Introduction
Prostate cancer is a common type of tumor in elderly
males, and is the second most common cancer in males worldwide
(1). Prostate cancer accounts for
11% of all types of tumor in males, and ~9% of the total
mortalities in males with cancer (2). The therapy of prostate cancer is
interlinked with clinical staging, mainly comprising active
surveillance, radical surgery, radiotherapy and endocrine therapy.
As the early stage of prostate cancer is always accompanied by a
lack of clinical symptoms, the majority of patients miss out on the
opportunity of surgical treatment due to the detection of their
prostate cancer at the advanced stage, which leads to a poor
prognosis. Therefore, it is necessary to explore novel anticancer
drugs.
As a novel biological treatment strategy, oncolytic
viruses have become a topic of particular interest in the
laboratory and they are specifically targeted to kill tumor cells
while leaving normal cells intact (3). Through intensive investigation, the
oncolytic potentials of viruses have been discovered in six major
viral families: reovirus type 3, flaviviruses, papillomaviruses,
hepadnaviruses, retroviruses and herpesviruses (4,5).
Among these, reovirus and smallpox virus are in phase II clinical
trials at present (6,7). However, as the majority of current
oncolytic viruses undergoing study are modified viruses, the
exploration of novel natural oncolytic viruses with little toxicity
is urgently required. A study has revealed that reoviruses
preferentially replicate in cells that have an activated Ras
pathway due to either Ras mutation or upregulated levels of
epidermal growth factor receptor signaling (8). As Ras activation exists in 30–40% of
human tumors but not in normal cells (9), the use of oncolytic viruses presents
an attractive prospect. Bluetongue virus (BTV) is of the
Orbivirus (ring or circle in Greek) genus in the Reoviridae
family, which also includes reoviruses (10). BTV is the causal agent of
bluetongue disease in domestic cattle and wild ruminants and is
non-pathogenic to humans; thus, humans do not have pre-existing
antibodies to BTV (11). BTV is a
natural oncolytic virus that has been confirmed to exert powerful
oncolytic activity against numerous types of cancer cell (12). A study has shown that reoviruses
exert effective cytotoxicity in prostate cancer (13).
Radiation therapy is a frequently used treatment for
prostate adenocarcinoma. However, despite significant improvements
in delivery technologies, numerous patients develop recurrence
following treatment with curative intent. As prostate cancer
progresses, the current therapeutic options for advanced prostate
cancer are limited to androgen deprivation and/or the cytotoxic
effects of high-dose radiation on the surrounding tissues with the
aim to extend the survival time of the patient while maintaining
quality of life (14). Therefore,
it may be hypothesized that the combination of radiation with BTV
to target prostate cells represents an attractive treatment option.
In the present study, the RM-1 mouse prostate cancer cell line was
used to investigate the effect of BTV in combination with
radiation.
Materials and methods
Cell lines
BTV was preserved by our own lab (Central Laboratory
of Renmin Hospital Of Wuhan University, Wuhan, China). RM-1 (mouse
prostate cancer cell line) and PC-3 (human prostate cancer cell
line) cells were obtained from the China Center for Type Culture
Collection (Wuhan, China). Vero (African green monkey kidney cell
line) cells were preserved by our lab and used for replication of
BTV. Human umbilical vein endothelial cells (HUVECs) were provided
by Professor Jing-Yue Hu of the Central Laboratory of Renmin
Hospital of Wuhan University (Wuhan, China). Smooth muscle cells
(SMCs) were separated from rat aortic smooth muscle and maintained
by Dr Zong-li Ren (Department of Cardiothoracic, Renmin Hospital Of
Wuhan University). All cell lines, with the exception of Vero, were
cultured in RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA)
containing 10% (v/v) fetal bovine serum (FBS; HyClone Laboratories,
Inc., Logan, UT, USA) and 1% (v/v) penicillin/streptomycin. The
Vero cells were maintained in Dulbecco’s Modified Eagle’s Medium
(DMEM; Gibco-BRL) supplemented with 10% (v/v) FBS and 1% (v/v)
penicillin/streptomycin. The cell lines were maintained at 37°C in
an atmosphere containing 5% CO2. Irradiation and BTV
infection was conducted in DMEM containing 2% (v/v) FBS and 1%
(v/v) penicillin/streptomycin.
Replication of BTV and infection
The BTV stock was initially diluted to the highest
multiplicity of infection (MOI) that was to be used in the
experiment (100 MOI) and subsequently serially diluted to the
various MOIs required for each individual experiment. BTV-sensitive
Vero cells were used for replication of the BTV. The Vero cells
were cultured in the DMEM medium with high sugar containing 10% FBS
in 25-ml culture flasks. The cells were grown until they covered
70% of the bottom of the flask, and then the culture medium was
discarded and the cells were inoculated with 1 ml purified virus
suspension. Subsequently, the cells were incubated with the virus
for 1–2 h at 37°C and 5% CO2, after which time the
medium was aspirated and replaced with fresh medium containing 2%
FBS. The cells were observed every 6 h and by the time the
cytopathic effect (CPE) reached 90%, the cells were frozen and
thawed three times, and then centrifuged at 4°C and 1,600 × g for
15 min. The lysate was collected in 1-ml tubes and maintained at
−80°C.
