Introduction
Rheumatoid arthritis (RA) is an autoimmune
inflammatory disease that has been shown to be associated with the
destruction of articular cartilage and loss of the function of
joints (1). A study demonstrated
that complex cytokine networks exist in RA, the biological effects
of which are associated with the relative serum concentrations of
inflammatory cytokines and their inhibitors. The interactions of
cytokines play an important role in inflammation, adhesion,
neovascularization and decreased bone density (2).
Leptin is a type of hormone constitutively secreted
by adipose tissue with a molecular weight of 16 kDa. It was
originally described as a regulator of food intake and energy
expenditure (3). A study revealed
that leptin plays an important role in autoimmune diseases through
proinflammatory functions on T-helper type 1 (Th1) cells (4). Patients with RA in the acute phase
exhibit increased serum leptin levels and the leptin concentration
in the joint fluid was lower than with that in the serum (5). A positive correlation between the
levels of leptin and proinflammatory cytokines, including tumor
necrosis factor-α (TNF-α) and interleukin (IL)-6, has been
identified (6). However, the
association between leptin levels and RA activity levels of has not
been fully elucidated. The Janus kinase (JAK)/signal transducer and
activator of transcription (STAT) signal transduction pathway is a
major mediator of the biological effects of leptin, including
proinflammation and immunological regulation. When leptin combines
with a leptin receptor, the corresponding molecules are activated
and transported to the cell nucleus, which promotes the
transcription of target genes that involves two key molecules:
Phosphorylated (p)-STAT1 and p-STAT3. Therefore, lowering leptin
levels may be an important strategy in the treatment of RA
(7).
RA is a chronic disease that requires the intake of
drugs, including antirheumatics, non-steroidal anti-inflammatory
drugs and biological agents (8).
Patients are prone to discontinue treatment due to the side-effects
of the drugs. It has been reported that 33–75% of RA patients
consider food to play an important role in their severity of their
symptoms and 20–50% have tried dietary manipulation in an attempt
to relieve suffering (9,10). It has been indicated that vitamins
with antioxidant properties may help to treat RA. Antioxidants,
including vitamin A (VitA) and vitamin E (VitE), have been
demonstrated to manifest inhibitory effects on inflammatory
cytokines in vivo (11,12).
However, there are no studies concerning the effects of VitA and
VitE on the leptin levels of rats with RA. Therefore, the present
study aimed to examine the effects of VitA and VitE on the levels
of leptin and other related experimental and clinical indices in
rats with collagen-induced arthritis (CIA) and to explore the
possible mechanisms of these effects associated with the signal
transduction pathway of leptin.
Materials and methods
Animals and treatments
Male Wistar rats (147±15 g) from Southern Medical
University Laboratory Animal Co. Ltd. (Guangzhou, China) were used
in the experiments. The animal care and study protocols employed
were in accordance with the guidelines of the Animal Care and Use
Committee of Southern Medical University (Guangzhou, China) and the
Organization for Economic Co-operation and Development (13). The rats were housed in cages in a
climate-controlled room with a 12-h light-dark cycle. Throughout
the study, the animals were allowed access to regular standard rats
chow and water ad libitum.
After a one-week acclimation period, the animals
were administered an intradermal injection (100 μl) of bovine type
II collagen emulsified in incomplete Freund’s adjuvant or 0.9%
normal saline (the model and control groups, respectively). Two
weeks later, the rats were administered a booster intradermal
injection. At the end of the fourth week, the arthritis index
(14) was applied to evaluate paw
swelling. Each paw was graded on a scale of 0–4 as follows: 0,
normal, without any macroscopic signs of arthritis; 1, mild, but
definite redness and swelling of the ankle or apparent redness and
swelling limited to individual digits, regardless of the number of
affected digits; 2, moderate redness and swelling of the ankle; 3,
redness and swelling of the entire paw including the digits; and 4,
maximally inflamed limb with involvement of multiple joints. The
four paw scores for each animal were summed. The rats with a score
of >6 were used in the following experiments.
The model group was divided into four subgroups: i)
VitA [42.86 μg retinol equivalents/kg body weight (b.w.)] (n=6);
ii) VitE (200 mg/kg b.w.) (n=6); iii) ibuprofen (50 mg/kg b.w.)
(n=6) and iv) untreated model groups (n=6). The rats in the VitA,
VitE and ibuprofen groups received intragastric administration of
VitA, VitE and ibuprofen, respectively, once daily for four weeks.
