Introduction
Mucormycosis is a rare and life-threatening invasive
fungal infection caused by fungi of the Zygomycetes class and
Mucorales order. Rhizopus, Mucor and
Rhizomucor are the genera of Mucorales that are most
frequently identified in human infections (1). Based on the clinical presentation and
involvement, mucormycosis is classified as six major forms, namely,
rhinocerebral, pulmonary, cutaneous, gastrointestinal (GI),
disseminated and miscellaneous (2,3),
with rhinocerebral and pulmonary being the common forms. GI
mucormycosis is rare, accounting for only 7% of all cases; however,
the mortality rate is as high as 85% (1). GI mucormycosis may occur in any part
of the alimentary tract, with the stomach being most commonly
involved, followed by the colon and ileum (4,5). GI
mucormycosis occurs more frequently in patients with diabetes
mellitus and other conditions associated with immunodeficiency,
including hematologic malignancies, solid organ transplantation,
glucocorticoid therapy, chronic renal failure, liver cirrhosis and
malnutrition in infants and children (6–8).
There are only a few reports of successfully treated invasive
gastric mucormycosis due to its high mortality rate (9–11).
Gastric mucormycosis in patients with liver cirrhosis has rarely
been reported (12). Therefore,
the present study reports a case of successfully treated invasive
gastric mucormycosis in a patient with alcoholic liver cirrhosis,
and reviews the associated studies.
Case Report
A 55-year-old male with alcoholic liver cirrhosis
was referred to the emergency department of Keimyung University
Dongsan Medical Center (Daegu, Republic of Korea) from a local