TCID50 assays
Vero cells were plated at a density of
1×104 cells per well in a 96-well plate. The viral
suspension was sequentially diluted in 10-fold series, from
10−3 to 10−8. When the cells attached to the
bottom of the plates, various MOIs of 100 μl BTV were added to each
well (each MOI was applied to eight parallel wells). To the control
cells were added 100 μl growth liquid and 100 μl cell suspension.
Following incubation at 37°C for 3 h, the lysates or viral
suspensions were removed and replaced with DMEM containing 2% (v/v)
FCS and 1% (v/v) glutamine, and the cells were then incubated for a
further 6–7 days at 37°C. The change in the CPE of each well was
observed every 12 h, and the viral titer was calculated using the
Karber statistical method when the results were steady.
Targeting character of BTV
RM-1 and PC-3 cells, HUEVCs and SMCs were plated in
six-well plates at a density of 1×106 cells per well
individually. When the cells covered 70% of the bottom of the
culture flasks, the cells were infected with BTV at a MOI of 1.0
for 2 h and then the medium was replaced with fresh medium
containing 2% (v/v) FBS and 1% (v/v) penicillin/streptomycin. The
corresponding mock-infected cultures were subjected to the same
procedure using virus-free phosphate-buffered saline (PBS) instead
of the viral suspensions. Changes in the cells were observed every
8 h and then images were captured using a microscope (Olympus,
Tokyo, Japan). After 24 h, cell survival was assessed by a lactate
dehydrogenase (LDH) release bioassay. The LDH release assay was a
colorimetric assay for the quantification of cell death and cell
lysis. The LDH assay was performed using LDH Cytotoxicity Assay kit
(cat. no. C0016, Beyotime, Haimen, China). After cells infected by
BTV 24 h later, 200 ml LDH release reagent was added to each well
in six-well plates to incubate for 45 min. Afterwards, the cell
culture plate was centrifuged in microplate-centrifuge at 400 × g
for 5 min, and the 120 μl supernatant of each group was added to
96-wells plate respectively. The absorbance was read at 492 nm by
the microplate spectrophotometer (Perkin Elmer, Akron, OH, USA).
Cell survival= (2-Abstreated cells/Absuntreated
cells) ×100%.
Cytotoxic activity of the combination of
irradiation and BTV infection
RM-1 cells were plated in 96-well flat-bottom plates
in 1 ml media. The cells were treated with the media alone (control
wells), radiation alone, BTV alone or combination therapy using
radiation therapy and BTV. The BTV infection was conducted at MOIs
of 10−3, 10−2, 10−1, 1, 10 and 100
in a total volume of 100 μl medium. The radiotherapy was performed
using serial dilutions of radiation (2, 4, 5, 6, 8 and 10 Gy). To
assess the combined effect of the two agents, 5 Gy of radiation was
used and cells were infected with various MOIs. The effect of the
sequence of the combined treatment was evaluated via two protocols.
In the first schedule, the RM-1 cells were infected with BTV at 0.1
MOI and radiation doses of 0, 4 and 5 Gy were administered 24 h
later. In the second schedule, the order of treatments was reversed
such that the same radiation doses (0, 4 and 5 Gy) were delivered
24 h prior to infection of the cells with BTV at 0.1 MOI. The
percentage survival for each group was determined on each day 24 h
after treatment by the Cell Counting Kit-8 (CCK-8) method and the
absorbance (A) was measured at 450 nm. The cell survival rate was
calculated using the following formula: Cell survival rate (%) = (A
of the experimental group − A of the blank control group)/(A of the
control group − A of the blank control group).
Fluorescence-activated cell sorting
(FACS) analysis of cell survival using Annexin V/propidium iodide
(PI)
The RM-1 cells were treated with 5 Gy radiation
alone, BTV (0.1 MOI) alone, the combination treatment (0.1 MOI BTV
24 h after 5 Gy), or with mock-infection and mock-radiation
(control). All cell cultures were harvested 24 h post-infection and
the media were collected, then re-suspended at a density of
1×106 cells in 500 μl binding buffer. The cells were
stained with 10 μl PI and 5 μl Annexin V-fluorescein isothiocyanate
by incubation for 5 min. A total of 10,000 events were collected
and the proportion of apoptotic cells was analyzed with a
FACSCalibur flow cytometer (Becton-Dickinson, Franklin Lakes, NJ,
USA).
Cell cycle analysis by FACS
The procedure of the cell treatment for the cell
cycle analysis was the same as that of the FACS analysis of cell
survival. Subsequently, the cells were collected, washed twice with
100 μl PBS and fixed in 70% ice-cold ethanol at 4°C overnight. On
the following morning, 5 μl RNase was added to the cells and then
they were incubated at 37°C. After 20 mins, the cells underwent
further PI staining and the final concentration of PI staining was
40 mg/ml. All samples were analyzed on a FACSCalibur flow cytometer
using FlowJo software (TreeStar Inc., Ashland, OR, USA).
Antitumor effect of BTV and radiation on
RM-1 tumor volume in vivo
C57BL6 mice were purchased from the Beijing HFK
Bioscience Co., Ltd. (Beijing, China). The care and use of the
animals followed the recommendations and guidelines of the National
Institutes of Health (IACUC; approval number, 2011006) and they
were reviewed and approved by the Institutional Animal Care and Use
Committee of Wuhan University. The animals were maintained under
specific pathogen-free conditions and fed a strictly sterile diet.
