Introduction
Osteoarthritis (OA) is a type of chronic progressive
osteoarthropathy that is common in middle-aged individuals. The
prevalence of OA is increasing annually and has become the leading
cause of joint pain and disability in the elderly. OA causes an
enormous economic burden to patients, the family of patients and to
society. Thus, an effective prevention of OA has become a major
public health issue. A number of methods, including systemic
treatment, medication and surgery, have been adopted over the past
years, however, the clinical efficacy requires improvement
(1).
In recent years, motion therapy has been applied
extensively for OA treatment. Clinical and animal experiments have
shown that regular motion therapy can relieve the symptoms of knee
OA and improve knee function (2–4).
However, research into motion therapy for the treatment of OA is
limited, and there are a number of limitations associated,
including whether exercise is effective in all types of OA or only
to a particular type of OA. In addition, the effect of strength,
frequency, opportunity and mechanism of motion therapy on OA
treatment is unknown.
Running is a common exercise. In the present study,
an OA degeneration model was constructed using knee fracture. A
PT98 rat running device was used to simulate running exercise for
early- and middle-stage OA. Staining techniques, namely,
hematoxylin and eosin (HE), toluidine blue and
immunohistochemistry, as well as transmission electron microscopy
(TEM), were used to observe the effect of proper passive motion on
cartilage thickness, the cartilage collagen matrix, protein
proteoglycan content and the morphological structure of the
cartilage in the OA rat model. The effect of proper passive motion
on OA degenerative cartilage and other aspects were investigated,
as well as the possible mechanism.
Materials and methods
Experimental animals
The study was approved by the Ethics Committee of
Tongji University (Shanghai, China) and the study followed the
animal care guidelines of Tongji University. In total, 40
Sprague-Dawley male rats (age, 8 weeks; weight, 200–220 g) that
were specific-pathogen free, were provided by the Experimental
Animal Center of Tongji University. Knee fracture (5) was used to construct early- and
middle-stage rat articular OA degeneration models, which were fixed
for three and six weeks, with 20 rats assigned for each group. The
animals were divided randomly into exercise and control groups for
each stage, from which each group was divided into three- and
six-week exercise subgroups. In the exercise group, a PT98 electric
animal treadmill was used at 15 m/min for 1 h/day. By contrast,
rats in the control group were free following removal of the
plaster. During the experimental process, the animals were housed
separately and were provided with natural light and free access to
a diet. Feeding conditions were maintained at 20–23°C, with a
relative humidity of 50–55%.
Sampling and handling for light
microscopy
All the rats in the experimental groups were
sacrificed at a set time. Cartilage tissues in the internal
epicondyle of the femur were extracted and subjected to fixation,
decalcification and progressive dehydration. The samples were
sliced following embedding for HE, toluidine blue and
immunohistochemical staining detection.
Sampling and handling for TEM
Cartilage specimens were extracted by obtaining a
1×1×1 mm tissue section from the weight-bearing area of the condyle
from the femur. The specimen was sliced into ultra-thin sections
and was subjected to fixation, decalcification, gradient
dehydration and embedment. Next, the specimen was observed with
TEM, following double staining with uranygl acetate and 70% lead
nitrate.
HE staining
Surfaces of the articular cartilage, chondrocytes,
cartilage matrix and the tidemark were observed with light
microscopy. Two independent observers prepared slice ratings
according to the Mankin rating (6), which were averaged to derive Mankin’s
scores. Images were observed with light microscopy (magnification,
×10 and ×40) and were stored into an Image-Pro Plus 6.0 image
analysis system (Media Cybernetics, Inc., Rockville, MD, USA) to
determine the thickness of the cartilage layer (distance between
the cartilage surface and the junction of the calcified cartilage
and bone). In total, five specimens were obtained for each sample,
five visual fields were collected for each specimen and the average
value was computed.
