four human leukemia cell lines, K562, HL-60, U937
and THP1, were obtained from the American Type Culture Collection
(Manassas, VA, USA). ATO was purchased from Beijing SL
Pharmaceutical Co., Ltd. (Beijing, China) and the Cell Counting
kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto,
Japan). An Annexin V-fluorescein isothiocyanate (AV) Apoptosis
Detection kit and TRIzol reagent were purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). A first-strand cDNA
Quantscript RT kit and Taq DNA Polymerase were purchased
from Tiangen Biotech Co., Ltd. (Beijing, China). Rabbit anti-EVI-1,
anti-JNK, anti-phosphorylated-JNK (p-JNK), anti-Bax, anti-Bcl-2 and
anti-B-cell lymphoma-extra large (Bcl-xL) and mouse anti-caspase-3
antibodies were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Mouse anti-β-actin antibody was purchased from
Abmart (Arlington, MA, USA). Horseradish peroxidase-conjugated
secondary antibodies were purchased from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA).
Ethics and dissemination
The clinical investigation using primary human
material from one healthy volunteer was conducted according to the
principles of the 1996 Declaration of Helsinki and was approved by
the Joint Committee on Clinical Investigation of Ren Ji Hospital
(Shanghai, China). Written informed consent was obtained from the
volunteer prior to inclusion in the study.
Cell culture
Cell lines, K562, U937 and THP1, were cultured in
RPMI 1640 medium (Gibco-BRL, Carlsbad, CA, USA), while the HL-60
cell line was cultured in Dulbecco’s modified Eagle’s medium
(Gibco-BRL), supplemented with 100 U/ml penicillin, 100 μg/ml
streptomycin and 10% (v/v) fetal bovine serum (Gibco-BRL), with or
without ATO, in a humidified atmosphere of 5% CO2 at
37°C.
Isolation of mononuclear cells
A 10-ml peripheral blood sample was donated by one
healthy volunteer from the laboratory. Peripheral blood mononuclear
cells (PBMCs) were separated using Ficoll-Hypaque density gradient
centrifugation. The isolated PBMCs were then used promptly.
Reverse transcription polymerase chain
reaction (RT-PCR)
For the extraction of total RNA, ~1×106
cells were harvested using TRIzol reagent, according to the
manufacturer’s instructions. RNA samples were assessed for
integrity by agarose-gel electrophoresis and quantified using the
optical density values (OD) values at 260/280 nm. cDNA was
synthesized from equal amounts of total RNA (500 ng) using the
first-strand cDNA Quantscript RT kit, followed by PCR using
Taq DNA Polymerase. Primers and reaction conditions for
RT-PCR are shown in Table I. PCR
products were examined by 1.5% agarose gel electrophoresis
(containing nucleic acid dye), scanned for signals using a gel
imaging analysis system and the peak gray value of each band was
analyzed with ImageJ software. The gray ratio of each group was
calculated as follows: Gray ratio = EVI-1 gray value/β-actin gray
value. The relative mRNA expression level of EVI-1 was calculated
as follows: Expression (%) = (experimental group gray ratio/control
group gray ratio) × 100%, and the relative mRNA expression level of
EVI-1 in the control group was designated as 100%.
 | Table IPrimer sequences and reaction
conditions for RT-PCR. |
Table I
Primer sequences and reaction
conditions for RT-PCR.
| Gene | Primer sequence | Product size
(bp) | Annealing temperature
(°C) | Cycles |
|---|
| EVI-1 |
5′-ATATCGCTGCGAAGACTGTGACCA-3′
5′-TGAAGGTTGCTAGGGTCCGTGAAA-3′ | 381 | 62 | 38 |
| β-actin |
5′-CATGTACGTTGCTATCCAGGC-3′
5′-CTCCTTAATGTCACGCACGAT-3′ | 195 | 60 | 32 |
Cell viability assay and cell
morphology
Cell suspensions of THP1 (90 μl; 2×105
cells/ml), supplemented with various concentrations of ATO (0, 1,
3, 5 μM) in culture medium, were seeded in 96-well plates for 24,
48 and 72 h, followed by the addition of 10 μl CCK-8 solution. The
cells were then incubated for ~4 h at 37°C. The absorbance of each
well was determined at 490 and 630 nm using a microplate reader
(Thermo Fisher Scientific, Waltham, MA, USA). The cell viability
was calculated as follows: Cell viability (%) = experimental group
OD/control group OD × 100%. For morphological observations, the
cells were centrifuged onto slides by cytospin and stained with
hematoxylin and eosin. Images were captured using an Olympus
microscope (Olympus Corporation, Tokyo, Japan).
Analysis of apoptosis by flow
cytometry
Cells were treated with ATO in six-well culture
plates (2×105 cells/ml), washed twice in ice-cold
phosphate-buffered saline and resuspended in 1X binding buffer.
Next, 5 μl AV and 1 μl propidium iodide (PI; 100 μg/ml) were added
to 100-μl cell suspensions. The samples were then incubated at room
temperature for 15 min. Finally, 400 μl 1X binding buffer was added
to each sample and the samples were immediately evaluated by flow
cytometry using a FACScalibur system (BD Biosciences, Franklin
Lakes, NJ, USA), which was followed by analysis using CellQuest Pro
software (BD Biosciences). The proportion of apoptotic cells was
represented as early apoptotic cells (AV+/PI−
staining) and late apoptotic cells (AV+/PI+
staining).