In all cases, tumors were established by injection of RM-1 cells
suspended in 100 μl PBS in the right flank and the tumor size of
all mice was observed every two days. Approximately 12–14 days
following the injection, the mice with tumors of 6–8 mm in diameter
were selected to be involved in the experiment. In total, 24 mice
were allocated to the study and randomly divided into four groups:
i) Mock; ii) radiation alone (10 Gy in five fractions); iii) BTV
alone [intratumoral injection of 1×107 plaque-forming
units (PFU) every other day for seven times]; and iv) BTV plus
radiation (10 Gy in five fractions and seven doses of intratumoral
BTV, administered as 2-Gy doses of radiation followed by a single
intratumoral injection of 1×107 PFU BTV 24 h later). The
longest diameter, d1 (mm), and shortest diameter, d2 (mm), of the
tumors were measured every other day using Vernier calipers for 20
days. The tumor volume, V, was calculated from the following
formula: V = d1 × d22/2. The mice were sacrificed if the
largest tumor dimension was >4 cm or there was ulceration of the
skin.
Statistical analysis
Experiments were repeated at least three times and
the results are expressed as the mean ± standard deviation.
Comparative analyses between groups were performed using post-hoc
analysis or Wilcoxon Z test. The software SPSS, version 14.0 (SPSS,
Inc., Chicago, IL, USA) was used to perform the statistical
analysis and GraphPad Prism, version 5.0 (GraphPad Software, Inc.,
La Jolla, CA, USA) was used for drawing the figures. The effect of
the combined therapy on cell proliferation was measured by
calculating the combination index (CI) values using CalcuSyn
software (Biosoft, Cambridge, UK). Based on the median-effect
principle of Chou and Talalay (15), the CI affords a quantitative
measure of the degree of interaction between two or more agents.
CI>1 signifies antagonism, CI<1 signifies synergy and CI=1
signifies an additive interaction.
Results
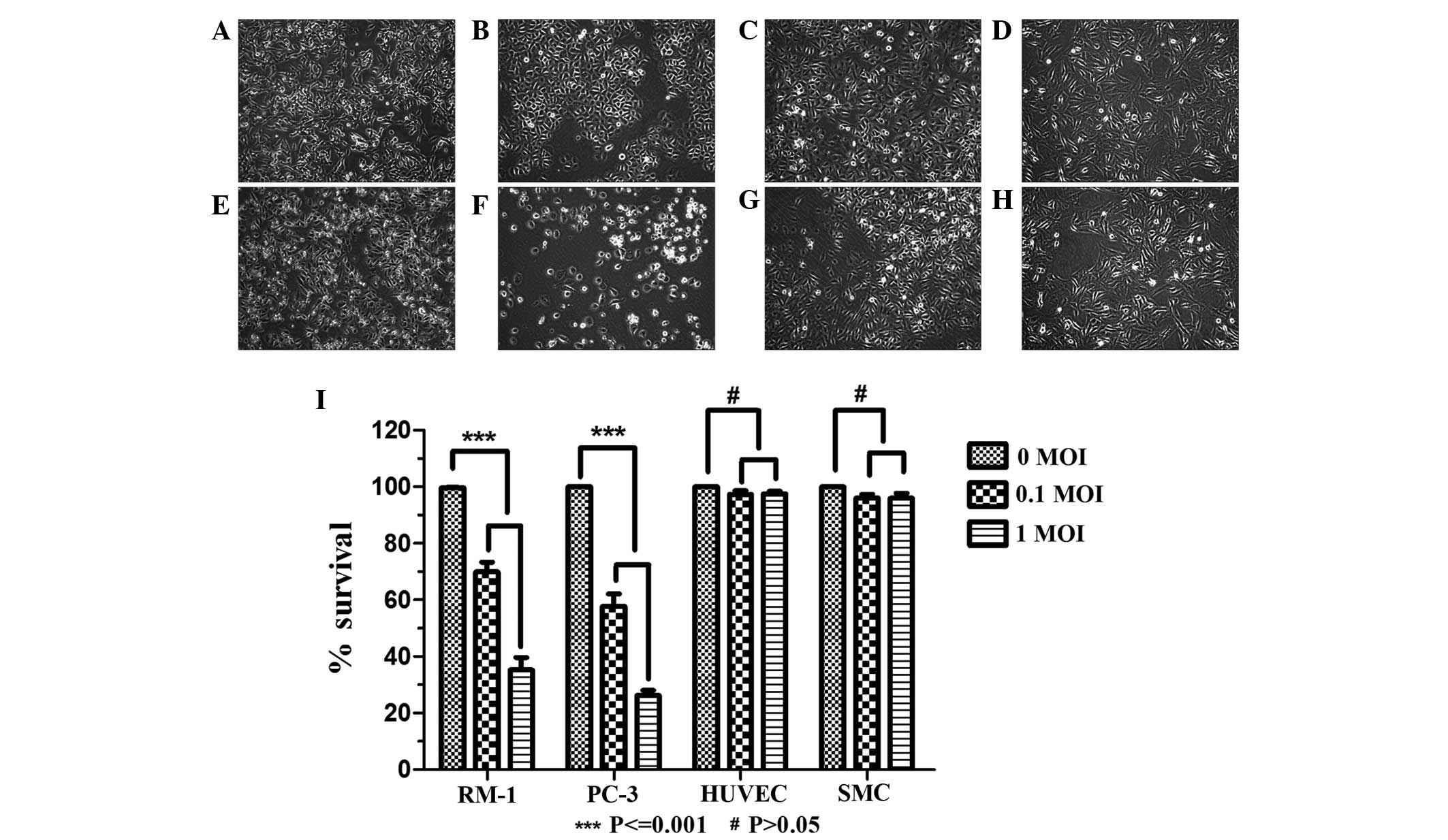
BTV targets cancer cells
The effect of BTV infection in a range of cell lines
was assessed. RM-1 and PC-3 cells, HUVECs and SMCs were infected
with BTV at 0.1 MOI or 1.0 MOI. The results demonstrated that in
the RM-1 and PC-3 tumor cells, CPEs were observed and the CPEs
reached 90% 48 h later (data not shown). Various pathological
changes of the cells occurred and included the following:
Intracellular appearance of a large number of particles, the
rounding and reduction in size of a number of cells, expansion of
the intervals among cells, enhancement of the cell contours and
loss of inherent morphological characteristics under normal
conditions. A large amount of exfoliated cells and cell debris
floating in the medium were observed (Fig. 1A–H). In the normal HUVEC and SMC
groups, CPEs were not observed in the treatment group, with no
difference in appearance compared with that of the control group.