Toluidine blue staining,
immunohistochemistry and terminal deoxynucleotidyl transferase dUTP
nick end labeling (TUNEL) apoptosis assay
A type II collagen polyclonal antibody SP
immunohistochemical staining kit and a TUNEL in situ
apoptosis detection kit (Wuhan Boster Biological Technology, Co.,
Ltd., Wuhan, China) were used according to the manufacturer’s
instructions.
An Image-Pro Plus 6.0 image analysis system was
adopted and five visual fields were selected for each specimen. The
average absorbance value of the positive staining in the unit area
was measured to express the proteoglycan content in the cell matrix
by semiquantitative measurements conducted with light microscopy
(magnification, ×10 and ×40).
TEM observations
TEM (JEM-1230; JEOL, Tokyo, Japan) was used to
observe the cartilage surface, fiber and ultrastructure of the
cells; images were captured.
Biomechanical test
Knee articulation was applied to prepare a
combination specimen of the femur and tibia (retention lengths of
the femur and tibia were 3–4 cm from the knee articulation, soft
tissues were removed and only the medial accessory ligament was
retained). A CSS-44010 type electronic universal testing machine
(Changchun Institute of Mechanical Science, Co., Ltd, Changchun,
China) was used to stretch the specimen to cause rupture, from
which the maximum load was obtained and was measured in
Newtons.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the
mean ± standard deviation. Comparisons between two groups were
performed using the t-test, where P<0.05 was considered to
indicate a statistically significant difference.
Results
Thickness of the articular cartilage and
the Mankin rating
Thicknesses of the articular cartilage and the
Mankin ratings of all the groups following passive motion are shown
in Table I.
 | Table IComparison of cartilage thicknesses
and Mankin ratings among the groups (mean ± SD). |
Table I
Comparison of cartilage thicknesses
and Mankin ratings among the groups (mean ± SD).
| Early-stage OA | Middle-stage OA |
|---|
|
|
|
|---|
| Cartilage thickness
(μm) | Mankin rating | Cartilage thickness
(μm) | Mankin rating |
|---|
|
|
|
|
|
|---|
| Subgroup | Control group | Exercise group | Control group | Exercise group | Control group | Exercise group | Control group | Exercise group |
|---|
| 3-week | 223±23 | 244±20 | 4.39±0.97 | 3.93±1.16 | 217±25 | 233±19 | 8.93±1.73 | 8.39±1.63 |
| 6-week | 224±20 | 271±28 | 6.35±1.57 | 4.02±1.19 | 211±19 | 249±21 | 10.75±3.12 | 10.02±2.57 |
Results for the early-stage OA rats demonstrated
that the cartilage thickness of the three-week group increased
significantly when compared with the control group (P<0.05). In
addition, the cartilage thickness of the six-week group increased
significantly compared with the control and three-week groups
(P<0.05). These results indicated that proper passive motion
significantly increased the thickness of the articular cartilage in
rats with early-stage OA that had been caused by knee fracture, and
the effect was improved with prolonged exercise duration.
Mankin ratings of the two experimental early-stage
OA groups were significantly lower compared with the respective
control groups (P<0.05). However, no significant difference was
observed between the two experimental groups. These results
indicated that proper passive motion repaired and improved the
structure and function of chondrocytes in rats with early-stage OA
caused by knee fracture, as well as delayed the degeneration of
articular cartilage.
In the middle-stage OA group, the cartilage samples
in the three- and six-week groups were thicker compared with the
control groups (P<0.05), indicating that proper passive motion
increased the thickness of the cartilage in rats with middle-stage
OA caused by knee fracture.
Mankin ratings of the two exercise middle-stage OA
groups did not increase or decrease compared with the respective
control groups (P>0.05). However, statistically significant
differences were observed between the six- and three-week control
groups, and between the six- and three-week experimental groups
(P<0.05). These results indicated that proper passive motion
improved the thickness of articular cartilage in rats with
middle-stage OA caused by knee fracture. However, the repairing
effect and improvements to the structure and function of the
chondrocytes were not significant.