At the end of the eighth week, all rats were anaesthetized and
sacrificed, and blood samples and joint synovium tissue were
extracted. The tissue was stored at −80°C until it was used for
western blot analysis.
Determination of serum leptin levels and
other related experimental and clinical indices
Serum was isolated from the blood samples by
centrifugation at 11.1 × g for 10 min and then maintained at −20°C
prior to the following assays. The levels of leptin, TNF-α, IL-6,
IL-10, IL-4, C-reactive protein (CRP) and rheumatic factor (RF)
were measured by ELISA using commercial kits (R&D Systems,
Inc., Minneapolis, MN, USA). The erythrocyte sedimentation rate
(ESR) was also determined. ESR was determined by automated
erythrocyte sedimentation rate analyzer (ELECTA LAB S.r.l, Via
Balzella, Italy).
Western blot analysis of p-STAT1, p-STAT3
and leptin expression levels
The tissue samples were homogenized in complete
radioimmunoprecipitation assay lysis buffer (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). The total protein was
quantified with a bicinchoninic acid protein assay kit. All
preparations were performed at 4°C. For western blotting, a total
of 15 μl of the mixture of protein and sample buffer was loaded per
lane and the proteins were electrophoretically separated on an
SDS-PAGE gel. The protein bands were transferred to a
