Introduction
Currently, the incidence of cervical cancer is
second only to that of breast cancer, being the second most
prevalent female malignancy worldwide (1). The morbidity and mortality rates of
cervical cancer patients are gradually increasing, and the disease
is demonstrating a marked tendency to occur in individuals at
younger ages (2). Surgical
treatment is normally used at the early stages, while radiotherapy
is mainly applied for patients at the advanced stages. Furthermore,
cervical cancer has a high recurrence rate (3). Therefore, the elucidation of the
molecular pathogenesis of cervical cancer is likely to be conducive
for the diagnosis and treatment of the disease.
micro (mi)RNA are a class of endogenous non-coding
RNA with only 19–21 nucleotides. miRNA participates in the
regulation of numerous biological functions, including cell cycle,
proliferation, differentiation or apoptosis, by degrading its
associated proteins or inhibiting their expression through
downstream target genes at the post-transcriptional level (4). Previously, the abnormal expression of
miRNA has been found in cervical cancer, including miR-214
(5), miR-21 and miR-143 (6). These miRNAs may be involved in
cervical cancer proliferation, cycle or invasion, metastasis and
other tumorigenic processes. miR-145 has an important role as a
tumor suppressor gene, with low expression in esophageal (7), bladder (8), colorectal and numerous other human
cancer types, and affects the biological functions of tumor cells
by regulating the expression of numerous downstream genes. However,
to the best of our knowledge, no study has reported the role of
miR-145 in cervical cancer until now. In the present study, an
miR-145 expression vector was constructed using the eukaryotic
expression vector pcDNA™6.2-GW, and was transfected into HeLa
cervical cancer cells to examine the regulatory effect of miR-145
on its downstream target gene cyclin-dependent protein kinase 6
(CDK6).
Materials and methods
Plasmids, cells and reagents
The eukaryotic expression vector pcDNA6.2-GW, E.
coli DH5α and the HeLa cervical cancer cell line were provided
by the Department of Cell Biology and Genetics of the Xi’an
Jiaotong University Health Science Center (Xi’an, China). The
reverse transcription kit (PrimeScript® RT) and SYBR
Premix Ex TaqTM II were purchased from Takara Bio Inc.
(Dalian, China). The transfection reagents were purchased from
Roche (Basel, Switzerland). TRIzol was purchased from Invitrogen
Life Technologies (Carlsbad, CA, USA). Radio-immunoprecipitation
(RIPA)lysis buffer and an SDS-PAGE gel configuration kit were
purchased from Beyotime Institute of Biotechnology (Beijing,
China). Luminata Classico Western HRP substrate and PVDF membrane
were obtained from Millipore Corporation (Billerica, MA, USA).
Rabbit anti-human CDK6 antibody was purchased from Beijing Bioss
Biotechnology Ltd., (Beijing, China). Mouse anti-human β-actin was
obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA,
USA).
The blank group consisted of untreated HeLa cells.
The normal control (NC) group was transfected with the pcDNA6.2-GW
plasmid. The miR-145 group was transfected with a
pcDNATM6.2-GW-miR-145 recombinant plasmid. The
proliferation of HeLa cells was detected at 24, 48 and 72 h
following transfection.
Construction of miR-145 eukaryotic
expression vector
Two types of single-stranded DNA were synthesized,
one was:
AATTCCACCTTGTCCTCACGGTCCAGTTTTCCCAGGAATCCCTTAGATGCTAAGATGGGGATTCCTGGAAATACTGTTCTTGAGGTCATGG,
and the other was:
AGCTTAACCATGACCTCAAGAACAGTATTTCCAGGAATCCCCATCTTAGCATCTAAGGGATTCCTGGGAAAACTGGACCGTGAGGACAAGG.
The two single-stranded DNA sequences were annealed to synthesize
double-stranded DNA, which was inserted into the pcDNA6.2-GW
eukaryotic expression vector and amplified by E. coli
DH5α.
MTT assay
HeLa cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. The
cells were incubated in a thermostatic incubator at 37°C in an
atmosphere with 5% CO2. The cells were seeded on a
96-well plate at a density of 3,000 cells/well and were transfected
with liposomal transfection reagent (Roche). At 24, 48 and 72 h
after transfection, cell proliferation was analyzed using MTT
(Sigma Aldrich, St. Louis, MO, USA). Briefly, 20 μl MTT was added
to the 96-well plate and incubated at 37°C for 4 h until purple
precipitate was visible. Then, the culture supernatant was
discarded and 150 μl dimethylsulfoxide was added. The 96-well plate
was oscillated for 10 min until the purple precipitate was
dissolved. The absorbance was measured at 492 nm on a microplate
reader (Bio-Rad Laboratories, Hercules, CA, USA). Cell
proliferation was calculated based on these absorbance values.