Levels of proteoglycans and type II
collagen fibers
Absorbance values from the toluidine blue and
immunohistochemical staining of all the groups following proper
passive motion are shown in Table
II.
 | Table IIComparison of toluidine blue
absorbance and immunohistochemical staining following passive
motion (mean ± SD). |
Table II
Comparison of toluidine blue
absorbance and immunohistochemical staining following passive
motion (mean ± SD).
| Early-stage OA | Middle-stage OA |
|---|
|
|
|
|---|
| Proteoglycans | Type II collagen
fibers | Proteoglycans | Type II collagen
fibers |
|---|
|
|
|
|
|
|---|
| Subgroup | Control group | Exercise group | Control group | Exercise group | Control group | Exercise group | Control group | Exercise group |
|---|
| 3-week | 0.33±0.06 | 0.48±0.05 | 0.36±0.05 | 0.42±0.08 | 0.29±0.04 | 0.35±0.09 | 0.32±0.03 | 0.37±0.09 |
| 6-week | 0.31±0.05 | 0.55±0.04 | 0.35±0.05 | 0.54±0.09 | 0.28±0.05 | 0.39±0.06 | 0.38±0.02 | 0.43±0.05 |
Statistically significant differences were observed
in the levels of proteoglycans and type II collagen fibers in the
cartilage among all the groups following proper passive motion for
the early- and middle-stage OA rats (P<0.05). Thus, proper
passive motion significantly increased the content of proteoglycans
and type II collagen fibers, and the effect was enhanced in cases
of prolonged exercise for six weeks. In addition, statistically
significant differences were identified in the proteoglycan and
type II collagen fiber content between the early- and middle-stage
OA experimental groups (P<0.05). Thus, proper passive motion
exhibits better repairing effects on articular cartilage in
early-stage OA compared with middle-stage OA at the same strength
and duration.
Chondrocyte apoptosis TUNEL staining
Absorbance values from the TUNEL staining apoptosis
assays of all the experimental groups following proper passive
motion are shown in Table III.
Statistically significant differences were identified in the
chondrocyte apoptotic rates among all the groups following passive
motion in the early-stage OA rats (P<0.05). This result
indicated that passive motion significantly reduced the chondrocyte
apoptotic rate, and the apoptotic rate was reduced significantly
within six weeks. For the middle-stage OA rats, the chondrocyte
apoptotic rates in the experimental groups were lower compared with
the respective control groups following passive motion, however, no
statistically significant differences were observed (P>0.05). In
addition, no marked difference was observed between the two
experimental groups. These results indicated that six weeks of
proper passive motion did not significantly increase the effect of
reducing apoptosis with prolonged exercise for the middle-stage OA
rats. Statistically significant differences were observed between
the early- and middle-stage OA experimental groups (P<0.05),
which indicated that proper passive motion had better repairing
effects on chondrocytes in early-stage OA compared with
middle-stage OA at the same strength and duration.
 | Table IIIComparison of TUNEL staining
absorbances of the articular cartilages following passive motion
(mean ± SD). |
Table III
Comparison of TUNEL staining
absorbances of the articular cartilages following passive motion
(mean ± SD).