polyvinylidene fluoride membrane using a Trans-Blot SD Semi-Dry
Electrophoretic Transfer cell (Santa Cruz Biotechnology, Inc.). The
gels were then incubated with primary antibodies (Santa Cruz
Biotechnology, Inc.) against p-STAT1, p-STAT3 and leptin overnight
at 4°C. Subsequently, the gels were incubated with secondary
antibodies (Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The films were scanned and analyzed using Quantity One
(Bio-Rad Laboratories Inc., Berkeley, CA, USA) to quantify the
protein levels. The relative protein levels were counted by
comparison with the beta-actin control.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical differences between groups were compared by one-way
analysis of variance with SPSS software for statistical analysis,
version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of VitA and VitE on the levels of
leptin
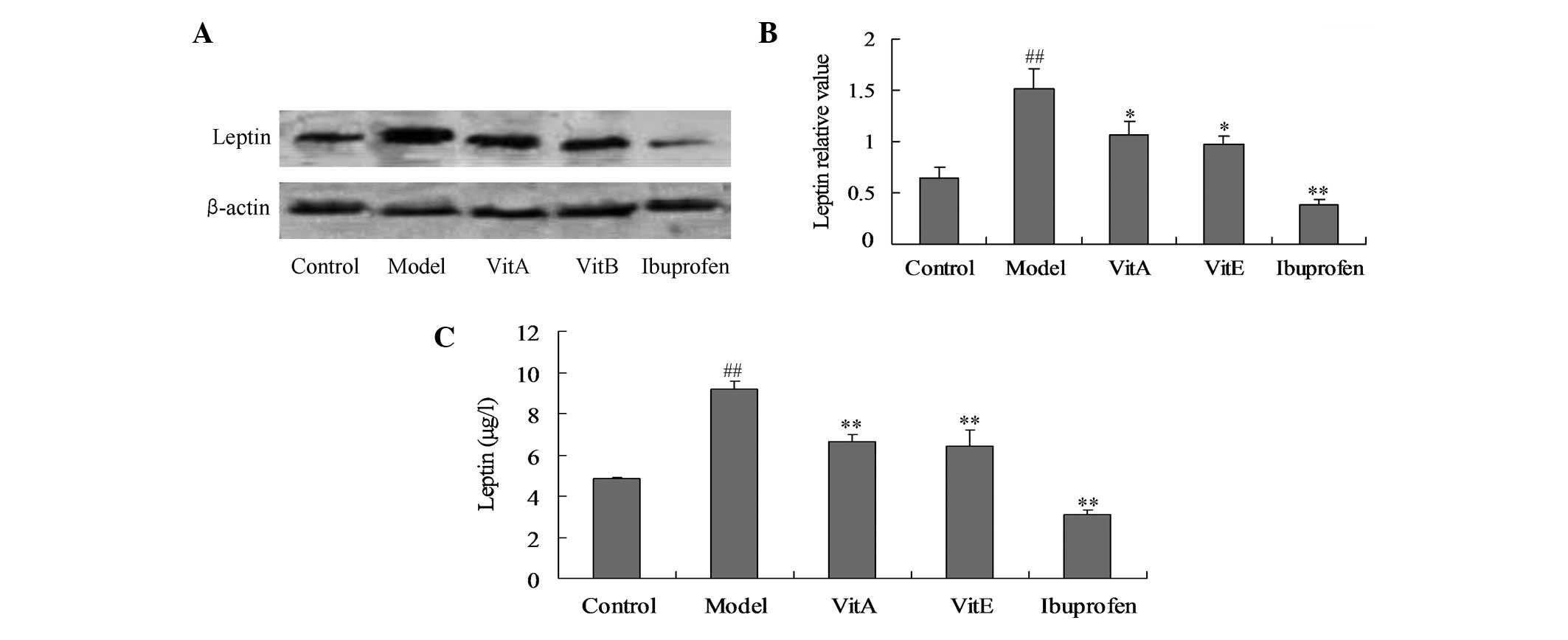
The leptin levels in the joint synovium tissue and
serum were detected by western blot analysis and ELISA,
respectively. Compared with the those of the control animals, the
leptin levels were significantly increased in the untreated model
animals, as detected by the western blot analysis and ELISA
(Fig. 1; P<0.01). Four-week
administration of VitA and VitE significantly reduced the levels of
leptin compared with those of the untreated model animals (Fig. 1; P<0.05).
Effects of VitA and VitE on the levels of