Quantitative polymerase chain reaction
(qPCR)
HeLa cells were cultured in DMEM supplemented with
10% fetal bovine serum. The cells were seeded on a 6-well plate at
a density of 2–6×105 cells/well and were transfected
with liposomal transfection reagent. The RNA was extracted from
these cells at 24 h after transfection using TRIzol. The
PrimeScript® RT kit was used for reverse transcription.
The reverse primer was designed as:
GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAGGGATT. The qPCR
primers were as follows: forward, 5′-CAGTGCGTGTCGTGGAGT-3′; and
reverse, 5′-AGGTCCAGTTTTCCCAGG-3′. U6 was selected as the internal
standard. The CDK6 primers were as follows: forward,
5′-TGGAGACCTTCGAGCACC-3′; and reverse, 5′-CACTCCAGGCTCTGGAACTT-3′.
β-actin was selected as the internal standard. SYBR Premix Ex Taq™
II was used for reaction. Briefly, the 20 μl qPCR system contained
1 μl reverse transcription product, 10 μl SYBR Premix Ex
TaqTM II, 1 μl forward primer (10 μM) and 1 μl reverse
primer (10 μM). The reaction was performed on an FTC-3000 qPCR
system (Funglyn Biotech Inc., Toronto, ON, Canada). The following
PCR program was used: Pre-denaturation at 95°C for 1 min, followed
by 40 cycles of 95°C for 10 sec and 60°C for 40 sec. The
2−ΔΔCt method was used to quantify the expression of
miR-145 and CDK6.
Western blotting analysis
HeLa cells were cultured in DMEM supplemented with
10% fetal bovine serum. The cells were seeded on a 6-well plate at
a density of 2–6×105 cells/well and were transfected
with liposomal transfection reagent. The proteins were extracted
from these cells 24 h after transfection. According to the
manufacturer’s instructions, the proteins were extracted with RIPA
assay lysis buffer and examined by electrophoresis. Equal amounts
of the proteins were separated on 10% SDS-PAGE and were
electrophoretically transferred onto nitrocellulose membranes
(Millipore Corporation), which were blocked in phosphate-buffered
saline with Tween (PBST) containing 5% milk for 2 h. The film was
incubated with primary antibody (anti-CDK6 1:100; anti-β-actin
1:3,000) at 4°C overnight. Following being washed with PBST and
then incubated with the secondary antibody (HRP-conjugated goat
anti-rabbit/mouse IgG; Pierce Biotechnology Inc., Rockford, IL,
USA) at room temperature for another 1.5 h, the film was then
washed with Tris-buffered saline and Tween-20, and the protein
expression was visualized with chemiluminescence from the Luminata
Classico Western HRP substrate.
Statistical analyses
SPSS software, version 13.0 was used for data
analysis (SPSS, Inc., Chicago, IL, USA). Comparisons of data
between the groups were performed using a t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Successful construction of the miR-145
eukaryotic expression vector
To identify whether miR-145 was expressed in the
HeLa cervical cancer cells, qPCR was used. Data in Fig. 1A demonstrate that the expression
level of miR-145 in the miR-145 group was significantly higher than
that in the blank group (P=0.001), while no significant difference
was observed in the expression level of miR-145 between the NC
group and blank group (P=0.412). This demonstrates that the
eukaryotic expression vector was successfully constructed and had a
high transfection efficiency in the HeLa cells.