hospital, complaining of severe, constant pain throughout the whole
abdomen for 6 h. According to the patient’s past medical history,
the patient had been treated with medication for gastric ulcers for
one month immediately prior to admission to Keimyung University
Dongsan Medical Center. The patient also had liver cirrhosis due to
excessive ingestion of alcohol for 35 years. Upon arrival at the
emergency department, the patient appeared acutely ill and mildly
dehydrated. However, the vital signs were within normal range as
sufficient volumes of fluid had been administered at the local
hospital. The patient did not exhibit jaundice, hepatosplenomegaly
or signs of chronic liver disease. Abdominal examination revealed
severe tenderness and rebound tenderness throughout the whole
abdomen with rigidity and hypoactive bowel sounds. Furthermore, the
laboratory data on arrival noted reduced levels of hemoglobin,
measuring 3.6 g/dl (normal, 12–18 g/dl), and the other laboratory
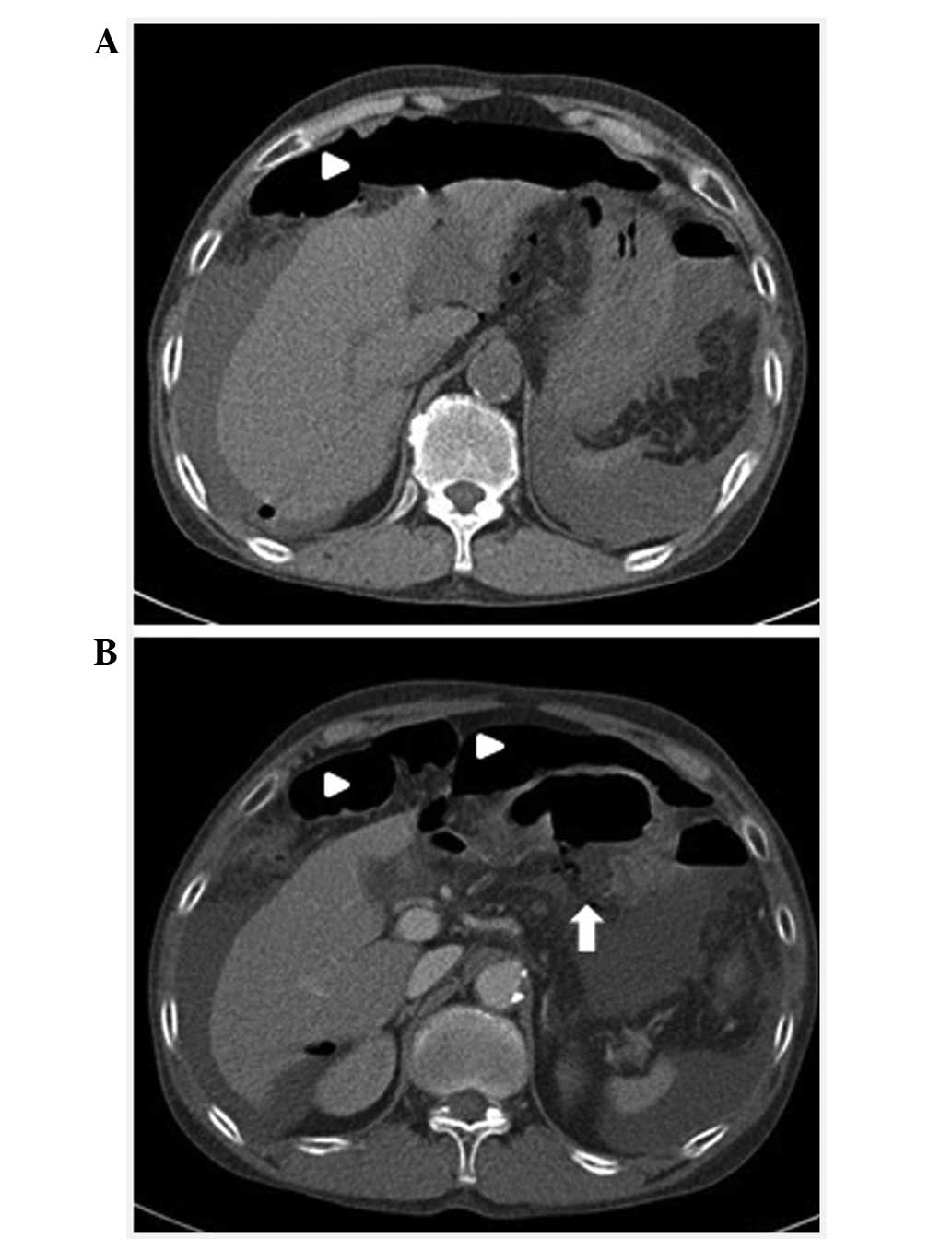
tests were normal. An abdominal computed tomography (CT) scan
revealed pneumoperitoneum and hemoperitoneum due to gastric ulcer
perforation (Fig. 1).
Subsequently, the patient underwent an emergency laparotomy.
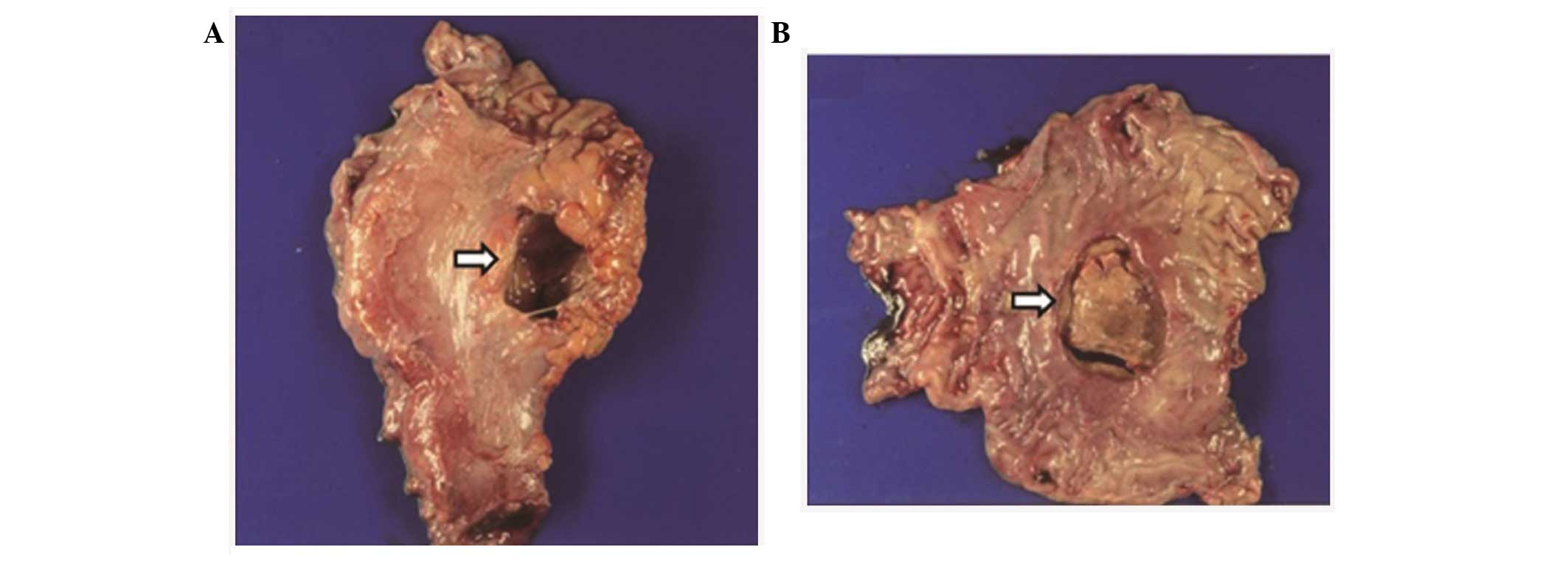
The surgical findings showed an unusually large
perforation, measuring 5.0×3.7 cm in gastric angle (Fig. 2), with ~2,000 ml ascitic fluid
collection in the abdominal cavity which was not bloody. Severe
cirrhotic changes of the liver were also noted. A subtotal
gastrectomy and gastrojejunostomy with massive irrigation in the
abdominal cavity was performed. The patient was then transferred to
the intensive care unit for further management.