| Early-stage OA | Middle-stage OA |
|---|
|
|
|
|---|
| Group | 3-week | 6-week | 3-week | 6-week |
|---|
| Control | 0.41±0.03 | 0.47±0.05 | 0.53±0.04 | 0.58±0.07 |
| Experimental | 0.26±0.02 | 0.22±0.02 | 0.49±0.07 | 0.55±0.03 |
Ultrastructure of the articular
cartilage
TEM observations of the articular cartilage
ultrastructure following passive motion for all the experimental
groups are shown in Fig. 1. In the
three-week early-stage OA control group, the cartilage surface
exhibited a wave-like, low, flat and rough surface. The number of
cytoplasmic organelles was reduced, while the number of thread-like
or finely granular matters increased. Projections on the surface of
the cells were reduced and the content of collagen fibers
decreased. In the six-week early-stage OA control group, partial
fracture of the cartilage surface and degeneration of the
chondrocytes were observed. In addition, the size of the cells had
decreased and cell nuclei were deformed, with chromatin
distribution uneven and visible fat droplets. In the three-week
early-stage OA experimental group, no apparent nude chondrocytes
and collagen were observed. The number of cellular projections
increased on the chondrocyte surface, as well as the number of
cytoplasmic organelles, with the matrix fibers arranged more
tightly. In the six-week early-stage OA exercise group, the
chondrocyte surfaces were basically complete and the chondrocyte
volume had increased. Projections in the endoplasmic reticulum and
on the surface of the cells increased, with a higher frequency of
thicker collagen fibers. In the three-week middle-stage OA control
group, the collagen texture was indistinct and the connections were
blocked. A large gap existed between the chondrocytes and lacuna,
and the number of projections on the cell surface had decreased. In
the six-week middle-stage OA control group, partial fractures
existed on the chondrocyte surfaces and vague collagen texture was
observed. The cell outline was unclear or missing, and cell nuclei
were deformed or not present. In the three-week middle-stage OA
experimental group, the cartilage surface exhibited wave-like, low
and flat characteristics, and basically clear collagen texture with
an even distribution and connections. Chondrocytes were smaller,
showing a small number of mitochondria, endoplasmic reticulum and
Golgi bodies. In the six-week middle-stage OA experimental group,
fewer cells were observed. A number of the chondrocyte outlines
were not distinct and exhibited cracks, and the number of
projections on the cell surface were reduced. Unclear collagen