Western blotting
Equal amounts of protein extract were loaded onto
6–12% SDS-polyacrylamide gels, electrophoresed and transferred to
nitrocellulose membranes (Millipore Corporation, Billerica, MA,
USA). The membranes were then blocked with 5% non-fat milk in
Tris-buffered saline Tween-20. The membranes were then incubated
with primary antibodies (1:2,000), which was followed by incubation
with secondary horseradish peroxidase-conjugated antibodies
(1:3,000). Signals were detected by chemiluminescence and the peak
gray value of each band was analyzed with ImageJ software.
Statistical analysis
All the experiments were repeated at least three
times and the data are expressed as the mean ± standard deviation.
The treatment groups were compared using analysis of variance
followed by the Student-Newman-Keul’s multiple comparison tests
with SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). Curves and
histograms were constructed using GraphPad Prism 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
High mRNA expression of EVI-1 in THP1
cells
Relative EVI-1 mRNA expression levels were measured
in four leukemia cell lines, K562, HL-60, U937 and THP1. Expression
levels were also measured in PBMCs from a healthy adult, which was
used as a control. The mRNA expression of EVI-1 in the PBMCs from
the healthy adult was very low. Among the four leukemia cell lines,
THP1 cells exhibited the highest EVI-1 expression (Fig. 1). Thus, the THP1 cell line was
selected for further study. Through gene sequencing analysis and
comparison with Genbank, the THP1 cell line was confirmed to
contain the full length of the EVI-1 gene with 21 exons (data not
shown).
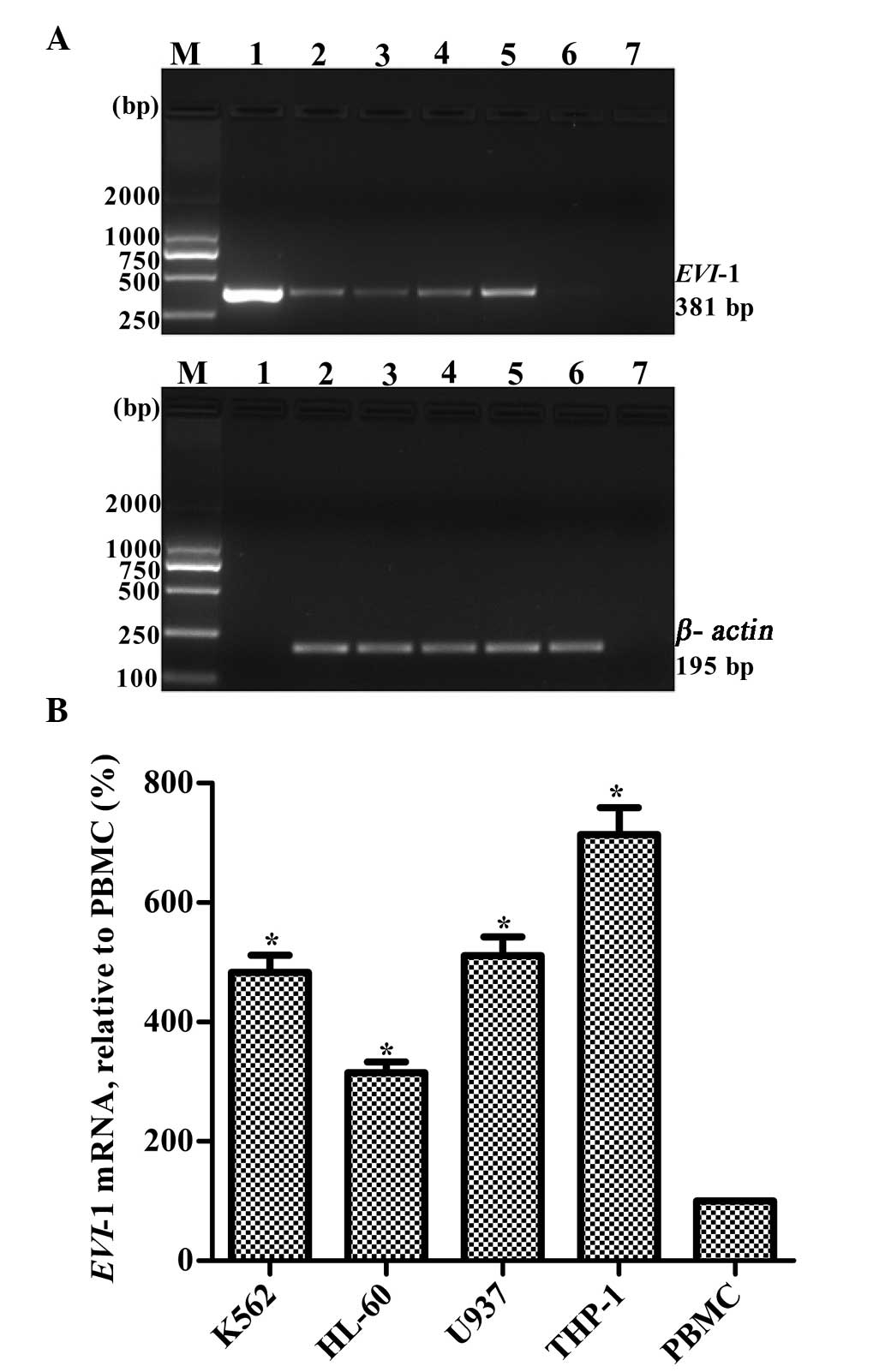 | Figure 1Relative mRNA expression levels of
EVI-1 in four leukemia cell lines. (A) Representative RT-PCR
results. Lanes: M, marker; 1, plasmid containing EVI-1 gene
(positive control); 2, K562; 3, HL-60; 4, U937; 5, THP1; 6, PBMCs
from a healthy adult; and 7, blank control without template. (B)
Quantitative results are expressed as the mean ± standard deviation
(n=3). *P<0.01, vs. PBMCs. EVI-1, ecotropic viral
integration site-1; RT-PCR, reverse transcription polymerase chain
reaction; PBMCs, peripheral blood mononuclear cells. |
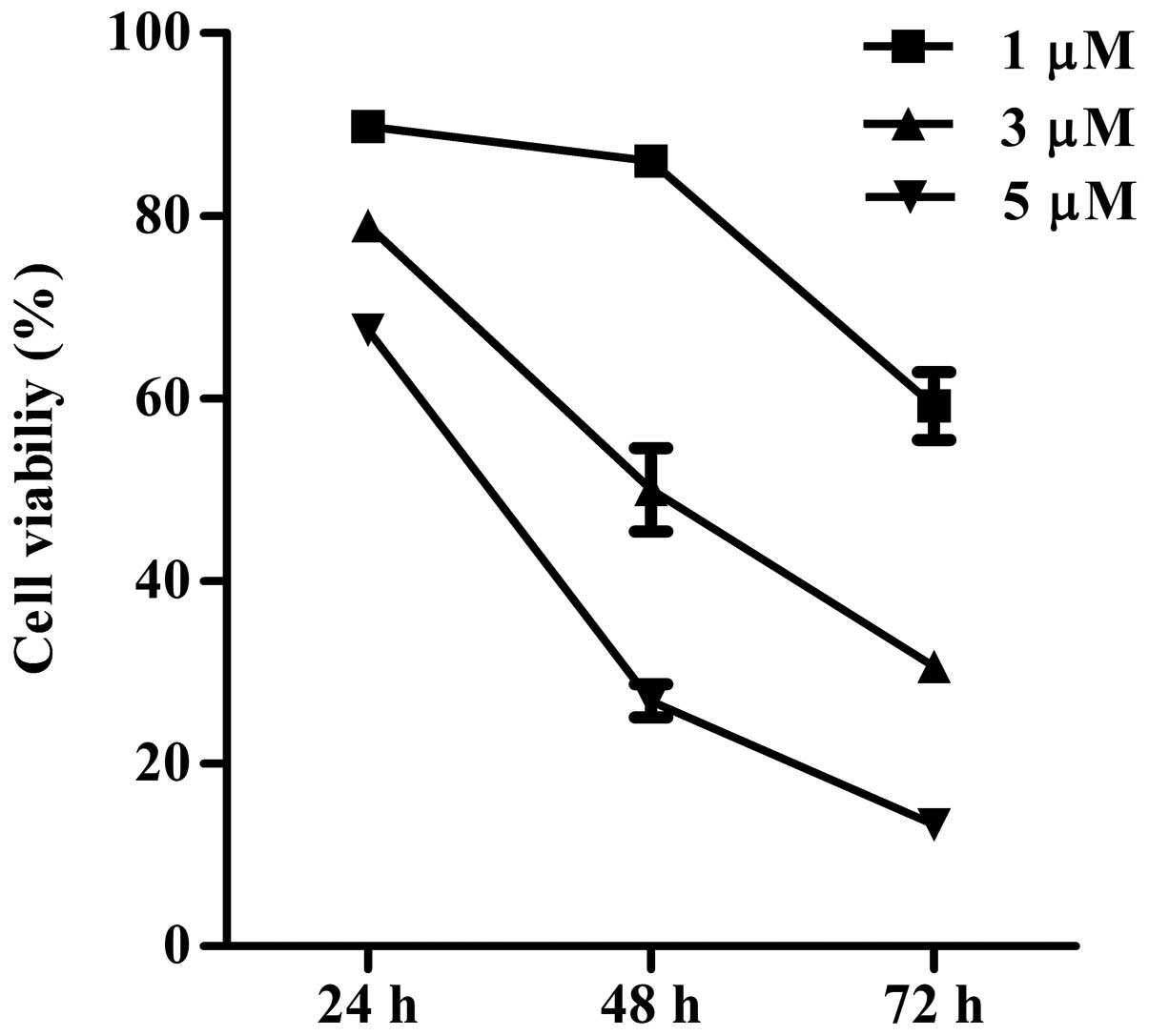
Inhibition of THP1 cell proliferation by
ATO
CCK-8 was used to analyze the effect of ATO on the
viability of the THP1 cell line. The growth of THP1 cells was
inhibited by ATO in a dose- and time-dependent manner (two-way
analysis of variance, P<0.01; Fig.