Subsequently, a LDH release bioassay was used to detect cell
survival following infection with BTV. The results showed that BTV
had a significant inhibitory effect on the RM-1 and PC-3 tumor
cells (P<0.001), and no effect on the normal HUVECs and SMCs
(P>0.05) compared with the survival of the untreated cells
(Fig. 1I).
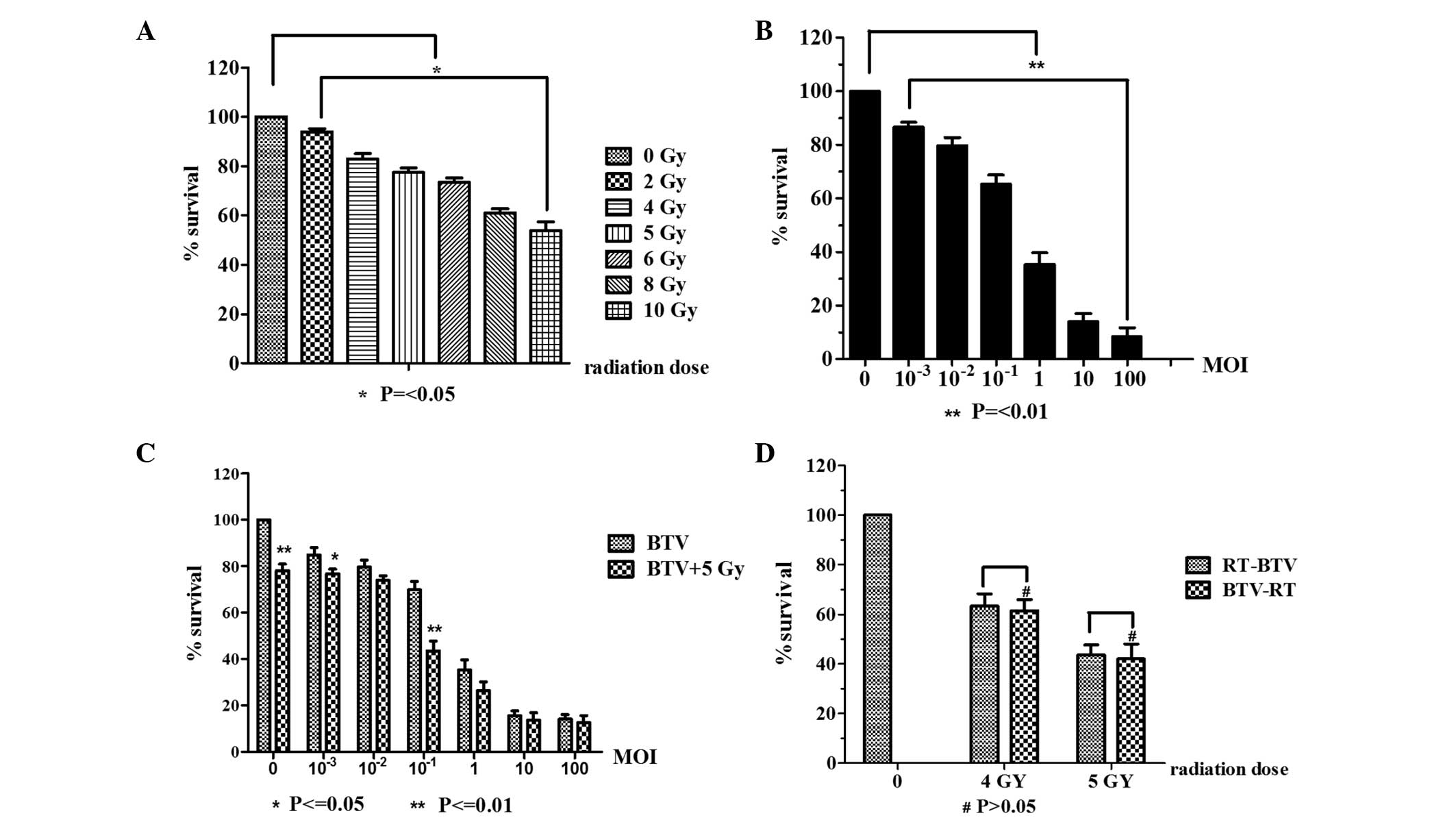
Combination of irradiation and BTV
infection
In the initial experiments, the cytotoxicity of
radiotherapy or BTV infection alone was tested prior to examining
the combination treatment using CCK-8. As shown in Fig. 2A, the cells received 2, 4, 5, 6, 8
or 10 Gy of radiation and 24 h later, the cell survival was
measured. The control group received mock irradiation. As shown in
Fig. 2A, it was observed that as
the dose of radiation increased, the inhibitory effect was
increasing evident. When the radiation dose was 10 Gy, the survival
rate of the cells was 53.75±7.32%. Statistically significant
differences existed between the control and irradiated groups.