On the fourth day in the hospital, gastric
mucormycosis was cited in the preliminary pathology report.
Antifungal therapy was promptly initiated using amphotericin B
deoxycholate (0.6 mg/kg/day). However, following administration of
the agent for 3 h, the vital signs became unstable (blood pressure
80/60 mmHg and tachycardia), so the amphotericin B deoxycholate was
replaced with liposomal amphotericin B (5 mg/kg/day). On the
seventh day in hospital, the pathologic diagnosis was confirmed as
invasive gastric mucormycosis (Fig.
3).
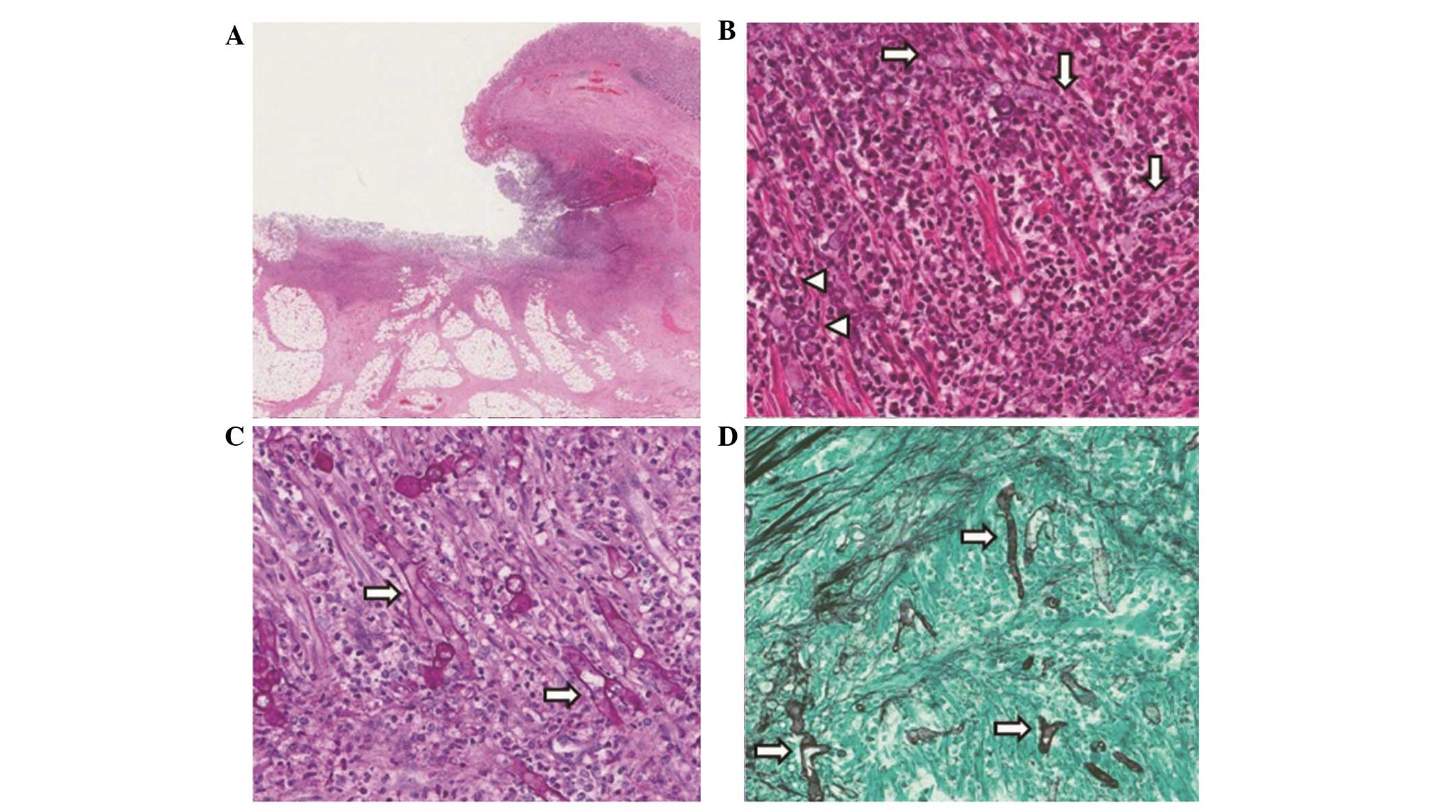 | Figure 3Microscopic findings of the gastric
ulcer. (A) H&E staining showed a deep gastric ulcer covered
with exudates was observed (staining, H&E; magnification, ×40).