2). The 48-h IC50 value for ATO in the THP1 cells
was determined as 2.99±0.09 μM.
Visualization of apoptotic cells using
light microscopy
Compared with the control groups, cells treated with
3 μM ATO for 24, 48 and 72 h exhibited typical apoptotic
characteristics, as observed in the light microscope images.
Observations included a large number of vacuoles in the cytoplasm,
higher intensity of nuclear staining, karyopyknosis and the
formation of crescents and apoptotic bodies (Fig. 3).
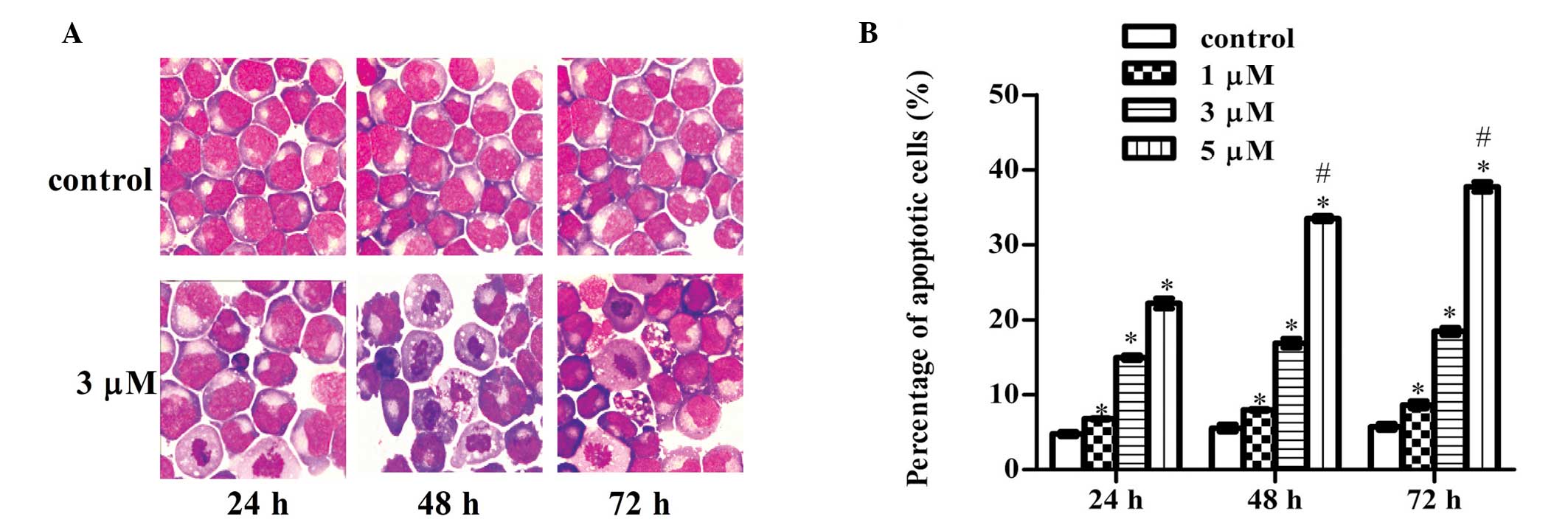 | Figure 3Induction of apoptosis by ATO in the
THP1 cell line. (A) Morphological changes in the THP1 cell line
treated with ATO were observed using light microscopy (hematoxylin
and eosin staining; magnification, ×1,000). Compared with the
control group, cells treated with 3 μM ATO for 24, 48 and 72 h
exhibited typical apoptotic characteristics, including a large
number of bubbles in the cytoplasm, higher intensity of nuclear
staining, karyopyknosis and the formation of crescents and
apoptotic bodies. (B) Fluorescence-activated cell sorting analysis
was conducted to analyze the apoptosis rate induced by ATO in THP1
cells. *P<0.05, vs. previous concentration at the
same time point; #P<0.05, vs. previous time point at
the same concentration. Results are expressed as the mean ±
standard deviation (n=3). ATO, arsenic trioxide. |
Induction of apoptosis
To clarify whether the reduction in THP1 cell
viability was due to apoptosis, fluorescence-activated cell sorting
analysis was performed. THP1 cells were treated with ATO at
concentrations of 0, 1, 3 and 5 μM for 24, 48 and 72 h. As shown in
Fig. 3, the percentage of
apoptotic THP1 cells increased significantly in a dose- and
time-dependent manner (two-way analysis of variance, P<0.01;
Fig. 3).
ATO downregulates EVI-1 mRNA and protein
expression in the THP1 cell line
RT-PCR and western blotting were used to detect the
expression levels of EVI-1 mRNA and protein, respectively, in THP1
cells treated with various concentrations of ATO (0, 1, 3 and 5 μM)
for 24, 48 and 72 h. Exposure to increasing concentrations of ATO
for increasing lengths of time was associated with gradual
inhibition in the expression of EVI-1 mRNA (Fig. 4) and protein (Fig. 5).