texture was enhanced, and the number of collagen fibers was
reduced.
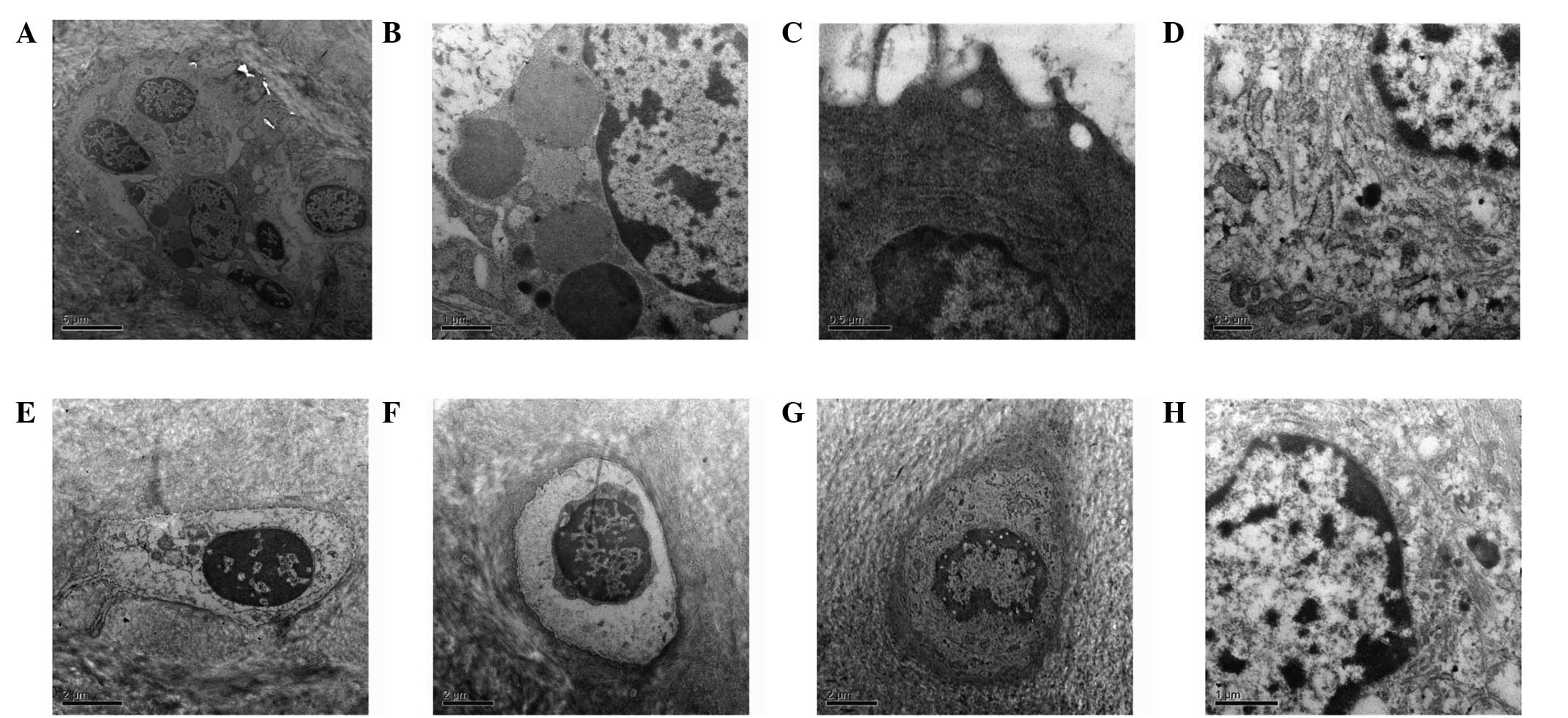 | Figure 1TEM images of the articular cartilage
ultrastructure following passive motion in the (A) three-
(magnification, ×5,000) and (B) six-week early-stage OA control
(magnification, ×12,000), (C) three- and (D) six-week early-stage
OA experimental (magnification for both, ×20,000), (E) three- and
(F) six-week middle-stage OA control and (G) three- (magnification
for all three, ×8,000) and (H) six-week middle-stage OA
experimental groups (magnification, × 12,000). TEM, transmission
electron microscopy; OA, osteoarthritis. |
Stretching resistance of the medial
collateral ligament of the knee joint combination
Stretching resistances of the knee femur-medial
collateral ligament-tibia combination in the rats in all the groups
following passive motion are shown in Table IV.
 | Table IVComparison of medial collateral
ligament combination stretching resistances following passive
motion (mean ± SD). |
Table IV
Comparison of medial collateral
ligament combination stretching resistances following passive
motion (mean ± SD).
| Early-stage OA | Middle-stage OA |
|---|
|
|
|
|---|
| Stretching force
(N) | Weight (g) | Stretching force
(N) | Weight (g) |
|---|
|
|
|
|
|
|---|
| Subgroup | Control group | Exercise group | Control group | Exercise group | Control group | Exercise group | Control group | Exercise group |
|---|
| 3-week | 14.91±1.45 | 18.62±1.19 | 225±15 | 221±16 | 14.25±1.07 | 16.51±1.22 | 279±25 | 278±18 |
| 6-week | 15.20±1.61 | 20.25±1.22 | 261±16 | 266±22 | 13.92±1.16 | 18.37±1.16 | 310±35 | 313±37 |
No statistically significant difference was
identified in the animal weight between the experimental and
control groups. Therefore, the difference in ligament tension
caused by weight difference was discounted.
In the early- and middle-stage OA models, the
ligament tension in the three-week experimental groups increased
significantly compared with the respective control groups
(P<0.05). In addition, the ligament tension of the six-week
experimental groups increased significantly compared with the
respective control and three-week groups (P<0.05). These
observations indicated that proper passive motion significantly
increased the medial collateral ligament tension in the knee joint
and enhanced the stability of the knee joint of rats with OA caused
by knee fracture. In addition, the effect was improved with
prolonged exercise duration.
Discussion
The repair of articular cartilage in OA is an issue
of great concern to clinicians, since no conclusive treatment for
OA has been established to date.