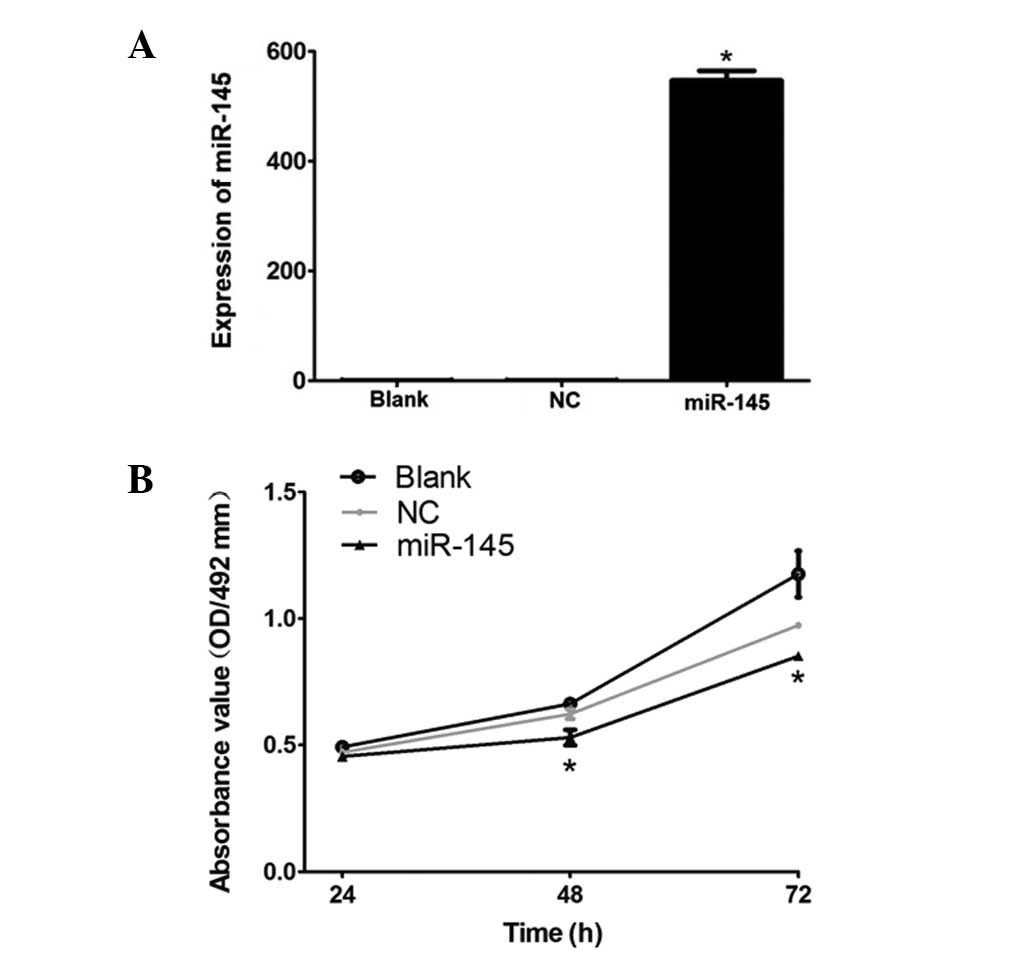 | Figure 1(A) mRNA expression level of miR-145
in each group. HeLa cells were divided into three groups,
specifically, the untransfected blank group, empty vector group and
miR-145 group, and were seeded into 6-well plates with a density of
2–6×105 cells/well. After 24 h, quantitative polymerase
chain reaction was employed to measure the expression levels of
miR-145 in the three groups. *P<0.01, statistically
significant difference compared with the blank group. (B) Cell
proliferation of each group. The three groups of HeLa cells were
seeded into 96-well plates with a density of 3,000 cells/well.
After 24, 48 and 72 h, the MTT assay was repeated three times to
measure the cell proliferation of each group.
*P<0.05, statistically significant difference
compared with the blank group (P<0.05). miR, microRNA; OD,
optical density. |
miR-145 inhibits cervical cancer HeLa
cell proliferation
To examine whether miR-145 inhibits HeLa cervical
cancer cell proliferation 24, 48 and 72 h following transfection,
an MTT assay was conducted. As demonstrated in Fig. 1B, the proliferation of
miR-145-transfected HeLa cells was significantly lower than that of
the blank group (P<0.05), whereas no marked difference was
observed between the NC group and the blank group (P>0.05). This
indicates that the high expression level of miR-145 inhibited the
proliferation of HeLa cells.
miR-145 inhibits the expression of
CDK6
To investigate whether the overexpression of miR-145
in HeLa cells affects the expression of CDK6, qPCR and western
blotting analysis were employed. Following transfection by the
recombinant pcDNA6.2-GW-miR-145, the mRNA and protein levels of the
downstream CDK6 in the HeLa cells were significantly lower than
those in the NC and blank HeLa cells (P=0.001; Fig. 2). This demonstrates that miR-145
inhibited the mRNA and protein expression of CDK6 in the HeLa
cervical cancer cells at the transcriptional and translational
levels.