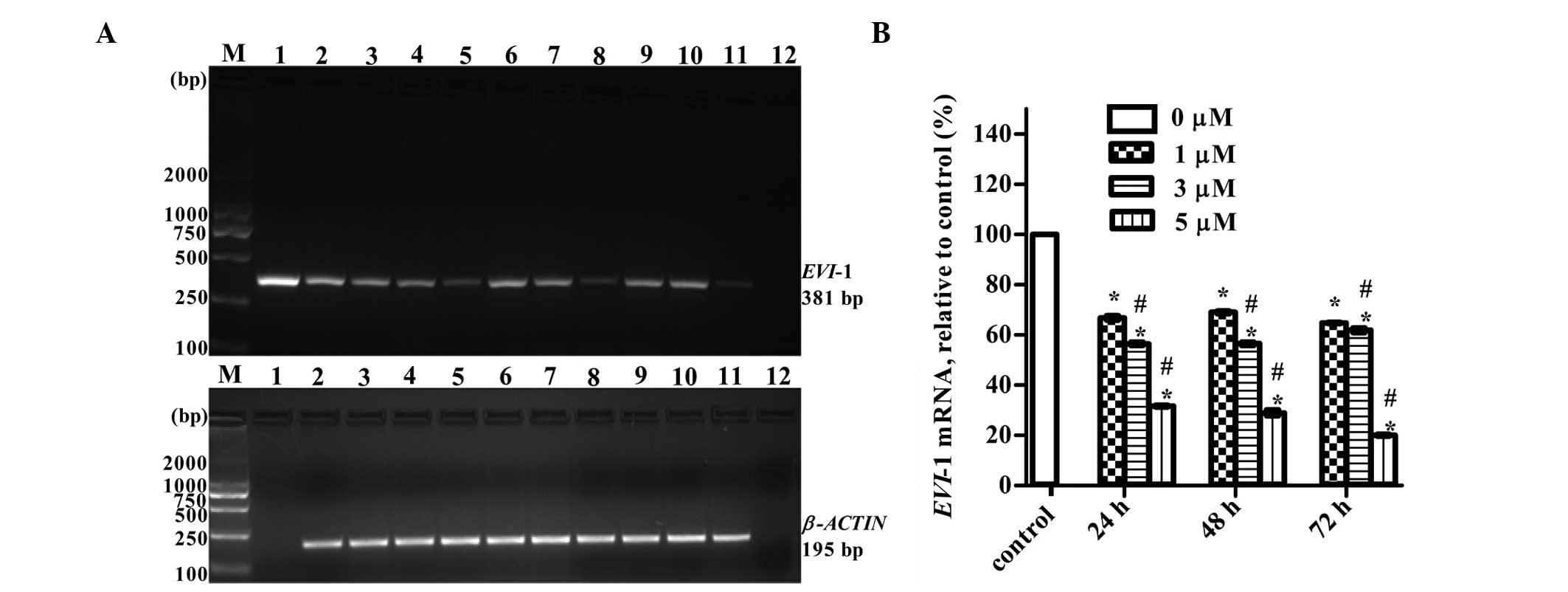 | Figure 4ATO downregulated EVI-1 mRNA
expression in the THP1 cell line. (A) Representative RT-PCR
results. Lanes: M, marker; 1, plasmid containing EVI-1 gene
(positive control); 2, control group without ATO; 3, 4 and 5, 1, 3
and 5 μM ATO for 24 h; 6, 7 and 8, 1, 3 and 5 μM ATO for 48 h; 9,
10 and 11, 1, 3 and 5 μM ATO for 72 h; and 12, blank control
without template. (B) Quantitative results are expressed as the
mean ± standard deviation (n=3). *P<0.01, vs. control
group; #P<0.05, vs. previous concentration at the
same point. ATO, arsenic trioxide; EVI-1, ecotropic viral
integration site-1; RT-PCR, reverse transcription polymerase chain
reaction. |
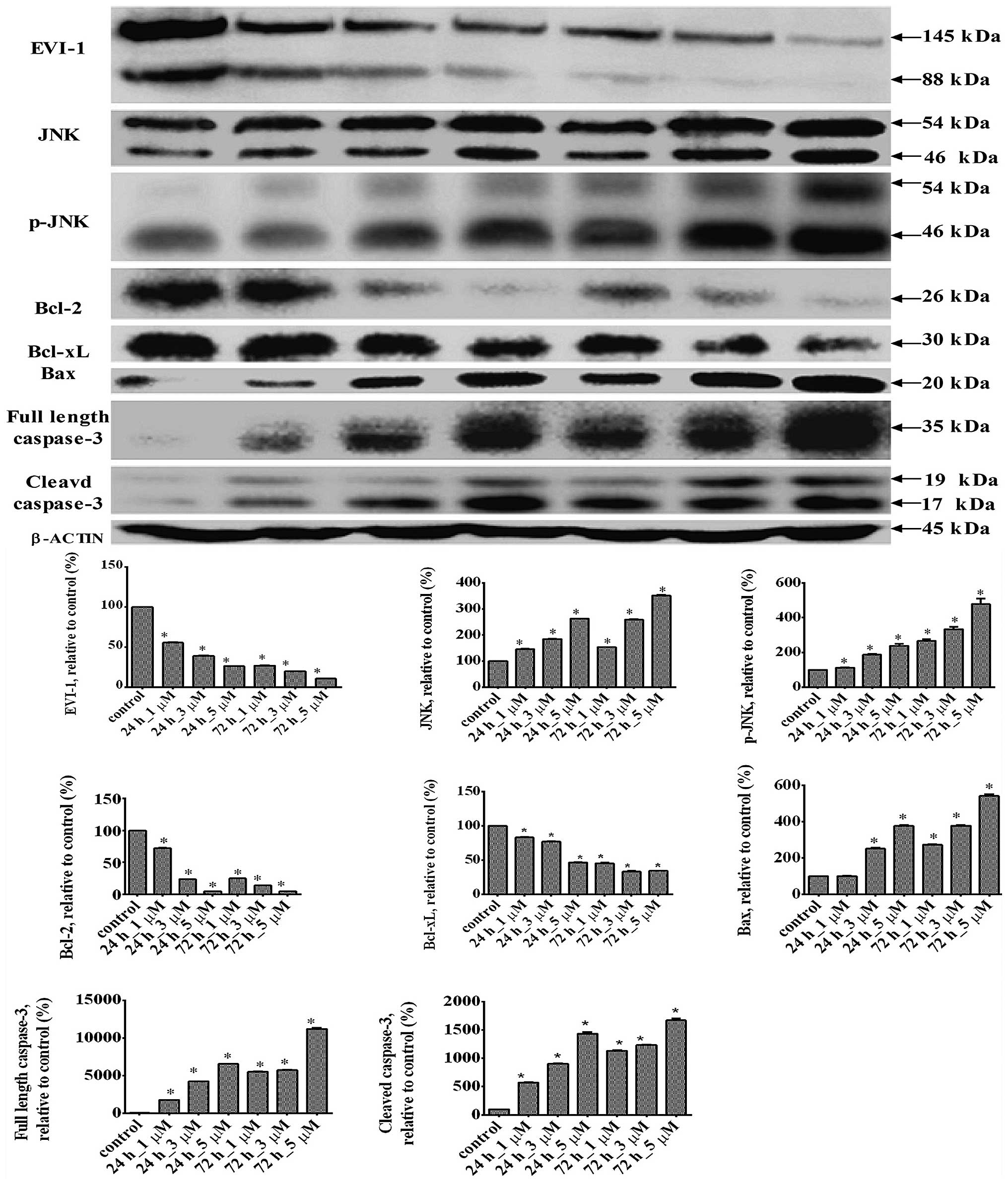 | Figure 5Changes in the protein expression
levels of EVI-1, JNK, p-JNK, Bcl-2, Bcl-xL, Bax, full-length
caspase-3 and cleaved caspase-3 in the THP1 cell line treated with
ATO at various concentrations. Representative western blot results
are shown. Results are expressed as the mean ± standard deviation
(n=3). *P<0.01, vs. control group. EVI-1, ecotropic
viral integration site-1; JNK, c-Jun N-terminal kinase; p-JNK,
phosphorylated JNK; Bcl-2, B-cell lymphoma 2; Bcl-xL, B-cell
lymphoma-extra large; ATO, arsenic trioxide. |
Changes in the expression levels of JNK,
p-JNK, Bcl-2, Bcl-xL, Bax, full length caspase-3 and cleaved
caspase-3 following ATO treatment
Western blotting was used to determine changes in
the expression levels of proteins located downstream of EVI-1. ATO
significantly increased the expression levels of JNK, p-JNK, Bax,
full length caspase-3 and cleaved caspase-3, but significantly
decreased the expression levels of Bcl-2 and Bcl-xL (Fig. 5).