Prominent BTV MOI-dependent cytotoxicity is shown in Fig. 2B. These data indicate that RM-1
cells have a high susceptibility to BTV infection. The effect of
BTV was most marked at the highest MOI (P<0.001).
In Fig. 2C, the
results indicated markedly enhanced cytotoxicity of the combination
of BTV and radiation compared with that of BTV alone. The effect of
the combination treatment was superior to that of various dilutions
of BTV or 5 Gy radiation alone (P<0.001), and the combined
effect was particularly evident at the lower MOIs (MOI<1) and
most marked at the MOI of 0.1 (P<0.001). By contrast, the effect
of the combined treatment was less evident compared with that of
BTV alone when BTV was used at the high MOIs (>10).
To evaluate the effects of the radiation schedule on
the cytotoxicity of BTV, a series of experiments was conducted in
which the interval between the two treatments was varied. When the
sequence of treatments was reversed and the BTV infection was
performed 24 h prior to irradiation with 4 and 5 Gy, no evident
difference was identified in comparison with BTV infection
following radiation for 4 Gy (63.4±11.8 vs. 61.4±11.3%; P>0.05)
and 5 Gy (44±10.3 vs. 42±15.0%; P>0.05; Fig. 2D).
For the combination treatment, analysis of the
interaction between radiation and BTV was conducted based on the
principle of Chou and Talalay. The CIs were computed and are shown
in Table I. Using this
methodology, a CI<1.0 is indicative of synergy, with a CI>1.0
denoting antagonism and CI=1 indicating an additive effect. From
the results, synergism (CI<1.0) was observed for the RM-1 cells
exposed to 5 Gy radiation combined with BTV at MOI>0.03, and
CI>1.0 was only observed when the BTV was at a low MOI. The
susceptibility of the RM-1 cells to radiation may have affected the
results.
 | Table IRequired doses of radiation and virus
to achieve various FA levels of RM-1 cells. |
Table I
Required doses of radiation and virus
to achieve various FA levels of RM-1 cells.
| FA | RT (Gy) | BTV (MOI) | 5Gy RT + BTV
(MOI) | CI |
|---|
| LD30 | 6.40 (5.93,6.91) | 0.03 (0.00,0.86) | 0.00 (0.00,0.10) | 1.11 (1.07,1.17) |
| LD40 | 8.44 (7.79,9.14) | 0.10 (0.00,2.64) | 0.02 (0.00,0.58) | 0.94 (0.89,1.00) |
| LD50 | 10.86
(9.99,11.83) | 0.29 (0.01,7.74) | 0.07 (0.00,1.85) | 0.83 (0.76,0.90) |
| LD60 | 13.99
(12.75,15.35) | 0.88
(0.03,23.50) | 0.26 (0.01,6.17) | 0.75 (0.67,0.84) |
| LD70 | 18.43
(16.64,20.41) | 2.90
(0.10,82.50) | 0.99
(0.04,24.24) | 0.70 (0.61,0.81) |
| LD80 | 25.79
(22.98,28.94) | 12.46
(0.39,403.55) | 5.13
(0.19,138.61) | 0.69 (0.58,0.82) |
| LD90 | 42.75
(37.26,49.06) | 111.51
(2.55,4873.04) | 60.98
(1.70,2191.79) | 0.73 (0.60,0.89) |
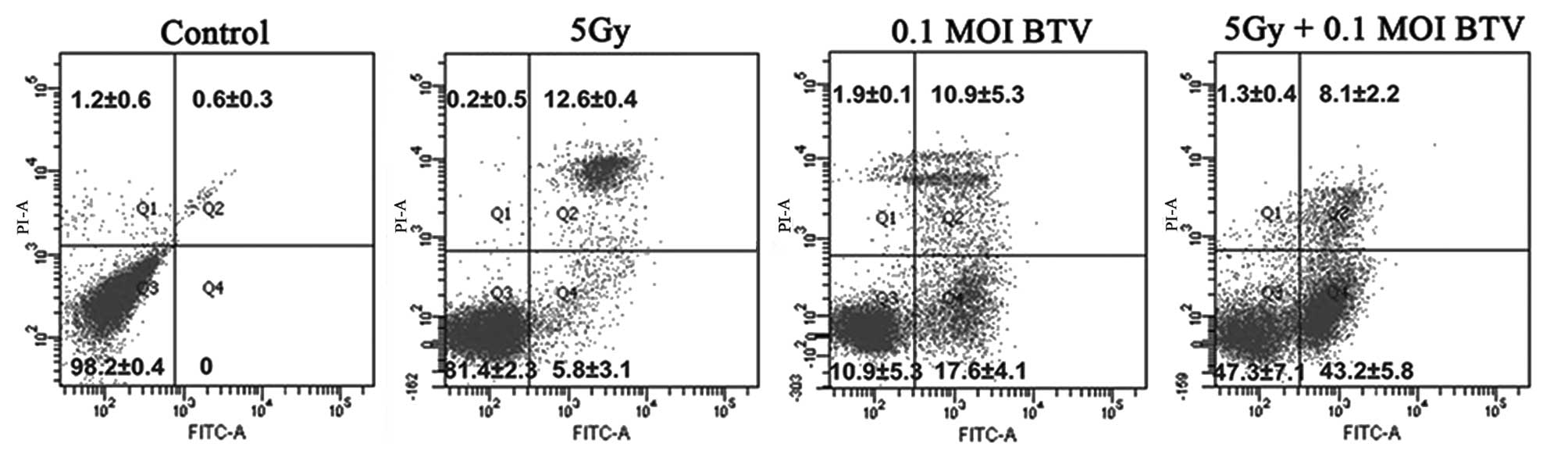
BTV and radiation individually induce
cell cycle arrest and together increase the levels of
apoptosis
The apoptosis-inducing effect of either irradiation
or BTV on RM-1 cells was detected by FACS analysis of the cell
survival using PI/Annexin-V staining. As shown in Fig. 3, there was no evident apoptosis in
the control group. Radiotherapy mainly lead to apoptosis at the
late stage (12.6±0.4%) whereas infection with BTV induced equal
levels of apoptosis at the early (17.6±4.1%) and late (10.9±5.3%)
stages. By contrast, the combination of BTV and radiation generated
the most prominent levels of apoptosis (51.3±3.2%) and the levels
of apoptosis at the early stage (43.2±5.8%) were the highest.