serum TNF-α, IL-6, IL-10 and IL-4
Compared with those of the control group, there were
significant increases in the serum TNF-α and IL-6 levels and
significant reductions in the serum IL-10 and IL-4 levels in the
untreated model group (P<0.01). Four weeks of VitA and VitE
administration significantly reduced the levels of TNF-α
(P<0.05) and IL-6 (P<0.01) compared with those of the
untreated model group. In addition, significant increases in the
serum IL-10 levels was identified in the VitA and VitE groups
compared with those of the untreated model group, but the changes
in the serum IL-4 levels were not found to be significant.
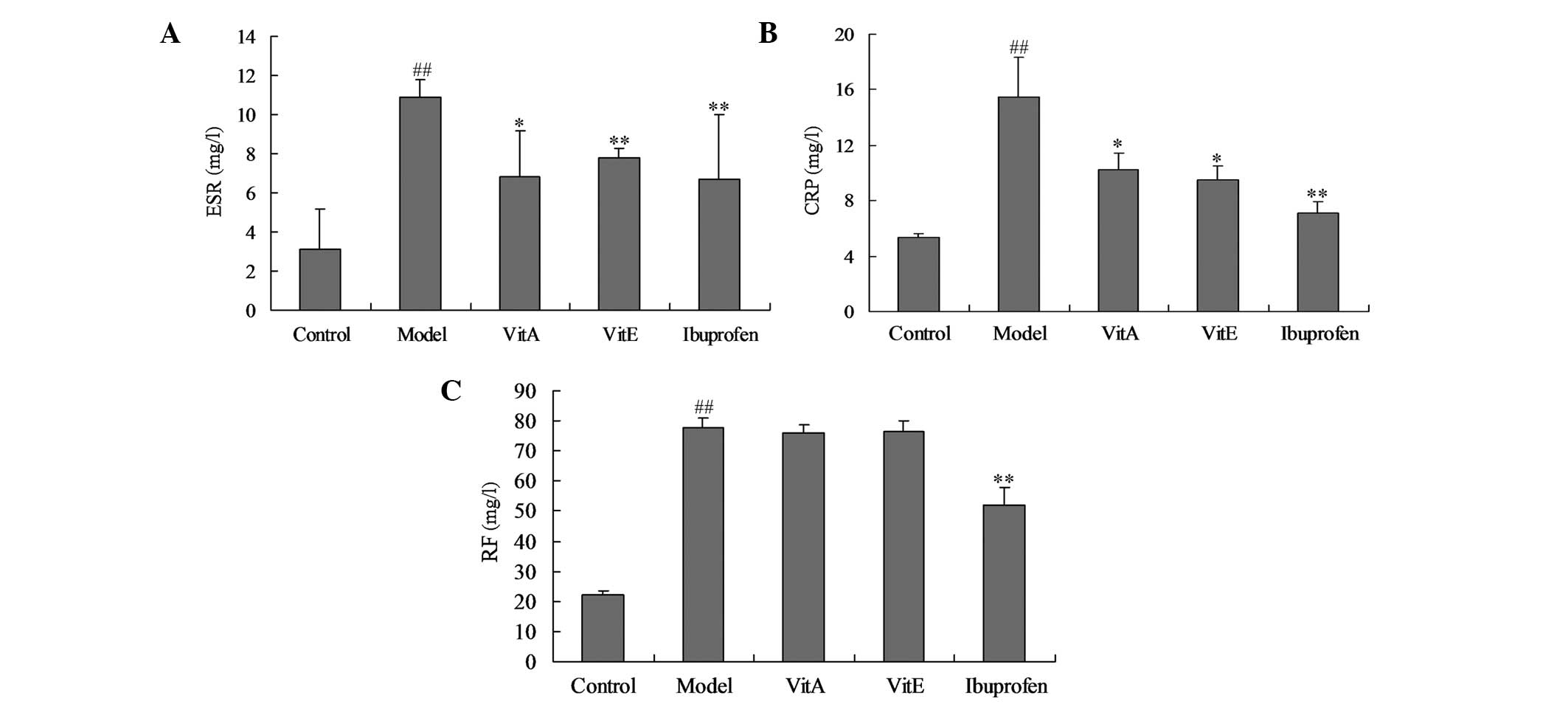
Effects of VitA and VitE on the ESR and
the levels of CRP and RF
ESR, CRP and RF are markers of the disease activity
index in RA. Fig. 3 shows the
changes in the levels of these markers. Compared with those of the
control group, the ESR, and the CRP and RF levels in the untreated
model group were significantly increased (P<0.01). Treatment
with VitA or VitE was associated with significant reductions in the
ESR and CRP levels (P<0.05), but the levels of RF were not found
to be significantly reduced compared with those of the untreated
model group.
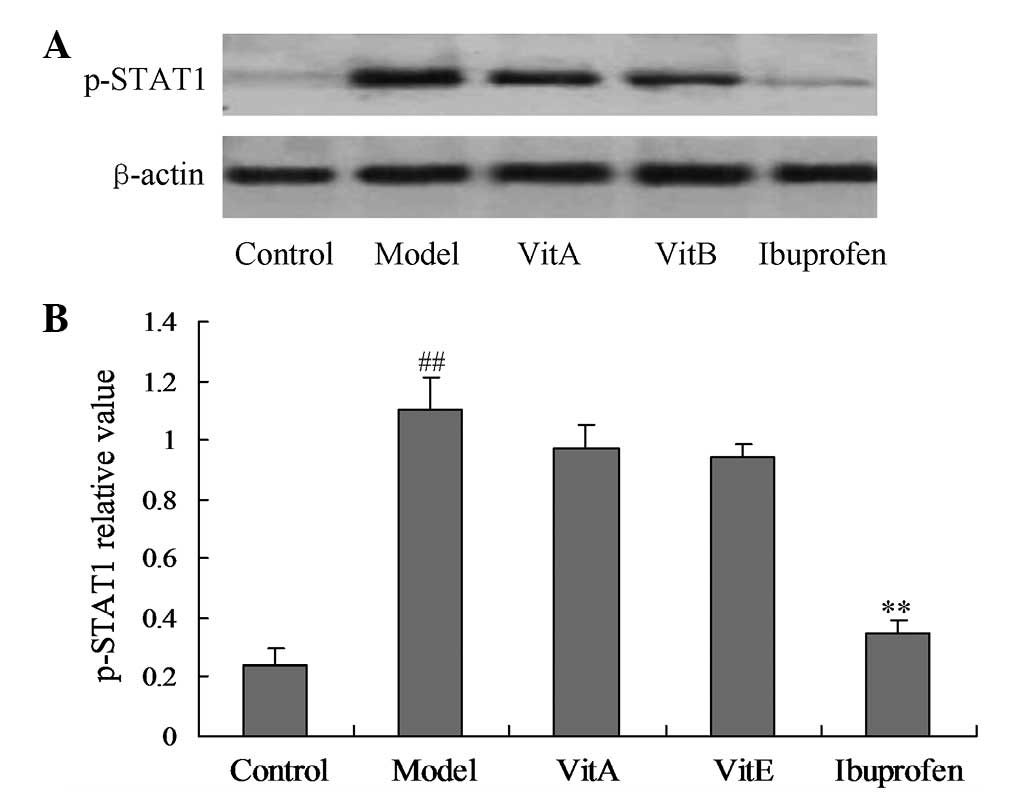
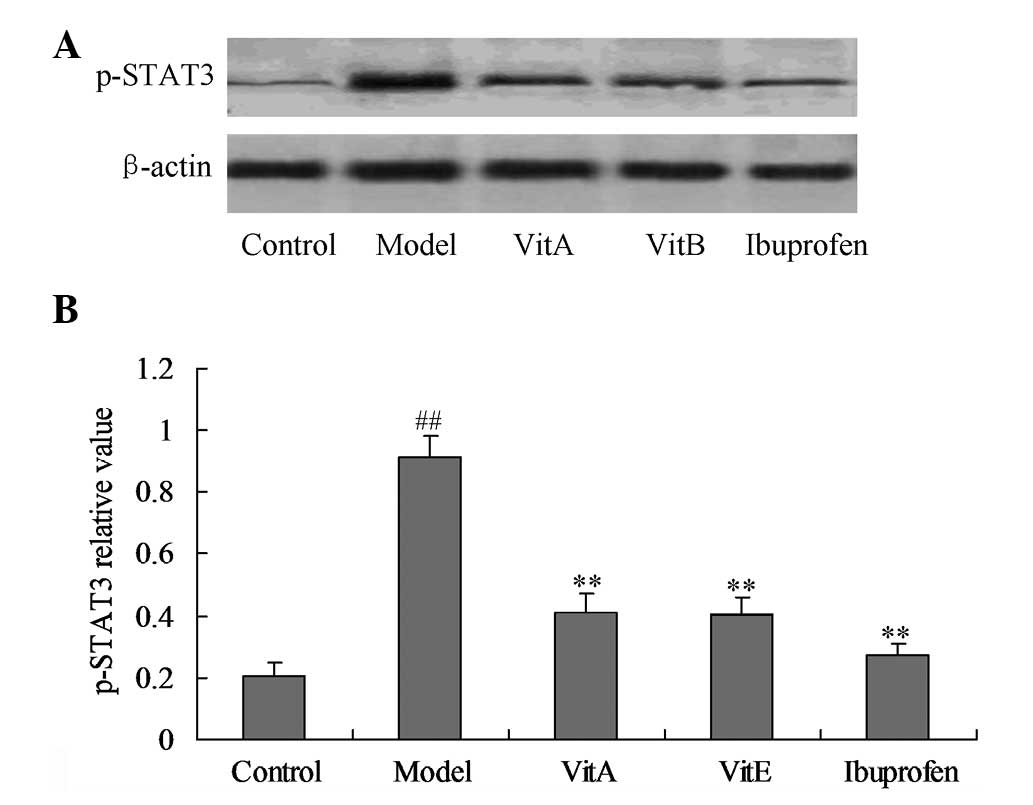
Effects of VitA and VitE on p-STAT1 and