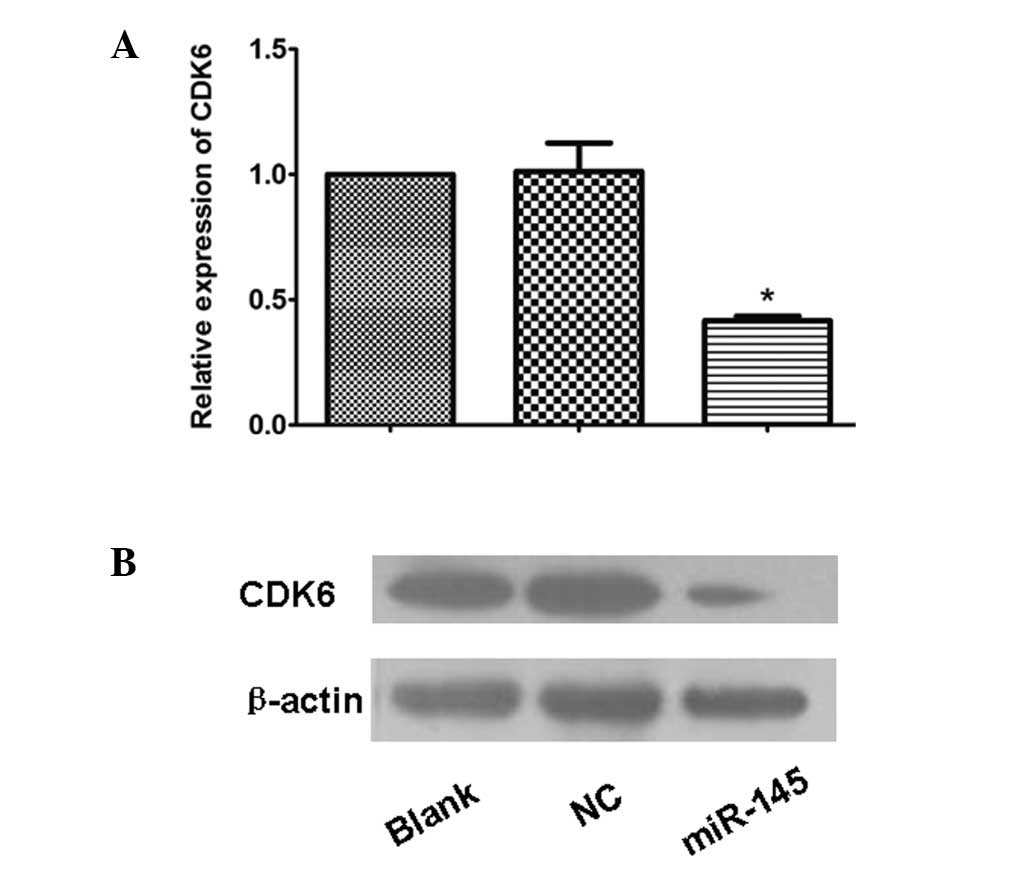 | Figure 2(A) The mRNA expression of CDK6 in
each group of cells. HeLa cells were divided into three groups,
specifically, the untransfected blank group, empty vector group and
miR-145 group, and were seeded into 6-well plates with a density of
2–6×105 cells/well. After 24 h, the total RNA was
isolated from the three groups of cells and quantitative polymerase
chain reaction was employed to measure the CDK6 mRNA expression
level in each group. *P<0.05, statistically
significant difference compared with the blank group (n=3). (B) The
protein expression of CDK6 in each group of cells. The three groups
of HeLa cells were seeded into 6-well plates with a density of
2–6×105 cells/well. After 24 h, the total protein was
isolated from the three groups of cells and western blotting
analysis was employed to detect the CDK6 protein expression in each
group. CDK6, cyclin-dependent protein kinase 6; NC, normal control;
miR, microRNA. |
Discussion
Abnormal miRNA expression is closely correlated with
the occurrence and development of prostate (9), lung (10), colon (11) and numerous other human tumor types.
Approximately half of the miRNA is located at tumor-associated
genomic regions or fragile sites (12). The miRNA expression profiles of
various tumor types are different (13). Currently, major miRNAs involved in
tumor formation are divided into two categories. One category is
oncogenic miRNA, which promotes tumorigenesis by inhibiting the
expression of tumor suppressor genes, such as miR-106a (14) and miR-21 (15); the other category is tumor
suppressor miRNA, which promotes tumor formation by activating
oncogenes to inhibit cell differentiation and the cell cycle, such
as miR-15b and miR-200b (16). At
present, miRNA-overexpression vectors are commonly constructed to
study the mechanisms of miRNAs and their downstream target genes in
a variety of tumor types.
A number of studies have suggested that
tumorigenesis may be caused by regulation disorders of cell
cycle-related proteins, including cyclins, CDKs and CDK inhibitors.