(B) H&E staining showed numerous fungal hyphae (arrows) and
yeasts (arrowheads) were admixed with acute inflammatory cells and
ulcer debris (staining, H&E; magnification, ×200). (C) PAS
staining showed numerous non septated fungal hypae (arrows) with
PAS positive thick walls (staining, PAS; magnification, ×200) (D)
GMS staining revealed numerous broad, irregular and non septated
fungal hypae (arrows) (staining, GMS; magnification, ×200).
H&E, Hematoxylin and eosin; PAS, periodic acid Schiff; GAS,
Grocott’s methenamine silver. |
Hepatotonics, fresh frozen plasma, platelets and
albumin were supplied following the surgery due to persistent
thrombocytopenia, hypocoagulopathy and hypoalbuminemia caused by
the underlying liver cirrhosis. Diuretics were also administered to
manage the large amount of ascites, of which ~800–1,000 ml was
drained for 10 days.
On the twenty-first day in hospital, treatment with
liposomal amphotericin B was ceased. All vital signs and laboratory
data were normal and the patient was in a satisfactory condition
without antifungal treatment. On the twenty-fourth day in hospital,
the patient was discharged to return home with a good health
status. Written informed consent was obtained from the patient. The
study was approved by the ethics committee of Dong San Medical
Center, Keimyung University School of Medicine, Daegu, Republic of
Korea.
Discussion
Mucormycosis is an uncommon type of fungal
infection, most frequently occurring in immunocompromised patients
and those with diabetes mellitus (1). Mucormycosis was first reported as a
cause of human disease by Paultauf in 1885 (13). Mucormycosis refers to any fungal
infections caused by fungi of the Mucorales order, which belongs to
the Zygomycetes class. Certain Zygomycetes genera, including
Rhizopus, Mucor and Rhizomucor, are frequently
observed in human infection and Saksenaea,
Cunninghamella, Absidia and Apophysomyces are
the genera less commonly identified in infections (14,15).
The diseases produced by the aforementioned organisms are almost
identical and, thus far, the diagnosis and therapy of mucormycosis
has not been influenced by identification of the specific species
of the pathogens. Thus, visualization of the characteristic hyphae
in sections of tissue pathologically is essential for the
definitive diagnosis of mucormycosis. The hyphae of Mucorales are
different from those of other types of mold. The hyphae of
Mucorales in tissue are irregular-shaped, broad (5–20 μm in
diameter) and have rare septation (14).
The clinical manifestation of mucormycosis has been
traditionally divided into six major forms, namely, rhinocerebral,
pulmonary, cutaneous, GI, disseminated and miscellaneous (including
endocarditis, osteomyelitis and renal infection) (2,3). The
common sites of mucormycosis have been reported as the sinuses
(39%), lungs (24%), skin (19%), brain (9%), GI tract (7%) and the
kidneys (2%), in addition to disseminated infection (3%) (1). The mode of transmission is mainly
airborne, thus the sinuses and pulmonary manifestations are more
frequent. Although it is unusual, mucormycosis of the GI tract may
occur as the result of ingestion of spores in food, including
fermented milk, dried bread products, and spore-contaminated herbal
and homeopathic remedies (7,16).