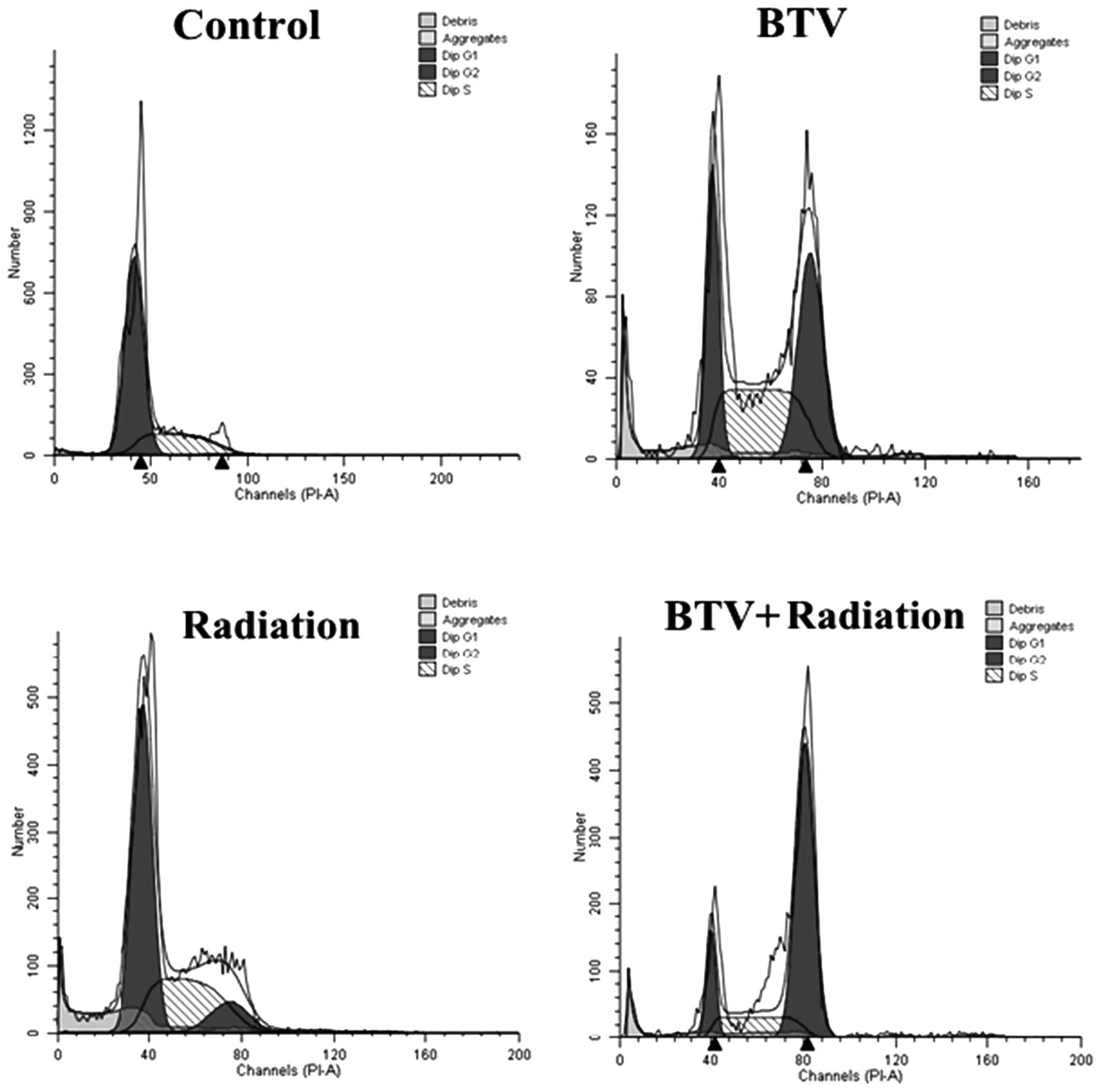
BTV and/or radiation induce changes in
the cell cycle
One of the most important potential mechanisms of
the radiation-enhanced cytotoxicity of BTV infection may be the
blockade of the cell cycle. Treatment with 5 Gy radiation induced
an increase in the number of RM-1 cells in the G2-M phase, while
infection with BTV resulted in an accumulation of cells in the S
and G2-M phases with a marked reduction in the number of cells in
G1 phase compared with those of the control group. By comparison,
when the RM-1 cells were infected with 1 MOI BTV following
treatment with 5 Gy radiation, the number of cells in the G2-M
phase increased the most compared with that in the other groups
(Table II; Fig. 4). This revealed that the combined
treatment induces increased levels of apoptosis compared with those
of either treatment alone.