Discussion
There is a long history for the use of ATO as a drug
in Chinese traditional medicine. Satisfactory results have been
achieved in the application of ATO for the treatment of APL. In the
present study, the effects of ATO on THP1 cells were investigated,
as well as the underlying mechanisms of action. The results
demonstrated that ATO effectively inhibited the proliferation of
THP1 cells. Furthermore, the present study demonstrated that ATO
induced THP1 cell apoptosis via the regulation of Bcl-2 family
proteins.
EVI-1, as an oncogene, exhibits different
characteristics from other antiapoptotic genes. Several studies
using mouse models have shown that EVI-1 is preferentially
expressed in hematopoietic stem cells (HSCs) and embryo tissues and
plays a key role in the proliferation and maintenance of HSCs. The
EVI-1 gene regulates HSC proliferation in a dose-dependent manner,
and the expression level of EVI-1 decreases with the
differentiation of HSC (7–9). Activation of EVI-1 leads to HSC
expansion and is involved in the transformation of leukemia cells
(8). Aberrant expression of EVI-1
has been found in a variety of human hematological malignancies and
solid tumors, including ovarian and colon cancers (2,4,5,10). A
previous study demonstrated that EVI-1 overexpression is an
independent negative prognostic indicator of survival in leukemia
patients (4). The results of the
present study revealed that PBMCs from the healthy adult expressed
extremely low levels of EVI-1 mRNA, however, the four leukemia cell
lines, K562, HL-60, U937 and THP1, overexpressed EVI-1 to varying
degrees, with the highest EVI-1 expression observed in THP1 cells.