CDK6, including 7 exons, is one of the CDK family members located
on human chromosome 7. It regulates the progress of the G1 phase by
combining with cyclin D to promote the phosphorylation of the tumor
suppressor gene, Rb, and by releasing the transcription factor,
E2F, into the nucleus to affect the promoters of the associated
genes (17), thereby promoting
tumorigenesis.
miRNA expression profiles are different in various
types of tumor. The same miRNA is able to regulate multiple target
genes. For example, miR-21 targets both programmed cell death
protein 4 (18) and myristoylated
alanine-rich C kinase substrate (9). Similarly, the same gene may be
regulated by a number of miRNAs. For instance, phosphatase and
tensin homolog may be regulated by either miR-205 (10) or miR-21 (11). Therefore, it is very important to
determine the corresponding downstream target genes for the late
mechanism study of a particular tumor miRNA. The present study
demonstrates that a good nucleotide complementary relationship
exists between miR-145 and the CDK6 3′ untranslated region, by
revealing that the expression of CDK6 is significantly inhibited by
miR-145 transfection. This suggests that miR-145 may directly
target the expression of CDK6 to inhibit the proliferation of
cervical cancer cells.
In summary, the expression of pcDNA6.2-GW-miR-145
downregulated the expression of CDK6 and inhibited the
proliferation of HeLa cervical cancer cells, providing a basis for
the further study of the effects of miR-145 on the biological
behaviors of cervical cancer cells and the associated
mechanisms.
Acknowledgements
This study was supported by the Science and
Technology Research and Development Program of Shanxi Province
(2011K13-02-06).
References
|
1
|
Ni J, Gao S, Cui LY and Li SW:
Intracranial arterial occlusive lesion in patients with Graves’
disease. Chin Med Sci J. 21:140–144. 2006.
|
|
2
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peiretti M, Zapardiel I, Zanagnolo V, et
al: Management of recurrent cervical cancer: a review of the
literature. Surg Oncol. 21:e59–e66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qiang R, Wang F, Shi LY, et al: Plexin-B1
is a target of miR-214 in cervical cancer and promotes the growth
and invasion of HeLa cells. Int J Biochem Cell Biol. 43:632–641.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deftereos G, Corrie SR, Feng Q, Morihara
J, Stern J, Hawes SE and Kiviat NB: Expression of mir-21 and
mir-143 in cervical specimens ranging from histologically normal
through to invasive cervical cancer. PloS One. 6:e284232011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kano M, Seki N, Kikkawa N, et al: miR-145,
miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in
esophageal squamous cell carcinoma. Int J Cancer. 127:2804–2814.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiyomaru T, Enokida H, Tatarano S, et al:
miR-145 and miR-133a function as tumour suppressors and directly
regulate FSCN1 expression in bladder cancer. Br J Cancer.
102:883–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li T, Li D, Sha J, Sun P and Huang Y:
MicroRNA-21 directly targets MARCKS and promotes apoptosis
resistance and invasion in prostate cancer cells. Biochem Biophys
Res Commun. 383:280–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai J, Fang L, Huang Y, et al: miR-205
targets PTEN and PHLPP2 to augment AKT signaling and drive
malignant phenotypes in non-small cell lung cancer. Cancer Res.
73:5402–5415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roy S, Yu Y, Padhye SB, et al:
Difluorinated-curcumin (CDF) restores PTEN expression in colon
cancer cells by down-regulating miR-21. PLoS One. 8:e685432013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA, Sevignani C, Dumitru CD, et al:
Human microRNA genes are frequently located at fragile sites and
genomic regions involved in cancers. Proc Natl Acad Sci USA.
101:2999–3004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mattick JS and Makunin IV: Non-coding RNA.
Hum Mol Genet. 15:R17–R29. 2006. View Article : Google Scholar
|
|
14
|
Xiao B, Guo J, Miao Y, et al: Detection of
miR-106a in gastric carcinoma and its clinical significance. Clin
Chim Acta. 400:97–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao W, Shen H, Liu L, Xu J, Xu J and Shu
Y: MiR-21 overexpression in human primary squamous cell lung
carcinoma is associated with poor patient prognosis. J Cancer Res
Clin Oncol. 137:557–566. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun L, Yao Y, Liu B, et al: MiR-200b and
miR-15b regulate chemotherapy-induced epithelial-mesenchymal
transition in human tongue cancer cells by targeting BMI1.
Oncogene. 31:432–445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sridhar J, Akula N and Pattabiraman N:
Selectivity and potency of cyclin-dependent kinase inhibitors. AAPS
J. 8:E204–E221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheedy FJ, Palsson-Mcdermott E, Hennessy
EJ, et al: Negative regulation of TLR4 via targeting of the
proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat
Immunol. 11:141–147. 2010. View
Article : Google Scholar : PubMed/NCBI
|
