p-STAT3 protein expression levels
p-STAT1 and p-STAT3 are molecules associated with
the JAK/STAT signal transduction pathway. Figs. 4 and 5 show the effects of VitA and VitE on the
p-STAT1 and p-STAT3 protein expression levels. The model group
showed a significant upregulation of the p-STAT1 and p-STAT3
protein expression levels compared with those of the control group
(Figs. 4 and 5; P<0.01). The rats treated with VitA
or VitE for four weeks showed significant reductions of the p-STAT3
protein expression levels (P<0.01), but the treatment effect was
not significant for the p-STAT1 protein expression levels compared
with those of the untreated model group.
Discussion
The present study indicates the role of leptin in
the pathogenesis of CIA in rats and the effects of VitA and VitE on
cytokine networks and other related indices. The study also
examined the effects of VitA and VitE on p-STAT1 and p-STAT3
protein expression levels, which are markers of the signal
transduction pathway of leptin.
An unbalanced cytokine network exists in the
pathogenesis of RA, which manifests as increased levels of
inflammatory cytokines and reduced levels of anti-inflammatory
cytokines (15). In the present
study, compared with those of the control group, rats with CIA had
higher levels of TNF-α and IL-6 and lower levels of IL-4 and IL-10,
which is consistent with the results in other studies (16,17).