Patients with GI mucormycosis may present abdominal
pain and distension, fever, and hematemesis and hematochezia due to
GI bleeding. The GI lesions are necrotic ulcers that may lead to
bowel bleeding and perforation, peritonitis, sepsis and hemorrhagic
shock (11,17). Therefore, the prognosis for all
patients with GI mucormycosis is poor and the majority of patients
are often diagnosed following mortality caused by the infection
(1).
GI mucormycosis may occur in any part of the
alimentary tract, with the most common site being the stomach
(58%), followed by the colon (32%), ileum and esophagus (4,5).
Gastric mucormycosis is primarily observed in patients with
diabetes mellitus, solid organ transplantation, glucocorticoid use,
liver cirrhosis, renal failure, deferoxamine administration,
prematurity and malnutrition (6,18).
In addition, alcoholism appears to be a risk factor of gastric
mucormycosis. Following a review of the case in the present study,
three previous studies on individual cases of gastric mucormycosis
in patients with alcoholism were identified (10,19,20).
Ho et al (20) reported
that the ingestion of fungal spores and their germination may be
harmful to a patient with alcoholism as ethanol may disrupt the
activation of macrophages and dendritic cells, which play crucial
roles in the immune reaction that eliminates fungal spores.
Also, as evidenced in the case in the present study,
chronic alcohol abuse may lead to liver cirrhosis. Liver cirrhosis
itself lowers systemic immunity, resulting in an increased
likelihood of gastric mucormycosis, and also causes coagulation
dysfunction and hypoalbuminemia, increasing the risk of
post-operative complications and resulting in an increased
mortality rate.
Management of gastric mucormycosis includes prompt
diagnosis, metabolic support, elimination of predisposing factors,
aggressive surgical debridement of involved tissues and antifungal
therapy. Prompt and extensive surgical debridement is, possibly,
the most important option for treatment, and the aim of the surgery
should be to remove all necrotic tissue. Also, the early initiation
of antifungal therapy is important for patients who have gastric
mucormycosis (8,17). Previously, a study has reported
that early initiation of amphotericin B therapy improves the
outcome of infection with mucormycosis (21).
Intravenous amphotericin B (including its
deoxycholate salt, lipid derivatives and liposomal formulations) is
the standard antifungal therapy for gastric mucormycosis. The
starting dose of amphotericin B deoxycholate is 1–1.5 mg/kg/day and
the usual starting dose of amphotericin B lipid complex or
liposomal amphotericin B is 5 mg/kg/day. The majority of clinicians
use a lipid formulation of amphotericin B or liposomal amphotericin
B in order to deliver a high dose with less nephrotoxicity. The
duration of amphotericin B therapy has not yet been defined, but is
guided by the resolution of associated symptoms and findings
(usually 6–8 weeks). Furthermore, a novel broad-spectrum oral azole
agent named posaconazole has a potential role in the treatment of
mucormycosis. Posaconazole is used as a step-down therapy for
patients who have responded to amphotericin B and rarely as salvage
therapy for patients who do not respond to or tolerate amphotericin
B (22,23). In the case in the present study,
only liposomal amphotericin B was used for 21 days without
posaconazole step-down therapy and no abnormal signs or symptoms
were observed after treatment with the antifungal agents ceased.
Therefore, further studies are required to determine the definite
criteria and duration of the use of antifungal agents to treat
gastric mucormycosis.
To the best of our knowledge, this is the first
study to report the successful treatment of gastric mucormycosis,
revealed by gastric ulcer perforation, in a patient with alcoholic
liver cirrhosis. To achieve the successful treatment of gastric
mucormycosis in a patient with alcoholic liver cirrhosis, we
consider that prompt diagnosis, aggressive surgical debridement of
the involved tissues, early use of amphotericin B and sufficient
metabolic support to prevent hepatic failure are essential and
ultimately improve the survival rate. Furthermore, further studies
on gastric mucormycosis in patients with liver cirrhosis are
required to design a treatment strategy to minimize the post
surgery complications and improve the survival rate.
References
|
1
|
Roden MM, Zaoutis TE, Buchanan WL, et al:
Epidemiology and outcome of zygomycosis: a review of 929 reported
cases. Clin Infect Dis. 41:634–653. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richardson MD and Koukila-Kahkola P:
Rhizopus, Rhizomucor, Absidia and other agents of systemic and
subcutaneous zygomycoses. Manual of Clinical Microbiology. Murray
PR: American Society for Microbiology; Washington, D.C: pp.