In recent years, motion therapy has been applied
extensively in the treatment of OA. Motion therapy is a technique
of relieving symptoms of patients or improving their function using
mechanical factors. This method is considered as an important
nonpharmacological therapy that can improve the repairing effect of
degenerated articular cartilage and promote the restoration of
articular cartilage morphology. Application of motion therapy can
improve muscle strength, increase the activity of the knee joint
and improve the motion dysfunction of OA patients (7). OA is a disease that primarily
involves the articular cartilage, thus, exercise is required to
enhance the metabolism of articular cartilage in order to repair
the damaged surface of the cartilage and remove the inflammatory
substances (8). The repairing
effect of exercise on degenerated articular cartilage has been
accepted by a number of scholars. However, the possible effect
mechanism of motion therapy on articular cartilage is not
well-defined.
In the present study, knee joint fracture was
applied to prepare an OA degeneration model. A PT98 rat running
device was used to investigate the possible mechanism and effect of
proper passive motion on the articular cartilage of rats with
various stages of OA. The results indicated that for early-stage
OA, following three weeks of exercise, the Mankin rating of the
articular cartilage decreased gradually and the thickness of the
cartilage layer increased when compared with the control group. In
addition, the strength resistance of the medial collateral ligament
increased progressively, and the proteoglycan content of the
cartilage matrix, type II collagen fibers and cell apoptotic rate
evidently decreased. TEM images revealed that the number of
projections on the surface of the chondrocytes increased, with
basically complete surfaces of cartilage and without nude
chondrocytes or collagen. Following six weeks of exercise, the
thickness of the cartilage layer and ligament tension, as well as
the levels of proteoglycans and type II collagen fibers, increased
when compared with the control and three-week experimental groups.
Additionally, the apoptotic rate decreased and the Mankin rating
was lower when compared with the control group. TEM observations
also revealed that the cartilage surface was repaired, since the
chondrocytes grew in number and size with markedly increased
projections on the cell surface, and matrix fibers were arranged
firmly with the number of collagen fibers increasing and becoming
thicker. The results demonstrated that proper running can repair
the surface of cartilage damaged by early-stage OA, and the
degenerated cartilage can be healed and regenerated through
exercise. Joint exercise may reduce the occurrence of OA by
promoting the regeneration and repair of chondrocytes, which may be
a key reason that the less severe the OA disease, the more complete
the repair. Salter et al (9) demonstrated that joint movement
prevented the complications caused by joint fracture and stimulated
a full layer of cartilage to be healed. Callus is similar to
hyaline cartilage in morphology. A previous study demonstrated that
joint movement can generate periodical pressure change in the
joint, favorable to the in-joint exchange of nutrients and liquid
via the synovial hole, which stimulates chondrocyte metabolism and
promotes the synthesis of cartilage matrix protein and internal
tissue reconstruction (10).
For the middle-stage OA rats, the cartilage layer
following three weeks of exercise became thicker than that of the
control group, with increased levels of proteoglycans and type II
collagen fibers. In addition, the Mankin rating was lower compared
with the control group, but the apoptotic rate was unchanged at
this stage. The cartilage layer following six weeks of exercise
became thicker than that of the control group, with a significant
increase in the levels of proteoglycans and type II collagen
fibers. The Mankin rating was also lower compared with the control
and three-week groups, although no statistically significant
differences were observed with regard to proteoglycan content in
the cartilage matrix, type II collagen fiber staining or Mankin
rating. The six-week experimental group exhibited increased
ligament tension when compared with the control and three-week
experimental group, but no significant reduction in the apoptotic
rate was observed. TEM observations revealed that the chondrocytes
remained smaller, with fewer round projections on the surface. In
addition, the cartilage surface was wave-like, low and flat, with
partial rough fractures and collagen fiber connections remaining
slightly disoriented. Therefore, proper running can improve
cartilage matrix damaged by middle-stage OA, although no marked
repair in the degenerated chondrocytes was observed. The possible
reason is dynamic mechanical loads on articular cartilage are
exerted against the effects of the inflammatory mediator, bacterial
lipopolysaccharide, and improve the expression of type II collagen
and proteoglycans (11). This
phenomenon aids the recovery of collagen and proteoglycan, as well
as improves the quality of articular cartilage rehabilitation.