The results of the present study indicated that TNF-α and IL-6 were
inflammatory cytokines while IL-4 and IL-10 were anti-inflammatory
cytokines in the pathogenesis of RA. The study also observed the
alterations in the levels of leptin. A previous study has
demonstrated increased serum leptin levels in patients with RA
(5). In the present study,
increased leptin levels existed in the rats with CIA, which
indicates that leptin plays an important role in RA and is
identifiable as an inflammatory cytokine. The possible mechanisms
involving leptin include two scenarios. One is that leptin
increases the secretion of inflammatory cytokines by the induction
of Th1-cell differentiation, and the other is that leptin
suppresses the apoptosis of senile cells and this results in the
deterrence of autoantigen clearance (18).
RA is a chronic disease that requires long-term
intake of drugs, including antirheumatics and non-steroidal
anti-inflammatory drugs. Patients with RA are prone to drop out of
drug treatment due to the adverse effects. In RA, free radicals are
associated with joint inflammation and damage. Antioxidant
supplements and diets have long been advocated for the treatment
and prevention of RA due to their protective role against free
radicals (19). It has been
reported that 33–75% of RA patients consider that food plays an
important role in the severity of their symptoms and 20–50% have
tried dietary manipulation in an attempt to relieve their suffering
(9,10). Epidemiological studies have shown
that a low intake of dietary antioxidants is associated with the
incidence of RA (20,21). Despite the fact that vitamins with
antioxidant properties have been demonstrated to be beneficial to
RA in a cellular study, there are contradicting results concerning
the effects of antioxidant vitamins on the development of RA in
animal and clinical studies (22–24).
In the present study, the effects of VitA and VitE on the
inflammatory cytokine networks were observed in CIA model rats.
When treated with VitA and VitE for four weeks, the levels of
leptin, TNF-α and IL-6 were significantly reduced while the levels
of IL-10 were significantly increased. This suggests that VitA and
VitE suppress the inflammatory reaction in RA by increasing the
levels of anti-inflammatory cytokines and reducing the levels of
inflammatory cytokines. Due to the complicated cytokine networks in
RA, cytokines interact by several signal transduction pathways.
Therefore, the stimulative effects of VitA and VitE on IL-4 levels
may be counteracted by the interaction between cytokines in RA.
This explains the observation that the levels of IL-4 were not
increased following treatment with VitA and VitE. Thus, the
interactions of cytokines in RA require further investigation.
The present study also examined the effects of VitA
and VitE on the ESR, and the levels of CRP and RF, which reflect
the levels of disease activity in RA. An elevated ESR indicates the
body is in a pathological status. Thus, the ESR is widely used in
the monitoring of the levels of disease activity in patients with
various ailments, including infection and inflammation (25). CRP is a reactive protein in the
acute phase response, which is observed at increased levels in a
number of ailments, including tissue damage, myocardial infarction
and malignant tumors (26). RF is
one of most widely used indicators in RA and although it has a low
specificity, a study has suggested that RF is one of the most
potent factors in the prognosis of RA due to its intimate
association with joint damage (27). The results of the present study
show that VitA and VitE reduce the ESR and CRP levels, the
indicators of disease activity. The evidence that the levels of RF
are not altered implies that RF is not a specific index of CIA in
rats.
The present study adds a novel dimension to the
protective effects of VitA and VitE on CIA rats by the assessment
of the levels of p-STAT1 and p-STAT3 proteins, two key molecules of
the JAK/STAT signal transduction pathway. The JAK/STAT signal
transduction pathway is a major mediator of the biological effects
of leptin, including cell proliferation and differentiation,
immunological regulation and inflammation in RA. Once leptin is
combined with a leptin receptor, the corresponding molecules are
activated and transported to the cell nucleus, which promotes the
transcription of the target genes p-STAT1 and p-STAT3 (7). In the present study, compared with
those of the control group, the expression levels of p-STAT1 and
p-STAT3 were significantly increased in the untreated model group,
which suggests they are involved in the pathogenesis of RA. In the
CIA model rats treated with VitA and VitE, the levels of p-STAT3
were significantly reduced, which is consistent with a previous
study that showed p-STAT3 expression levels are upregulated in
zymosan-induced arthritis (28).
However, this phenomenon was not observed for p-STAT1 (29,30).