 | Table IICell cycle distribution (%) induced by
BTV and/or radiation. |
Table II
Cell cycle distribution (%) induced by
BTV and/or radiation.
| Treatment | G0/G1 | S | G2/M |
|---|
| Control | 72.70±3.10 | 27.10±5.50 | 0.29±7.30 |
| BTV | 25.92±4.90 | 38.03±1.20 | 36.04±9.00 |
| Radiation | 58.55±2.80 | 32.91±10.30 | 10.54±1.00 |
| BTV + radiation | 6.46±3.20 | 32.55±4.40 | 60.99±8.30 |
Combined BTV and radiation treatment
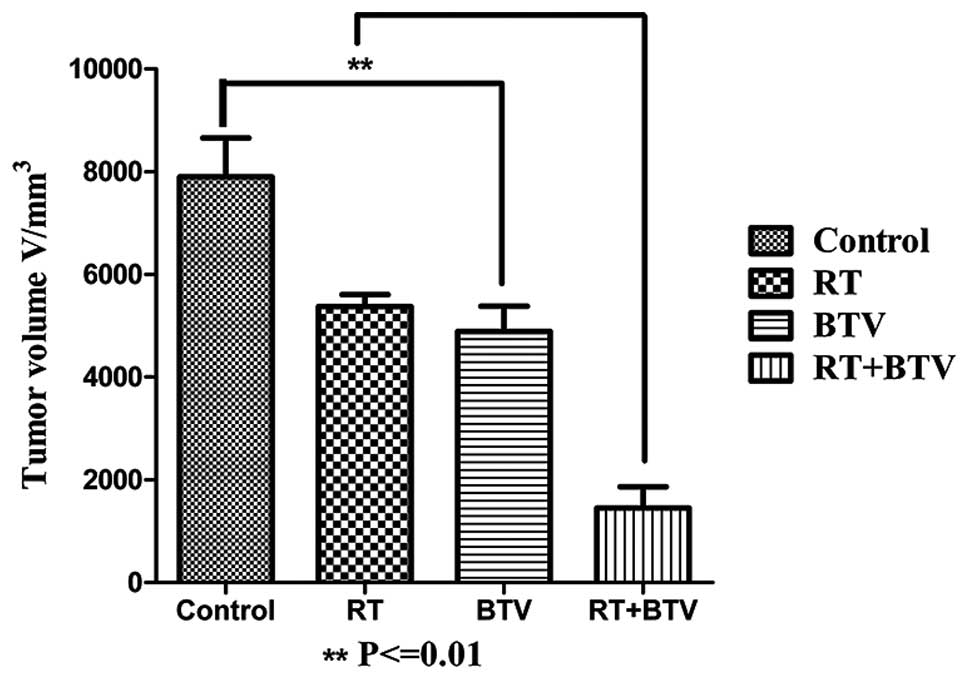
enhances the delay of tumor growth in vivo in C57BL6 mice
Following establishment of tumor-burdened mice for
7–10 days, 4–5 mm bumps were identified in the subcutaneous tissue
and they gradually increased in size. All mice tolerated the
radiation and no evident reduction in the physical quality and
mortality rate of the mice was observed in the study. The tumors in
the control group grew the most rapidly and in the other three
groups, the tumors grew slower than those of the control group.
Among them, the growth of the tumors in the combined group was the
slowest, and there were statistical differences compared with that
of the other three groups. Twenty days after treatment, the mean
tumor volume of the mice which received combination therapy was
reduced ~4-fold (compared with that of the control), 2-fold
(compared with that of radiation alone) and 1.9-fold (compared with
that of BTV alone) (P<0.01; Fig.
5). There was no evidence of exacerbation of the cutaneous
toxicity in the mice treated with the combination therapy compared
with that of radiotherapy alone.
Discussion
The combination of oncolytic viruses and
radiotherapy presents an emerging and promising novel approach for
the treatment of various types of malignant tumor. Numerous studies
have confirmed that combination therapies have shown synergistic
antitumor effects in preclinical models (16–19),
but the majority of them have used a gene-modified virus. BTV is a
type of natural targeted antitumor oncolytic virus that easily
multiplies and costs less compared with genetically modified
viruses. Radiation therapy is a standard treatment for patients
with prostate cancer, but long-term or high-dose rate radiation
inevitably induces many more side-effects. Therefore, combined
therapy may enable the dose of radiation to be reduced and thus
decrease the side-effects, or improve the efficiency of an equal
dose of radiation.