The rehabilitation effect of motion on degenerated
cartilage is more significant in a knee fracture model, which may
be associated with the pathogenesis (12). Articular cartilage degeneration in
a knee fracture model is a result of poor nutrition. Proper joint
motion can promote the secretion of synovial fluid, which is
required to maintain the normal metabolism of articular cartilage.
Passive and constant motion therapy is based on periodical press
joint (13). This therapy can
improve cartilage nutrition, enhance the strength, thickness and
elasticity of articular cartilage and inhibit surface rupture and
inflammation caused by cartilage denaturation or degeneration.
These conditions enhance and recover the maximum load on the
extension position of the knee and the stability of the knee joint,
as well as significantly relieve pain and benefit load. They can
also further increase and strengthen the knee stability and motion
function.
OA patients usually suffer from decreased muscle
strength around the joint, causing joint instability, which is a
risk factor for OA progression (14). Proper motion can increase the
muscle strength around the joint, enhance joint instability and
relieve pain. The present study demonstrated that the stretching
resistances of the medial ligament in the early and middle-stage OA
experimental rat models were markedly increased following passive
motion. In addition, the ligament tension increased with prolonged
exercise duration, which is favorable for the stability of the
joint. Deyle et al (15)
conducted a random control experiment with 83 knee OA patients. The
treatment group received four months exercise using a stationary
bike, and the results showed that the exercise markedly improved
the knee function rating, the degree of pain and stiffness, body
activity and the travel distance of the patients. A number of
similar studies have been conducted and consistent results have
been obtained (16–19). Furthermore, motion can promote
systemic and articular local blood circulation, as well as maintain
better functional status of the body movement, to further prevent
secondary damage to the joint (20).
Individuals with abnormal joint anatomical
structure, severe joint damage or past injury, poor joint
stability, joint or muscle innervation disorder, poor muscle
strength or who are overweight are more prone to suffering from OA.
These individuals may benefit from regular physical activity that
protects joints from damage, thereby maintaining or increasing
muscle strength, harmonization and the overall state of the joint
(21).
Thus, the results of the present study demonstrate
that proper passive motion can improve the repairing effect of
degenerated cartilage, promote the morphological rehabilitation of
articular cartilage, improve the metabolism of chondrocytes and
delay the degeneration progress of degenerated cartilage. The
repairing effect of motion on degenerated cartilage is more
significant if the disease is not severe, since joint motion may
reduce the occurrence of OA via the promotion of the rehabilitation
and repairing of early-stage OA. Proper passive motion has no
significant repairing effect on the degenerated chondrocytes for
middle- and late-stage OA, since the main effect is to improve the
damaged cartilage matrix and enhance the stability of the joint.
Thus, proper passive motion can effectively block OA through
providing better rehabilitation on the damaged cartilage.
References
|
1
|
Wang YB and Wang HF:
Rehabilitation-solution of full treatment of knee joint
osteoarthritis. Chin J Rehabil Med. 27:4–7. 2012.(In Chinese).
|
|
2
|
Gu Y, Dai K and Qiu S: A morphological
study of degenerative mechanism of articular cartilage by abnormal
high stress. Chinese Journal of Orthopaedics. 33:597–600. 1995.(In
Chinese).
|
|
3
|
Moran ME, Kim HK and Salter RB: Biological
resurfacing of full-thickness defects in patellar articular
cartilage of the rabbit. Investigation of autogenous periosteal
grafts subjected to continuous passive motion. J Bone Joint Surg
Br. 74:659–667. 1992.
|
|
4
|
Brismée JM, Paige RL, Chyu MC, et al:
Group and home-based tai chi in elderly subjects with knee
osteoarthritis: a randomized controlled trial. Clin Rehabil.