Through gene sequencing analysis, it was confirmed that the THP1
cell line contains the full length of the EVI-1 gene.
Alternatively spliced forms of the EVI-1 gene encode
at least three distinct proteins: EVI-1 (145 kDa), MDS1/EVI-1 (200
kDa) and EVI-1Δ324 (88 kDa) (11).
MDS1/EVI1, a longer alternatively spliced form of EVI-1, contains
188 additional amino acids at the N-terminus that encodes a
positive regulatory (PR) domain in addition to the entire EVI-1
sequence (12). The PR domain in
MDS1/EVI-1 prevents oligomerization and affects the biochemical
functions. A large body of evidence indicates that the
PR-containing form contributes to tumor suppression, while the
PR-absent forms are oncogenic (13,14).
The EVI-1Δ324 splicing variant encodes an 88-kDa protein that lacks
324 internal amino acids and has unknown functions. The western
blotting results indicated that the THP1 cell line expressed EVI-1
proteins encoded by the EVI-1 and EVI-1Δ324 variants, but not the
MDS1/EVI-1 splicing variant. ATO downregulated the expression of
the EVI-1 protein variants in the THP1 cell line. ATO also
inhibited the mRNA expression of EVI-1 in the THP1 cells.
Inconsistent with the results of the present study, the study by
Shackelford et al cloned the human EVI-1 gene in NIH 3T3
mouse fibroblasts and mouse bone marrow and found that ATO degraded
the EVI-1 protein without affecting EVI-1 mRNA (15). We hypothesized that the cause of
this discrepancy may be the result of the various gene regulation
mechanisms between humans and mice.
EVI-1 is a zinc finger-containing and site specific
DNA-binding transcription factor. JNKs belong to the superfamily of
mitogen-activated protein kinases that are involved in the
regulation of cell proliferation, differentiation and apoptosis
(16). In response to cell death
stimuli, JNKs are activated by distinct phosphorylation. p-JNKs
then activate apoptotic signaling by regulating
apoptotic-associated genes via the transcriptional activation of
specific transcription factors or by directly modulating the
activities of mitochondrial apoptosis-associated proteins through
distinct phosphorylation events (16). The EVI-1 oncoprotein inhibits JNKs
and prevents stress-induced cell death (17). In the present study, ATO was shown
to inhibit proliferation, induce apoptosis and suppress the mRNA
and protein expression of EVI-1 in THP1 cells, as well as increase
the expression levels of JNKs and p-JNKs. Therefore, it was
hypothesized that ATO induced apoptosis in THP1 cells by
downregulating the expression of the EVI-1 gene, thus, decreasing
the repression of EVI-1 on the JNK signaling pathway. To validate
this hypothesis, changes in the expression levels of
apoptosis-associated proteins located downstream of JNKs were
investigated.
The Bcl-2 family (particularly the BH3-only
subfamily) proteins play an important role in mitochondrial
intrinsic apoptotic pathways. According to their functions, the
proteins can be divided into two categories: Proteins with
proapoptotic activities, including Bax, and proteins with
antiapoptotic activities, including Bcl-2 and Bcl-xL. Bax forms
heterodimers with Bcl-2 and Bcl-xL, thus, antagonizes the
antiapoptotic effects and induces cell apoptosis (18). The sequential activation of
caspases plays an essential role in the execution phase of
apoptosis (19,20), and caspase-3 is a central
terminator of apoptotic pathways (21). Western blot analysis demonstrated
that ATO treatment significantly reduced the expression of Bcl-xL
and Bcl-2 and induced the expression of Bax, cleaved caspase-3 and
full length caspase-3, with a concurrent increase in p-JNK
expression and apoptosis. These results also indicated that p-JNKs
upregulated the expression level of proapoptotic proteins, such as
Bax and caspase-3, as well as downregulated the expression level of
antiapoptotic proteins, including Bcl-xL and Bcl-2.
In conclusion, ATO induced apoptosis in THP1 cells
by downregulating the expression of the EVI-1 gene, thus,
decreasing the repression of EVI-1 on the JNK signaling pathway.
Furthermore, the activated JNK signaling pathway regulated the
expression level of apoptosis-associated proteins, including
Bcl-xL, Bcl-2, Bax and caspase-3. These novel observations provide
mechanistic insights into the induction of apoptosis by ATO in
EVI-1-positive leukemia cell lines and may facilitate the
development of clinical strategies to improve the therapeutic
efficacy of the treatment of EVI-1-positive human cancers. In
addition, an EVI-1 transgenic zebrafish model has already been
established (22), and future
studies should focus on investigating the more specific mechanisms
underlying the effects of ATO on EVI-1 gene expression in
vitro and in vivo.