1839–1856. 2007
|
|
3
|
Lopes JO, Pereira DV, Streher LA, Fenalte
AA, Alves SH and Benevenga JP: Cutaneous zygomycosis caused by
Absidia corymbifera in a leukemic patient. Mycopathologia.
130:89–92. 1995. View Article : Google Scholar
|
|
4
|
Echo A, Hovsepian RV and Shen GK:
Localized cecal zygomycosis following renal transplantation.
Transpl Infect Dis. 7:68–70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geramizadeh B, Modjalal M, Nabai S, et al:
Gastrointestinal zygomycosis: a report of three cases.
Mycopathologia. 164:35–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martinez EJ, Cancio MR, Sinnott JT 4th,
Vincent AL and Brantley SG: Nonfatal gastric mucormycosis in a
renal transplant recipient. South Med J. 90:341–344. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ismail MH, Hodkinson HJ, Setzen G,
Sofianos C and Hale MJ: Gastric mucormycosis. Trop Gastroenterol.
11:103–105. 1990.
|
|
8
|
Spellberg B, Edwards J Jr and Ibrahim A:
Novel perspectives on mucormycosis: pathophysiology, presentation,
and management. Clin Microbiol Rev. 18:556–569. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shenoi S and Emery HM: Successful
treatment of invasive gastric mucormycosis in child with systemic
lupus erythematosus. Lupus. 19:646–649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park YS, Lee JD, Kim TH, et al: Gastric
mucormycosis. Gastrointest Endosc. 56:904–905. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Virk SS, Singh RP, Arora AS, Grewal JS and
Puri H: Gastric zygomycosis an unusual cause of massive upper
gastrointestinal bleed. Indian J Gastroenterol. 23:146–147.
2004.PubMed/NCBI
|
|
12
|
Rudler M, Barret M, Poynard T and Thabut
D: Gastric mucormycosis: a rare cause of gastrointestinal bleeding
in cirrhosis. Clin Res Hepatol Gastroenterol. 36:e32–e33. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paltauf A: Mycosis mucorina: ein Beitrag
zur Kenntnis der menschlichen Fadenpiltzerkrankungen. Virchows
Archiv A. 102:543–564. 1885.(In German).
|
|
14
|
Prabhu RM and Patel R: Mucormycosis and
entomophthoramycosis: a review of the clinical manifestations,
diagnosis and treatment. Clin Microbiol Infect. 10(Suppl 1): 31–47.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ribes JA, Vanover-Sams CL and Baker DJ:
Zygomycetes in human disease. Clin Microbiol Rev. 13:236–301. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Petrikkos G, Skiada A, Lortholary O,
Roilides E, Walsh TJ and Kontoyiannis DP: Epidemiology and clinical
manifestations of mucormycosis. Clin Infect Dis. 54(Suppl 1):
S23–S34. 2012. View Article : Google Scholar
|
|
17
|
Cherney CL, Chutuape A and Fikrig MK:
Fatal invasive gastric mucormycosis occurring with emphysematous
gastritis: case report and literature review. Am J Gastroenterol.
94:252–256. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Agha FP, Lee HH, Boland CR and Bradley SF:
Mucormycoma of the colon: early diagnosis and successful
management. AJR Am J Roentgenol. 145:739–741. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shahapure AG, Patankar RV and Bhatkhande
R: Gastric mucormycosis. Indian J Gastroenterol. 21:231–232.
2002.PubMed/NCBI
|
|
20
|
Ho YH, Wu BG, Chen YZ and Wang LS: Gastric
mucormycosis in an alcoholic with review of the literature. Tzu Chi
Med J. 19:169–172. 2007. View Article : Google Scholar
|
|
21
|
Chamilos G, Lewis RE and Kontoyiannis DP:
Delaying amphotericin B-based frontline therapy significantly
increases mortality among patients with hematologic malignancy who
have zygomycosis. Clin Infect Dis. 47:503–509. 2008. View Article : Google Scholar
|
|
22
|
van Burik JA, Hare RS, Solomon HF, Corrado
ML and Kontoyiannis DP: Posaconazole is effective as salvage
therapy in zygomycosis: a retrospective summary of 91 cases. Clin
Infect Dis. 42:e61–e65. 2006.PubMed/NCBI
|
|
23
|
Spanakis EK, Aperis G and Mylonakis E: New
agents for the treatment of fungal infections: clinical efficacy
and gaps in coverage. Clin Infect Dis. 43:1060–1068. 2006.
View Article : Google Scholar : PubMed/NCBI
|