In the present study, different types of cell
infected by BTV were examined, and the evidence demonstrated that
BTV selectively infected and was cytotoxic to RM-1 and PC-3 cancer
cells but not cultured normal primary cells. This is significant in
clinical therapy as no normal organs should be infected by BTV and
thus side-effects are not likely to occur in other systems.
The present study also demonstrated that BTV
exhibited a dose-dependent cytotoxicity to the RM-1 cells, as did
the radiation. When BTV and radiation were combined together, the
results showed a marked increase in the cytotoxicity compared with
that of each treatment alone in vitro, particularly at
moderate input MOIs of BTV. Furthermore, CIs were calculated to
examine the synergistic effect of BTV and radiation (CI<1). In
addition, the enhanced cytotoxicity was not associated with the
sequence of administration of the two agents. This may be of
relevance to clinical studies that use fractionated radiotherapy
schedules as it is likely that, if multiple viruses are used, BTV
administration will follow some fractions of radiation but precede
others. The data from the in vivo experiment in the present
study further confirmed that the combination of BTV and
radiotherapy notably increased the cytotoxicity compared with that
of each agent used alone, particularly at moderate MOIs of BTV.
Also, it was demonstrated that BTV is stable in irradiated tissues
and acts independently of the treatment schedule.
In agreement with our assumptions, the results of
the apoptosis analysis by FACS using PI/Annexin-V dye in the
present study indicate that the increased cytotoxicity may be due
to a notable increase in apoptosis caused by the combined
treatment. Cell cycle analysis for the combined therapy showed that
the G2-M phase dominates the cell cycle following the combination
treatment, and this may result in the arrest of RM-1 cells in the
G2/M phase and ultimately an antitumor effect.
In conclusion, the results of the present study
demonstrate that the combined treatment exerts a synergistic
antitumor effect on RM-1 cells in vivo and in vitro.
These data support the future clinical investigation of the use of
BTV combined with radiation for the therapy of prostate cancer with
the purpose of reducing toxicity while increasing efficacy.
Acknowledgements
This study was supported by a grant from the
Doctoral Discipline Foundation in the Higher Education Institutions
of Ministry of Education (no. 20130141110077).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Bray F, Sankila R, Ferlay J and Parkin DM:
Estimates of cancer incidence and mortality in Europe in 1995. Eur
J Cancer. 38:99–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hammill AM, Conner J and Cripe TP:
Oncolytic virotherapy reaches adolescence. Pediatr Blood Cancer.
55:1253–1263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cripe TP, Wang PY, Marcato P, Mahller YY
and Lee PW: Targeting cancer-initiating cells with oncolytic
viruses. Mol Ther. 17:1677–1682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
James Ou JH and Benedict Yen TS: Human
Oncogenic Viruses. World Scientific Publishing Co. Pte. Ltd;
Singapore: 2010
|
|
6
|
Kim JH, Oh JY, Park BH, et al: Systemic
armed oncolytic and immunologic therapy for cancer with JX-594, a
targeted poxvirus expressing GM-CSF. Mol Ther. 14:361–370. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
White CL, Twigger KR, Vidal L, et al:
Characterization of the adaptive and innate immune response to
intravenous oncolytic reovirus (Dearing type 3) during a phase I
clinical trial. Gene Ther. 15:911–920. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirasawa K, Nishikawa SG, Norman KL, et
al: Systemic reovirus therapy of metastatic cancer in
immune-competent mice. Cancer Res. 63:348–353. 2003.PubMed/NCBI
|
|
9
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gorman BM: The bluetongue viruses. Curr
Top Microbiol Immunol. 162:1–19. 1990.
|
|
11
|
Hu J, Dong CY, Li JK, Chen DE, Liang K and
Liu J: Selective in vitro cytotoxic effect of human cancer cells by
bluetongue virus-10. Acta Oncol. 47:124–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao AT, Dong CY, Li JKK, Chen DE, Liu J
and Zhang WY: Studies on the infectivity of bluetongue virus strain
HbC3 to several human and animal tumor cells. Virol Sin.
19:349–352. 2004.
|
|
13
|
Thirukkumaran CM, Nodwell MJ, Hirasawa K,
et al: Oncolytic viral therapy for prostate cancer: efficacy of
reovirus as a biological therapeutic. Cancer Res. 70:2435–2444.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teh BS, Amosson CM, Mai WY, McGary J,
Grant WH III and Butler EB: Intensity modulated radiation therapy
(IMRT) in the management of prostate cancer. Cancer Invest.
22:913–924. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lamfers ML, Grill J, Dirven CM, et al:
Potential of the conditionally replicative adenovirus
Ad5-Delta24RGD in the treatment of malignant gliomas and its
enhanced effect with radiotherapy. Cancer Res. 62:5736–5742.
2002.PubMed/NCBI
|
|
17
|
Geoerger B, Grill J, Opolon P, et al:
Potentiation of radiation therapy by the oncolytic adenovirus
dl1520 (ONYX-015) in human malignant glioma xenografts. Br J
Cancer. 89:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Idema S, Lamfers ML, van Beusechem VW, et
al: AdDelta24 and the p53-expressing variant AdDelta24-p53 achieve
potent anti-tumor activity in glioma when combined with
radiotherapy. J Gene Med. 9:1046–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nandi S, Ulasov IV, Tyler MA, et al:
Low-dose radiation enhances survivin-mediated virotherapy against
malignant glioma stem cells. Cancer Res. 68:5778–5784. 2008.
View Article : Google Scholar : PubMed/NCBI
|