Acknowledgements
The authors thank the members of the Leukemia
Research Institute in Ren Ji Hospital. The study was supported by
grants from the Shanghai Municipal Bureau of Health Major Subject,
the class of traditional Chinese medicine (no. ZYSNXD-CC-ZDYJ001),
the TCM Guide Project from the Natural Science Foundation of
Shanghai of China (no. 12401906700) and the National Natural
Science Funds of China (no. 81300405).
References
|
1
|
Liang R, Bai QX, Zhang YQ, et al: Reduced
tumor lysis syndrome with low dose chemotherapy for hyperleukocytic
acute leukemia prior to induction therapy. Asian Pac J Cancer Prev.
12:1807–1811. 2011.PubMed/NCBI
|
|
2
|
Krivtsov AV, Figueroa ME, Sinha AU, et al:
Cell of origin determines clinically relevant subtypes of
MLL-rearranged AML. Leukemia. 27:852–860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barjesteh van Waalwijk van
Doorn-Khosrovani S, Erpelinck C, van Putten WL, et al: High EVI1
expression predicts poor survival in acute myeloid leukemia: a
study of 319 de novo AML patients. Blood. 101:837–845.
2003.PubMed/NCBI
|
|
4
|
Konantz M, André MC, Ebinger M, et al:
EVI-1 modulates leukemogenic potential and apoptosis sensitivity in
human acute lymphoblastic leukemia. Leukemia. 27:56–65. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Weer A, Poppe B, Cauwelier B, et al:
Screening for EVI1: ectopic expression absent in T-cell acute
lymphoblastic leukemia patients and cell lines. Cancer Genet
Cytogenet. 171:79–80. 2006.PubMed/NCBI
|
|
6
|
Valk PJ, Verhaak RG, Beijen MA, et al:
Prognostically useful gene-expression profiles in acute myeloid
leukemia. N Engl J Med. 350:1617–1628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuasa H, Oike Y, Iwama A, et al: Oncogenic
transcription factor Evi1 regulates hematopoietic stem cell
proliferation through GATA-2 expression. EMBO J. 24:1976–1987.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goyama S, Yamamoto G, Shimabe M, et al:
Evi-1 is a critical regulator for hematopoietic stem cells and
transformed leukemic cells. Cell Stem Cell. 3:207–220. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoyt PR, Bartholomew C, Davis AJ, et al:
The Evi1 proto-oncogene is required at midgestation for neural,
heart, and paraxial mesenchyme development. Mech Dev. 65:55–70.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brooks DJ, Woodward S, Thompson FH, et al:
Expression of the zinc finger gene EVI-1 in ovarian and other
cancers. Br J Cancer. 74:1518–1525. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morishita K, Parganas E, Douglass EC and
Ihle JN: Unique expression of the human Evi-1 gene in an
endometrial carcinoma cell line: sequence of cDNAs and structure of
alternatively spliced transcripts. Oncogene. 5:963–971.
1990.PubMed/NCBI
|
|
12
|
Fears S, Mathieu C, Zeleznik-Le N, Huang
S, Rowley JD and Nucifora G: Intergenic splicing of MDS1 and EVI1
occurs in normal tissues as well as in myeloid leukemia and
produces a new member of the PR domain family. Proc Natl Acad Sci
USA. 93:1642–1647. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang GL and Huang S: The yin-yang of
PR-domain family genes in tumorigenesis. Histol Histopathol.
15:109–117. 2000.PubMed/NCBI
|
|
14
|
Nitta E, Izutsu K, Yamaguchi Y, et al:
Oligomerization of Evi-1 regulated by the PR domain contributes to
recruitment of corepressor CtBP. Oncogene. 24:6165–6173. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shackelford D, Kenific C, Blusztajn A,
Waxman S and Ren R: Targeted degradation of the AML1/MDS1/EVI1
oncoprotein by arsenic trioxide. Cancer Res. 66:11360–11369. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 27:6245–6251. 2008. View Article : Google Scholar
|
|
17
|
Kurokawa M, Mitani K, Yamagata T, et al:
The evi-1 oncoprotein inhibits c-Jun N-terminal kinase and prevents
stress-induced cell death. EMBO J. 19:2958–2968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garrison SP, Phillips DC, Jeffers JR, et
al: Genetically defining the mechanism of Puma- and Bim-induced
apoptosis. Cell Death Differ. 19:642–649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coiras M, López-Huertas MR, Mateos E and
Alcami J: Caspase-3-mediated cleavage of p65/RelA results in a
carboxy-terminal fragment that inhibits IkappaBalpha and enhances
HIV-1 replication in human T lymphocytes. Retrovirology. 5:1092008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun C, Guo XX, Zhu D, et al: Apoptosis is
induced in cancer cells via the mitochondrial pathway by the novel
xylocydine-derived compound JRS-15. Int J Mol Sci. 14:850–870.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang Y, Duan W, Liang Z, et al: Curcumin
attenuates endothelial cell oxidative stress injury through Notch
signaling inhibition. Cell Signal. 25:615–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin WJ, Shen LJ, Chen FY, Zhong M, Sun RL
and Zhong JH: The establishment of AML1-EVI1 transgenic zebrafish
model and regulation of hematopoietic transcription factors.
Journal of Internal Medicine Concepts & Practice. 3:192–196.
2013.(In Chinese).
|