In the present study, the difference in the effects of VitA and
VitE between p-STAT1 and p-STAT3 suggests that the two molecules
have disparate functions in RA. A clinical study involving 30
patients with RA identified that upregulation of p-STAT1 expression
levels increased the inflammation in the synovium of joints through
activation of the expression of related genes (29). Another cellular study showed that
increased p-STAT1 expression levels promote apoptosis of synovium
cells and suppress the inflammatory response (30). In the present study, p-STAT1
expression levels were not significantly reduced, which indicates
p-STAT1 may play a protective role by suppressing the proliferation
of synovial cells. The mechanism by which p-STAT3 expression levels
alone are reduced requires further study.
A limitation of the present study is that the
protective effects of VitA and VitE observed in the animal model of
RA may not be similar to those observed clinically. The
interactions of leptin and other cytokines require further study.
These results may have implications for the rational development of
antioxidant vitamins for the treatment of RA.
In conclusion, VitA and VitE reduced the levels of
serum leptin protein and other cytokines in a murine model of RA.
Furthermore, VitA and VitE also reduced the levels of p-STAT3
protein. The present study may provide a novel approach for the
treatment of RA.
References
|
1
|
Li WH, Li H, Song WQ, Hu YL, Liu YH, Da R,
Chen XB, Li Y, Ling H, Zhong ZH and Zhang FM: Differential
diagnosis of systemic lupus erythematosus and rheumatoid arthritis
with complements C3 and C4 and C-reactive protein. Exp Ther Med.
6:1271–1276. 2013.PubMed/NCBI
|
|
2
|
Gaston JS: Cytokines in arthritis - the
‘big numbers’ move centre stage. Rheumatology (Oxford).
47:8–12. 2008.
|
|
3
|
Maffei M, Halaas J, Ravussin E, Pratley
RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al:
Leptin levels in human and rodent: measurement of plasma leptin and
ob RNA in obese and weight-reduced subjects. Nat Med. 1:1155–1161.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sagiroglu T, Torun N, Yagci M, Yalta T,
Sagiroglu G and Oguz S: Effects of apelin and leptin on renal
functions following renal ischemia/reperfusion: An experimental
study. Exp Ther Med. 3:908–914. 2012.PubMed/NCBI
|
|
5
|
Bokarewa M, Bokarew D, Hultgren O and
Tarkowski A: Leptin consumption in the inflamed joints of patients
with rheumatoid arthritis. Ann Rheum Dis. 62:952–956. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lane ML and Vesely DL: Reduction of leptin
levels by four cardiac hormones: Implications for hypertension in
obesity. Exp Ther Med. 6:611–615. 2013.PubMed/NCBI
|
|
7
|
Bates SH, Stearns WH, Dundon TA, Schubert
M, Tso AW, Wang Y, Banks AS, Lavery HJ, Hag AK, Maratos-Flier E,
Neel BG, Schwartz MW and Myers MG Jr: STAT3 signalling is required
for leptin regulation of energy balance but not reproduction.
Nature. 421:856–859. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smolen JS, Landew R, Breedveld FC, et al:
EULAR recommendations for the management of rheumatoid arthritis
with synthetic and biological disease-modifying antirheumatic
drugs. Ann Rheum Dis. 69:964–975. 2010. View Article : Google Scholar
|
|
9
|
Martin RH: The role of nutrition and diet
in rheumatoid arthritis. Proc Nutr Soc. 57:231–234. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salminen E, Heikkilä S, Poussa T, Lagström
H, Saario R and Salminen S: Female patients tend to alter their
diet following the diagnosis of rheumatoid arthritis and breast
cancer. Prev Med. 34:529–535. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stone J, Doube A, Dudson D and Wallace J:
Inadequate calcium, folic acid, vitamin E, zinc, and selenium
intake in rheumatoid arthritis patients: results of a dietary
survey. Semin Arthritis Rheum. 27:180–185. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Canter PH, Wilder B and Emst E: The
antioxidant vitamins A, C, E and selenium in the treatment of
arthritis: a systematic review of randomized clinical trials.
Rheumatology (Oxford). 46:1223–1233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Doe JE, Lewis RW and Botham PA: Comments
on a scientific and animal welfare assessment of the OECD Health
Effects Test Guidelines for the safety testing of chemicals under
the European Union REACH system. Altern Lab Anim. 34:111–114.