21:99–111. 2007.PubMed/NCBI
|
|
5
|
Zhang H, Jiang HP and Wang DP: Discussion
of osteoarthritic animal model get from immobilized knees of rabbit
in full extension using plaster cast. China Journal of Modern
Medicine. 16:1843–1844. 1848.2006.(In Chinese).
|
|
6
|
Mankin HJ, Dorfman H, Lippiello L and
Zarins A: Biochemical and metabolic abnormalities in articular
cartilage from osteo-arthritic human hips. II Correlation of
morphology with biochemical and metabolic data. J Bone Joint Surg.
53:523–537. 1971.PubMed/NCBI
|
|
7
|
Maurer BT, Stern AG, Kinossian B, Cook KD
and Schumacher HR Jr: Osteoarthritis of the knee: isokinetic
quadriceps exercise versus an educational intervention. Arch Phys
Med Rehabil. 80:1293–1299. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang MH, Lin YS, Yang RC and Lee CL: A
comparison of various therapeutic exercises on the functional
status of patients with knee osteoarthritis. Semin Arthritis Rheum.
32:398–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salter RB, Simmonds DF, Malcolm BW, Rumble
EJ, MacMichael D and Clements ND: The biological effect of
continuous passive motion on the healing of full-thickness defects
in articular cartilage. An experimental investigation in the
rabbit. J Bone Joint Surg Am. 62:1232–1251. 1980.
|
|
10
|
Lee MS, Ikenoue T, Trindade MC, et al:
Protective effects of intermittent hydrostatic pressure on
osteoarthritic chondrocytes activated by bacterial endotoxin in
vitro. J Orthop Res. 21:117–122. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimizu T, Videman T, Shimazaki K and
Mooney V: Experimental study on the repair of full thickness
articular cartilage defects: effects of varying periods of
continuous passive motion, cage activity, and immobilization. J
Orthop Res. 5:187–197. 1987. View Article : Google Scholar
|
|
12
|
Sandoval R: Proximal femur fracture in a
patient referred to a physical therapist for knee pain. J Orthop
Sports Phys Ther. 41:7952011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bonutti P, Marulanda GA, McGrath MS, et
al: Static progressive stretch improves range of motion in
arthrofibrosis following total knee arthroplasty. Knee Surg Sports
Traumatol Arthrosc. 18:194–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mangani I, Cesari M, Kritchevsky SB, et
al: Physical exercise and comorbidity. Results from the Fitness and
Arthritis in Seniors Trial (FAST). Aging Clin Exp Res. 18:374–380.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deyle GD, Henderson NE, Matekel RL, Ryder
MG, Garber MB and Allison SC: Effectiveness of manual physical
therapy and exercise in osteoarthritis of the knee. A randomized,
controlled trial. Ann Intern Med. 132:173–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin SY, Davey RC and Cochrane T: Community
rehabilitation for older adults with osteoarthritis of the lower
limb: a controlled clinical trial. Clin Rehabil. 18:92–101. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foley A, Halbert J, Hewitt T and Crotty M:
Does hydrotherapy improve strength and physical function in
patients with osteoarthritis - a randomised controlled trial
comparing a gym based and a hydrotherapy based strengthening
programme. Ann Rheum Dis. 62:1162–1167. 2003. View Article : Google Scholar
|
|
18
|
Xuan Y, Lu YL and Li J: Exercise therapy
in osteoarthritis of the knee. Chinese Journal of Rehabilitation
Medicine. 18:523–525. 2003.(In Chinese).
|
|
19
|
Liu WM: The effect of isokinetic exercise
on function and symptoms of knee joint osteoarthritis patients.
Chinese Journal of Clinical Rehabilitation. 7:302–309. 2003.
|
|
20
|
Pincivero DM, Lephart SM and Karunakara
RG: Relation between open and closed kinematic chain assessment of
knee strength and functional performance. Clin J Sport Med.
7:11–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buckwalter JA and Mankin HJ: Articular
cartilage: degeneration and osteoarthritis, repair, regeneration,
and transplantation. Instr Course Lect. 47:487–504. 1998.PubMed/NCBI
|