2006.
|
|
14
|
Tanaka D, Kagari T, Doi H and Shimozato T:
Essential role of neutrophils in anti-type II collagen antibody and
lipopolysaccharide-induced arthritis. Immunology. 119:195–202.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McInnes IB and Schett G: Cytokines in the
pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 7:429–442.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kuroyanagi G, Yamada K, Imaizumi T,
Mizutani J, Wada I, Kozawa O, Tokuda H and Otsuka T: Leg lymphedema
caused by iliopectineal bursitis associated with destruction of a
rheumatoid hip joint: A case report. Exp Ther Med. 6:887–890.
2013.PubMed/NCBI
|
|
17
|
Wirjatijasa F, Dehghani F, Blaheta RA,
Korf HW and Hailer NP: Interleukin-4, interleukin-10, and
interleukin-1-receptor antagonist but not transforming growth
factor-beta induce ramification and reduce adhesion molecule
expression of rat microglial cells. J Neurosci Res. 68:579–587.
2002. View Article : Google Scholar
|
|
18
|
Martín-Romero C, Santos-Alvarez J, Goberna
R and Sanchez-Margalet V: Human 1eptin enhances activation and
proliferation of human circulation T 1ymphocytes. Cell Immunol.
199:15–24. 2000.PubMed/NCBI
|
|
19
|
Hagfors L, Leanderson P, Sköldstam L,
Andersson J and Johansson G: Antioxidant intake, plasma
antioxidants and oxidative stress in a randomized, controlled,
parallel, Mediterranean dietary intervention study on patients with
rheumatoid arthritis. Nutr J. 2:52003. View Article : Google Scholar
|
|
20
|
Wang Z, Chen Z, Yang S, Wang Y, Yu L,
Zhang B, Rao Z, Gao J and Tu S: (1)H NMR-based metabolomic analysis
for identifying serum biomarkers to evaluate methotrexate treatment
in patients with early rheumatoid arthritis. Exp Ther Med.
4:165–171. 2012.
|
|
21
|
Bae SC, Kim SJ and Sung MK: Inadequate
antioxidant nutrient intake and altered plasma antioxidant status
of rheumatoid arthritis patients. J Am Coll Nutr. 22:311–315. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edmonds SE, Winyard PG, Guo R, Kidd B,
Merry P, Langrish-Smith A, Hansen C, Ramm S and Blake DR: Putative
analgesic activity of repeated oral doses of vitamin E in the
treatment of rheumatoid arthritis. Results of a prospective placebo
controlled double blind trial. Ann Rheum Dis. 56:649–655. 1997.
View Article : Google Scholar
|
|
23
|
Wittenborg A, Petersen G, Lorkowski G and
Brabant T: Effectiveness of vitamin E in comparison with diclofenac
sodium in treatment of patients with chronic polyarthritis. Z
Rheumatol. 57:215–221. 1998.(In German).
|
|
24
|
Sakai A, Hirano T, Okazaki R, Okimoto N,
Tanaka K and Nakamura T: Large dose ascorbic acid administration
suppresses the development of arthritis in adjuvant-injected rats.
Arch Orthop Trauma Surg. 119:121–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Piva E, Fassina P and Plebani M:
Determination of the length of sedimentation reaction (erythrocyte
sedimentation rate) in non-anticoagulated blood with the Microtest
1. Clin Chem Lab Med. 40:713–717. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du Clos TW and Mold S: The role of C
reactive protein in the resolution of bacterial infection. Curr
Opin Infect Dis. 14:289–293. 2001.PubMed/NCBI
|
|
27
|
Erlandsen EJ and Randers E: Reference
interval for serum C-reactive protein in healthy blood donors using
the Dade Behring N Latex CRP mono assay. Scand J Clin Lab Invest.
60:37–43. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van der Pouw Kraan TC, van Gaalen FA,
Kasperkovite PV, et al: Rheumatoid arthritis is a heterogeneous
disease: evidence for differences in the activation of the STAT-1
pathway between rheumatoid tissues. Arthritis Rheum. 48:2132–2145.
2003.PubMed/NCBI
|
|
29
|
Krause A, Scaletta N, Ji JD and Ivashkiv
LB: Rheumatoid arthritis synoviocyte survival is dependent on
Stat3. J Immunol. 169:6610–6616. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nak SS, Lee S, Joo J, Kim HK, Sohn DR,
Kwon JT, Woo KM, Hong SJ and Kim HJ: Association of ADAMTS12
polymorphisms with rheumatoid arthritis. Mol Med Rep. 6:227–231.
2012.PubMed/NCBI
|